Improvement in Physiobiochemical and Yield Characteristics of Pea Plants with Nano Silica and Melatonin under Salinity Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Location and Treatments
- T1: control (plants irrigated with tap water under normal conditions).
- T2: plants treated with 50 mM NaCl.
- T3: plants treated with 50 mM NaCl and nano silica (50 mL L−1).
- T4: plants treated with 50 mM NaCl and melatonin (75 µM).
- T5: plants treated with 50 mM NaCl and nano silica (50 mL L−1) + melatonin (75 µM).
- T6: plants treated with 100 mM NaCl.
- T7: plants treated with 100 mM NaCl and nano silica (50 mL L−1).
- T8: plants treated with 100 mM NaCl and melatonin (75 µM).
- T9: plants treated with 100 mM NaCl and nano silica (50 mL L−1) + melatonin (75 µM).
2.2. Morphological Parameters
2.3. Biochemical and Physiological Parameters
2.3.1. Determination of Chlorophylls
2.3.2. Assay of Relative Water Content (RWC%)
2.3.3. Determination of Maximum Quantum Efficiency of PS II (Fv/Fm)
2.3.4. Assay of Electrolyte Leakage (EL%)
2.3.5. Determination of Lipid Peroxidation (MDA%)
2.3.6. Assay of Hydrogen Peroxide (H2O2) and Superoxide (O2−)
2.3.7. Determination of Proline Content
2.3.8. Determination of Total Phenolic Compounds
2.3.9. Determination of CAT, SOD and POX Activity
2.4. Yield Characteristics
2.5. Statistical Analysis
3. Results
3.1. Effect of Nano Silica and Melatonin on Plant Height (A), Leaves Number (B), Plant Dry Weight (g) and Flower Number Plant−1 (D) under Salinity Conditions
- T1: control (plants irrigated with tap water under normal conditions).
- T2: plants irrigated with 50 mM NaCl.
- T3: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1).
- T4: plants irrigated with 50 mM NaCl and treated with melatonin (75 µM).
- T5: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
- T6: plants irrigated with 100 mM NaCl.
- T7: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1).
- T8: plants irrigated with 100 mM NaCl and treated with melatonin (75 µM).
- T9: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
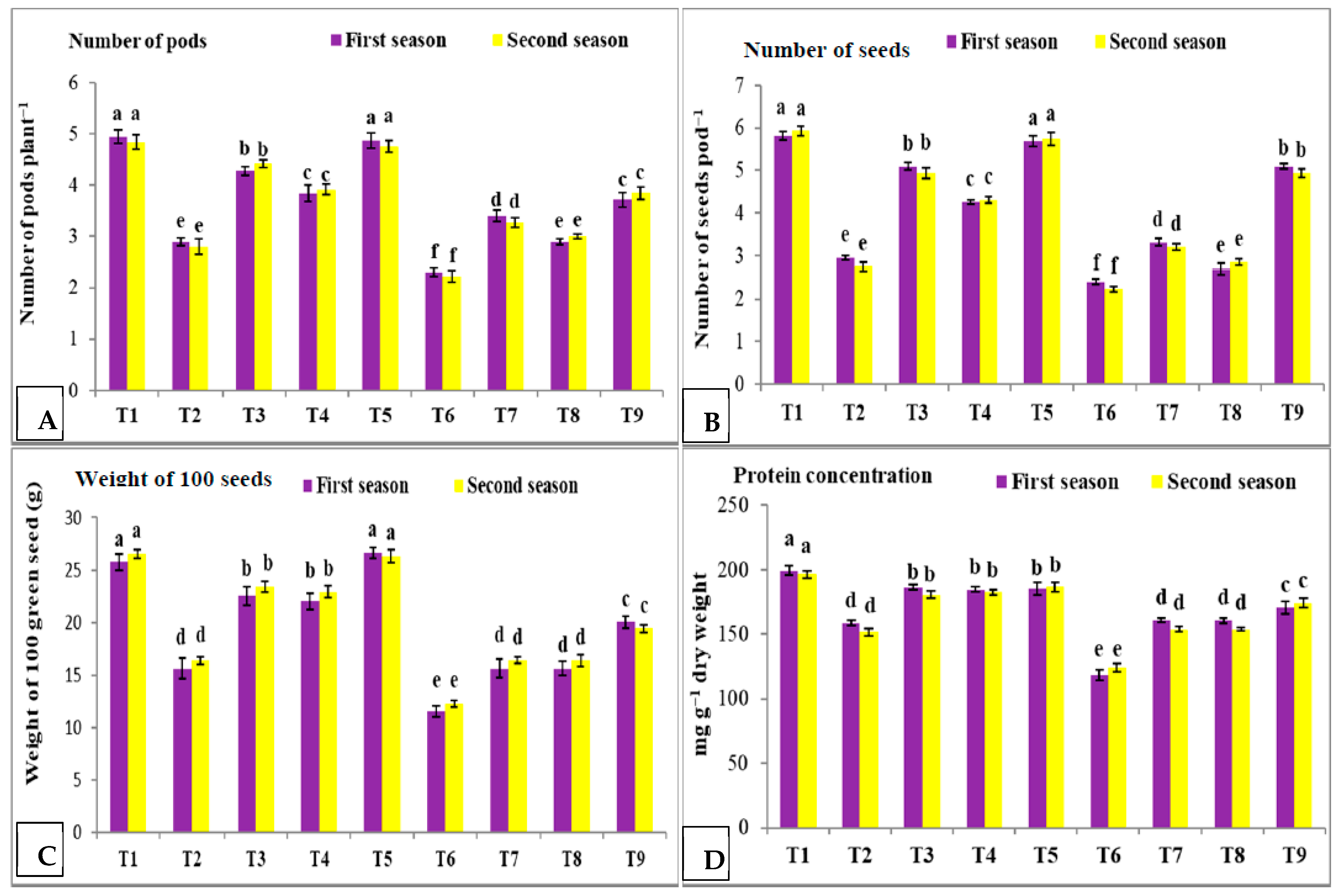
3.2. Effect of Nano Silica and Melatonin on Number of Pods Plant−1, Number of Seeds Pod−1, Weight of 100 Green Seeds and Protein Concentration in Pea Plants under Salinity Conditions
- T1: control (plants irrigated with tap water under normal conditions).
- T2: plants irrigated with 50 mM NaCl.
- T3: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1).
- T4: plants irrigated with 50 mM NaCl and treated with melatonin (75 µM).
- T5: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
- T6: plants irrigated with 100 mM NaCl.
- T7: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1).
- T8: plants irrigated with 100 mM NaCl and treated with melatonin (75 µM).
- T9: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
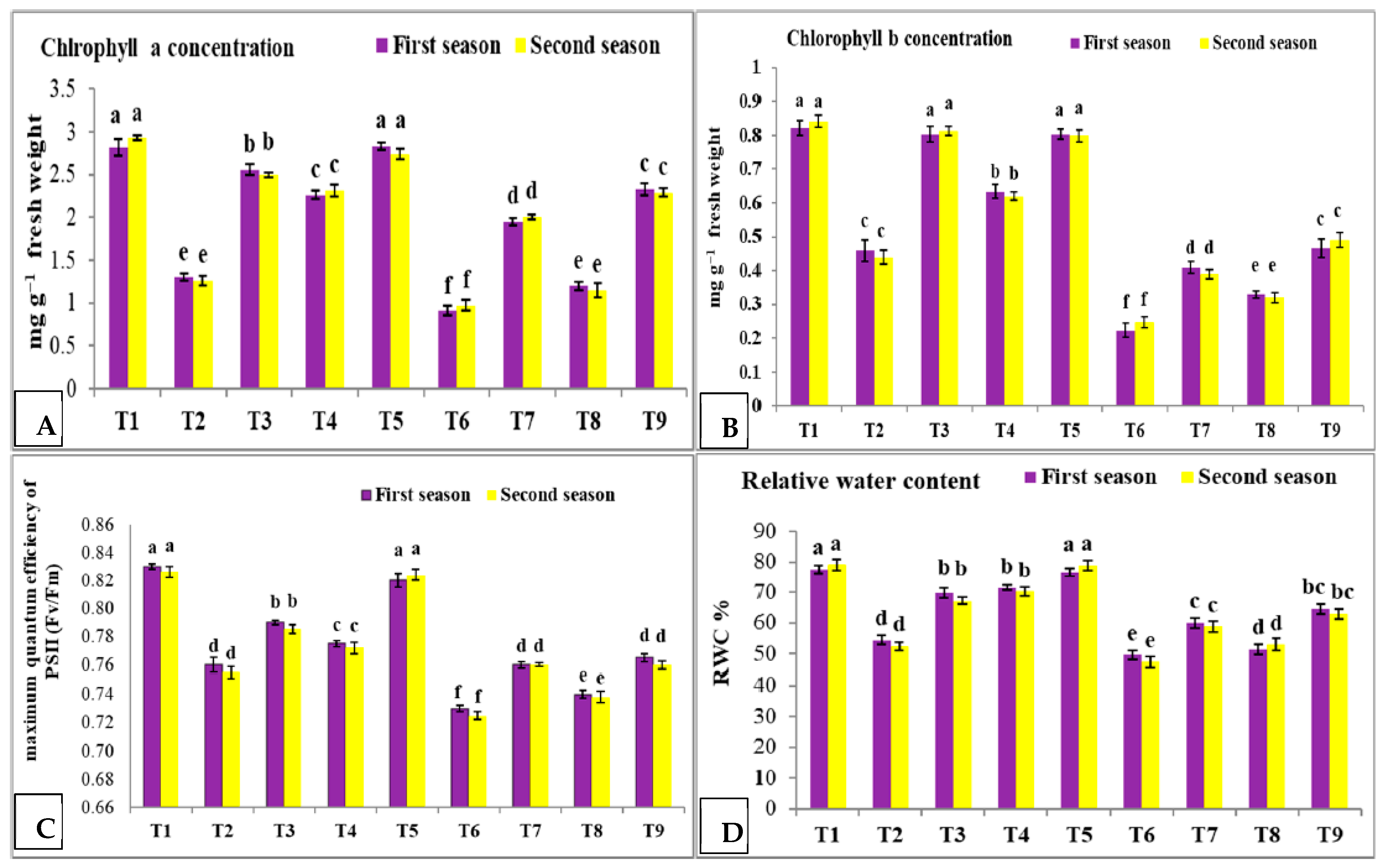
3.3. Effect of Nano Silica and Melatonin on Chlorophyll a and b, Maximum Quantum Efficiency of PS II and RWC in Pea Plants under Salinity Stress
- T1: control (plants irrigated with tap water under normal conditions).
- T2: plants irrigated with 50 mM NaCl.
- T3: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1).
- T4: plants irrigated with 50 mM NaCl and treated with melatonin (75 µM).
- T5: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
- T6: plants irrigated with 100 mM NaCl.
- T7: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1).
- T8: plants irrigated with 100 mM NaCl and treated with melatonin (75 µM).
- T9: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
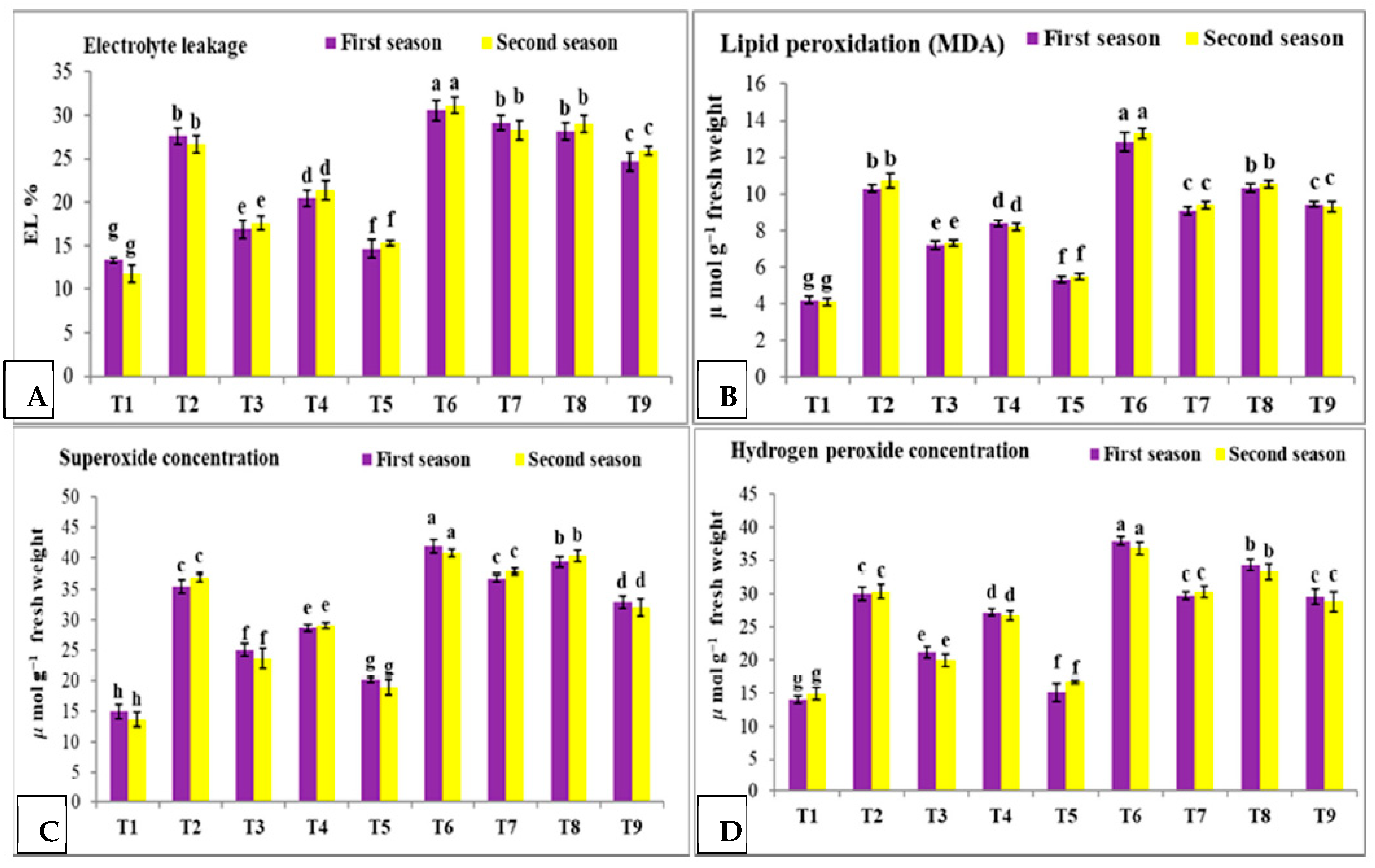
3.4. Effect of Nano Silica and Melatonin on EL%, MDA, Superoxide Concentration and H2O2 Concentration in Pea Plants under Salinity Stress
- T1: control (plants irrigated with tap water under normal conditions).
- T2: plants irrigated with 50 mM NaCl.
- T3: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1).
- T4: plants irrigated with 50 mM NaCl and treated with melatonin (75 µM).
- T5: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
- T6: plants irrigated with 100 mM NaCl.
- T7: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1).
- T8: plants irrigated with 100 mM NaCl and treated with melatonin (75 µM).
- T9: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
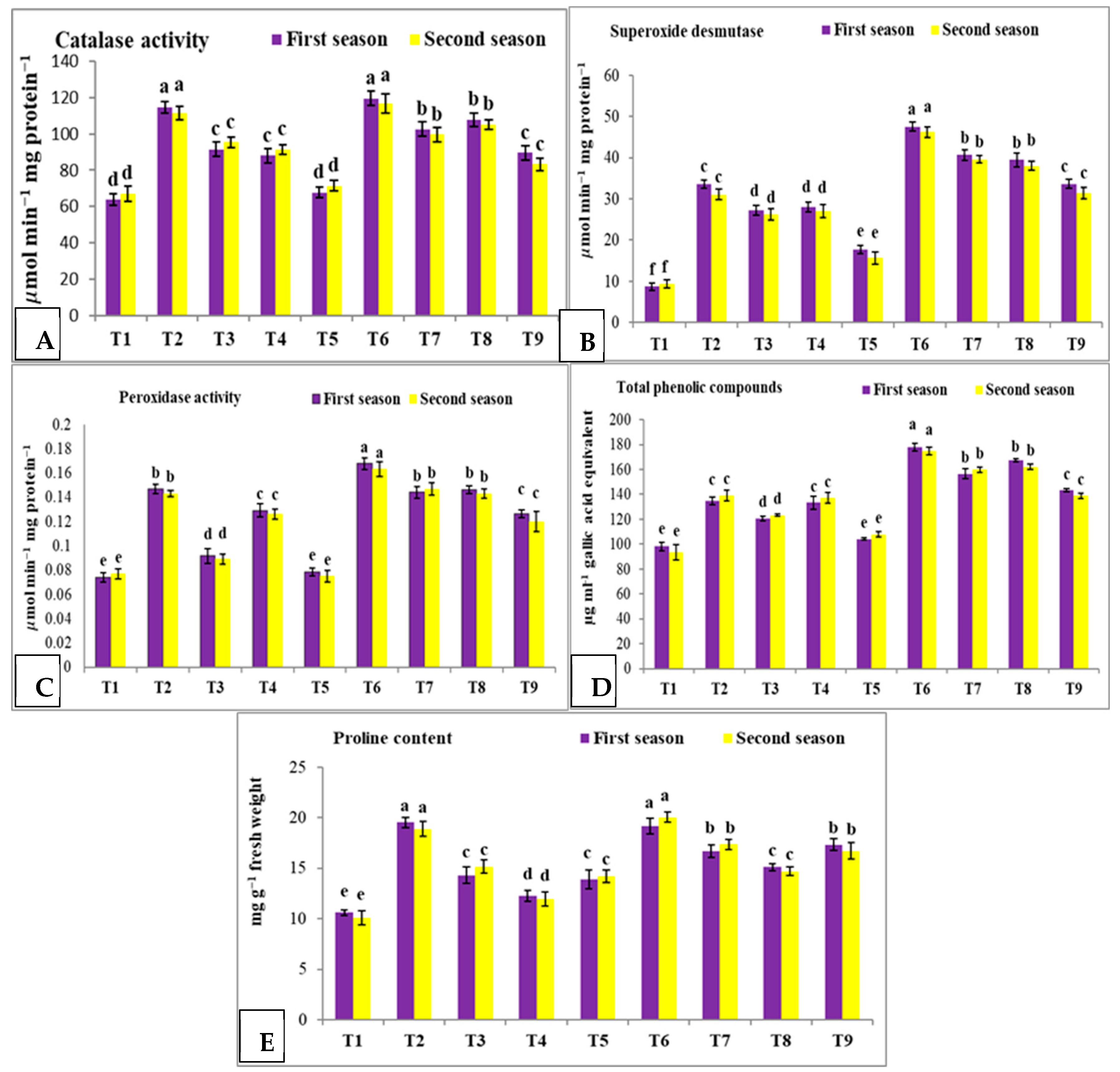
3.5. Effect of Nano Silica and Melatonin on CAT, SOD, POX, Total Phenolic Compounds and Proline Content in Pea Plants under Salinity Conditions
- T1: control (plants irrigated with tap water under normal conditions).
- T2: plants irrigated with 50 mM NaCl.
- T3: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1).
- T4: plants irrigated with 50 mM NaCl and treated with melatonin (75 µM).
- T5: plants irrigated with 50 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
- T6: plants irrigated with 100 mM NaCl.
- T7: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1).
- T8: plants irrigated with 100 mM NaCl and treated with melatonin (75 µM).
- T9: plants irrigated with 100 mM NaCl and treated with nano silica (50 mL L−1) + melatonin (75 µM).
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdelaal, K.; Hafez, Y.; Badr, M.; Youseef, W.; Esmail, S. Biochemical, histological and molecular changes in susceptible and resistant wheat cultivars inoculated with stripe rust fungus Puccinia striiformis f. sp. tritici. Egypt. J. Biol. Pest Control 2014, 24, 421–429. [Google Scholar]
- Hafez, Y.; Emeran, A.; Esmail, S.; Mazrou, Y.; Abdrabbo, D.; Abdelaal, K. Alternative treatments improve physiological characters, yield and tolerance of wheat plants under leaf rust disease stress. Fresenius Environ. Bull. 2014, 29, 4738–4748. [Google Scholar]
- Omara, R.I.; El-Kot, G.A.; Fadel, F.M.; Abdelaal, K.A.A.; Saleh, E.M. Efficacy of certain bioagents on patho-physiological characters of wheat plants under wheat leaf rust stress. Physiol. Mol. Plant Pathol. 2019, 106, 102–108. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; El-Shawy, E.-S.A.-A.; Hafez, Y.M.; Abdel-Dayem, S.M.A.; Chidya, R.C.G.; Saneoka, H.; El Sabagh, A. Nano-Silver and non-traditional compounds mitigate the adverse effects of net blotch disease of barley in correlation with up-regulation of antioxidant enzymes. Pak. J. Bot. 2020, 52, 1065–1072. [Google Scholar] [CrossRef]
- El-Nashaar, F.; Hafez, Y.M.; Abdelaal, K.A.A.; Abdelfatah, A.; Badr, M.; El-Kady, S.; Yousef, A. Assessment of host reaction and yield losses of commercial barley cultivars to Drechslera teres the causal agent of net blotch disease in Egypt. Fresenius Environ. Bull. 2020, 29, 2371–2377. [Google Scholar]
- Essawy, M.M.; Keratum, A.Y.; Abdallah, F.; Mohamed, H.M.; Mazrou, Y.; Hafez, Y.; Abdelaal, K.A.A. Susceptibility of some faba bean varieties to infestation with the main insect pests associated with physiological, biochemical and yield characters. Fresenius Environ. Bull. 2020, 29, 6147–6158. [Google Scholar]
- Abdelaal, K.; Essawy, M.; Quraytam, A.; Abdallah, F.; Mostafa, H.; Shoueir, K.; Fouad, H.; Fahmy, A.S.H.; Hafez, Y.M. Toxicity of Essential Oils Nanoemulsion against Aphis Craccivora and Their Inhibitory Activity on Insect Enzymes. Processes 2021, 9, 624. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Hossain, A.; Barutcular, C.; Islam, M.S.; Awan, S.I.; Galal, A.; Iqbal, A.; Sytar, O.; Yildirim, M.; Meena, R.S.; et al. Wheat (Triticum aestivum L.) production under drought and heat stress-adverse effects, mechanisms and mitigation: A review. Appl. Ecol. Environ. Res. 2019, 17, 8307–8332. [Google Scholar] [CrossRef]
- Abdelaal, K.; Elafry, M.; Abdel-Latif, I.; Elshamy, R.; Hassan, M.; Hafez, Y. Pivotal role of yeast and ascorbic acid in improvement the morpho-physiological characters of two wheat cultivars under water deficit stress in calcareous soil. Fresenius Environ. Bull. 2021, 30, 2554–2565. [Google Scholar]
- Abdelaal, K. Effect of Salicylic acid and Abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J. Plant Prod. Mansoura Univ. 2015, 6, 1771–1788. [Google Scholar] [CrossRef]
- Abdelaal, K.; Hafez, Y.M.; El-Afry, M.M.; Tantawy, D.S.; Alshaal, T. Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res. 2018, 25, 30199–30211. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.S.E.; M. Abu-Elsaoud, A.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Attia, K.A.; Alamery, S.; Ghazy, A.; Al-Dosse, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Abdelaal, K.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef]
- El Nahhas, N.; AlKahtani, M.; Abdelaal, K.; Al Husnain, L.; AlGwaiz, H.; Hafez, Y.; Attia, K.; El-Esawi, M.; Ibrahim, M.; Elkelish, A. Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol. Biochem. 2021, 166, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Helaly, M.N.; Mohammed, Z.; El-Shaeery, N.I.; Abdelaal, K.; Nofal, I.E. Cucumber grafting onto pumpkin can represent an interesting tool to minimize salinity stress. Physiological and anatomical studies. Middle East J. Agric. Res. 2017, 6, 953–975. [Google Scholar]
- El-Banna, M.F.; Abdelaal, K.A.A. Response of Strawberry Plants Grown in the Hydroponic System to Pretreatment with H2O2 Before Exposure to Salinity Stress. J. Plant Prod. Mansoura Univ. 2018, 9, 989–1001. [Google Scholar] [CrossRef]
- Alnusairi, G.S.H.; Mazrou, Y.S.A.; Qari, S.H.; Elkelish, A.A.; Soliman, M.H.; Eweis, M.; Abdelaal, K.; El-Samad, G.A.; Ibrahim, M.F.M.; ElNahhas, N. Exogenous Nitric Oxide Reinforces Photosynthetic Efficiency, Osmolyte, Mineral Uptake, Antioxidant, Expression of Stress-Responsive Genes and Ameliorates the Effects of Salinity Stress in Wheat. Plants 2021, 10, 1693. [Google Scholar] [CrossRef]
- El-Flaah, R.F.; El-Said, R.A.R.; Nassar, M.A.; Hassan, M.; Abdelaal, K.A.A. Effect of rhizobium, nano silica and ascorbic acid on morpho-physiological characters and gene expression of POX and PPO in faba bean (Vicia faba L.) under salinity stress conditions. Fresenius Environ. Bull. 2021, 30, 5751–5764. [Google Scholar]
- El-Shawa, G.M.R.; Rashwan, E.M.; Abdelaal, K.A.A. Mitigating salt stress effects by exogenous application of proline and yeast extract on morphophysiological, biochemical and anatomical characters of calendula plants. Sci. J. Flowers Ornament. Plants 2020, 7, 461–482. [Google Scholar] [CrossRef]
- ALKahtani, M.D.F.; Attia, K.A.; Hafez, Y.M.; Khan, N.; Eid, A.M.; Ali, M.A.M.; Abdelaal, K.A.A. Chlorophyll Fluorescence Parameters and Antioxidant Defense System Can Display Salt Tolerance of Salt Acclimated Sweet Pepper Plants Treated with Chitosan and Plant Growth Promoting Rhizobacteria. Agronomy 2020, 10, 1180. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Abdelaal, K.A.A.; Badr, M.M.; Esmaeil, R. Control of Puccinia triticina the causal agent of wheat leaf rust disease using safety resistance inducers correlated with endogenously antioxidant enzymes up-regulation. Egypt. J. Biol. Pest Control 2017, 27, 101–110. [Google Scholar]
- Abdelaal, K.; Alamrey, S.; Attia, K.; Elrobh, M.; Elnahhas, N.; Abou El-Yazied, A.; Ibrahim, M. The pivotal role of biochar in enhancement soil properties, morphophysiological and yield characters of barley plants under drought stress. Not. Bot. Horti Agrobot. 2022, 50, 12710. [Google Scholar] [CrossRef]
- Hafez, Y.; Mazrou, Y.; Shahin, A.; Nehiar, F.; Eid, M.; Abdelaal, K.A. Yield losses in wheat genotypes caused by stripe rust (Puccinia striifarmis f. sp. tritici) in North Delta, Egypt. Not. Bot. Horti Agrobot. 2022, 50, 12622. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Hossain, A.; Barutçular, C.; Abdelaal, K.A.A.; Fahad, S.; Anjorin, F.B.; Islam, M.S.; Ratnasekera, D.; Kizilgeçi, F.; Yadav, S.; et al. Sustainable maize (Zea mays L.) production under drought stress by understanding its adverse effect, Survival mechanism and drought tolerance indices. J. Exp. Biol. Agric. Sci. 2018, 6, 282–295. [Google Scholar] [CrossRef]
- Alharbi, K.; Haroun, S.A.; Kazamel, A.M.; Abbas, M.A.; Ahmaida, S.M.; AlKahtani, M.; AlHusnain, L.; Attia, K.A.; Abdelaal, K.; Gamel, R.M.E. Physiological Studies and Ultrastructure of Vigna sinensis L. and Helianthus annuus L. under Varying Levels of Nitrogen Supply. Plants 2022, 11, 1884. [Google Scholar] [CrossRef]
- Rashwan, E.; Alsohim, A.S.; El-Gammaal, A.; Hafez, Y.; Abdelaal, K.A.A. Foliar application of nano zink-oxide can alleviate the harmful effects of water deficit on some flax cultivars under drought conditions. Fresenius Environ. Bull. 2020, 29, 8889–8904. [Google Scholar]
- Khaffagy, A.E.; Mazrou, Y.S.A.; Morsy, A.R.; El-Mansoury, M.A.M.; El-Tokhy, A.I.; Hafez, Y.; Abdelaal, K.; Khedr, R.A. Impact of Irrigation Levels and Weed Control Treatments on Annual Weeds, Physiological Traits and Productivity of Soybean under Clay Soil Conditions. Agronomy 2022, 12, 1037. [Google Scholar] [CrossRef]
- AlKahtani, M.D.F.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.M.; Abdelaal, K. Evaluation of Silicon and Proline Application on the Oxidative Machinery in Drought-Stressed Sugar Beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; AlKahtani, M.D.F.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The pivotal role of plant growth promoting bacteria in alleviating the adverse effects of drought and facilitating sustainable agriculture. Biology 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; El-Afry, M.; Metwaly, M.; Zidan, M.; Rashwan, E. Salt tolerance activation in faba bean plants using proline and salicylic acid associated with physio-biochemical and yield characters improvement. Fresenius Environ. Bull. 2021, 30, 3175–3186. [Google Scholar]
- Abdelaal, K.; El-Okkiah, S.; Metwaly, M.; El-Afry, L. Impact of Ascorbic acid and proline application on the physiological machinery in soybean plants under salinity stress. Fresenius Environ. Bull. 2021, 30, 12486–12497. [Google Scholar]
- Hafez, Y.M.; Attia, K.A.; Kamel, S.; Alamery, S.; El-Gendy, S.; Al-Dosse, A.; Mehiar, F.; Ghazy, A.; Abdelaal, K. Bacillus subtilis as a bio-agent combined with nano molecules can control powdery mildew disease through histochemical and physiobiochemical changes in cucumber plants. Physiol. Mol. Plant Pathol. 2020, 111, 101489. [Google Scholar] [CrossRef]
- Khan, R.; Manzoor, N.; Zia, A.; Ahmad, I.; Ullah, A.; Shah, S.M.; Naeem, M.; Ali, S.; Khan, I.H.; Zia, D.; et al. Exogenous application of chitosan and humic acid effects on plant growth and yield of pea (Pisum sativum). Int. J. Biosci. 2018, 12, 43–50. [Google Scholar]
- Abdelaal, K.; Mazrou, Y.; Hafez, Y. Effect of silicon and carrot extract on morphophysiological characters of pea (Pisum sativum L.) under salinity stress conditions. Fresenius Environ. Bull. 2022, 31, 608–615. [Google Scholar]
- El-Afry, M.; Ahmed, H.; Mohsen, R.; Abd El-Kader, N.; Ismail, I.A.; Abdelaal, K.A.A. Infuence of some foliar treatments on growth, yield and quality of pea (Pisum sativum L.). Fresenius Environ. Bull. 2021, 30, 5765–5772. [Google Scholar]
- Arafa, S.A.; Attia, K.A.; Niedbała, G.; Piekutowska, M.; Alamery, S.; Abdelaal, K.; Alateeq, T.K.; Ali, M.A.M.; Elkelish, A.; Attallah, S.Y. Seed Priming Boost Adaptation in Pea Plants under Drought Stress. Plants 2021, 10, 2201. [Google Scholar] [CrossRef]
- Castigliona Monica, R.; Cremonini, R. Nanoparticals and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef]
- Rashwan, E.A.A.; Abdelaal, K. Effect of Nano Zink-oxide foliar application on some flax cultivars under different irrigation treatments. Egypt. J. Plant Breed. 2019, 23, 119–145. [Google Scholar]
- Torney, F.; Trewyn, B.G.; Lin, V.S.Y.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007, 2, 295–300. [Google Scholar] [CrossRef]
- Bao-Shan, L.; Chun-hui, L.; Li-jun, F.; Shu-chun, Q.; Min, Y. Effects of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef] [PubMed]
- Khedr, R.; Aboukhadrah, S.; El-Hag, D.; Elmohamady, E.; Abdelaal, K. Ameliorative effects of nano silica and some growth stimulants on water relations, biochemical and productivity of wheat under saline soil conditions. Fresenius Environ. Bull. 2023, 32, 375–384. [Google Scholar]
- Aly, M.A.; Zabat, R.M.O.; Gaber, M.K. The Efficiency of The Foliar Application Using Silica and Silver Nanoparticles on Duranta erecta under Salinity Conditions. Egypt. Acad. J. Biol. Sci. H. Bot. 2023, 14, 55–68. [Google Scholar]
- Hafez, Y.; Elkohby, W.; Mazrou, Y.S.A.; Ghazy, M.; Elgamal, A.; Abdelaal, K. Alleviating the detrimental impacts of salt stress on morpho-hpysiological and yield characters of rice plants (Oryza sativa L.) using actosol, Nano-Zn and Nano-Si. Fresenius Environ. Bull. 2020, 29, 6882–6897. [Google Scholar]
- Park, H.S.; Kazerooni, E.A.; Kang, S.M.; Al-Sadi, A.M.; Lee, I.J. Melatonin enhances the tolerance and recovery mechanisms in Brassica juncea (L.) Czern. under saline conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Abadia, J.; Marjani, M. Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Plant Physiol. Biochem. 2020, 149, 313–323. [Google Scholar] [CrossRef]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.; Imran, M.; Asaf, S.; Al-Harrasi, A.A. Melatonin: Awakening the defense mechanisms during plant oxidative stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef]
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in abiotic stress resistance and tolerance. In Plant Growth Regulators; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021; pp. 239–273. [Google Scholar] [CrossRef]
- Pardo-Hernández, M.; López-Delacalle, M.; Rivero, R.M. ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 2020, 9, 1078. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Wang, Z.; Feng, G.; Gao, Q.; Li, X. Induction of low temperature tolerance in wheat by pre-soaking and parental treatment with melatonin. Molecules 2021, 26, 1192. [Google Scholar] [CrossRef]
- Talaat, N.; Todorova, D. Antioxidant Machinery and Glyoxalase System Regulation Confers Salt Stress Tolerance to Wheat (Triticum aestivum L.) Plants Treated with Melatonin and Salicylic Acid. J. Soil Sci. Plant Nutr. 2022, 22, 3527–3540. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Synergistic effects of salicylic acid and melatonin on modulating ion homeostasis in salt-stressed wheat (Triticum aestivum L.) plants by enhancing root H+-pump activity. Plants 2022, 11, 416. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Y.; Rui, C.; Zhang, H.; Xu, N.; Dai, M.; Chen, X.; Lu, X.; Wang, D.; Wang, J.; et al. Melatonin improves cotton salt tolerance by regulating ROS scavenging system and Ca2+ signal transduction. Front. Plant Sci. 2021, 12, 693690. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Sanchez, F.J.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Growth of epicotyls, turgor maintenance and osmotic adjustment in pea plants (Pisum sativum L.) subjected to water stress. Field Crop. Res. 2004, 86, 81–90. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from plants and PGPR under biotic and abiotic stresses. Plant Growth Reg. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Huckelhoven, R.; Fodor, J.; Preis, C.; Kogel, K.H. Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation. Plant Physiol. 1999, 1119, 1251–1260. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- O’Mahony, M. Sensory Evaluation of Food: Statistical Methods and Procedures; CRC Press: Boca Raton, FL, USA, 1986; p. 487. [Google Scholar]
- Abdelaal, K.; Mazrou, Y.S.A.; Hafez, Y.M. Silicon Foliar Application Mitigates Salt Stress in Sweet Pepper Plants by Enhancing Water Status, Photosynthesis, Antioxidant Enzyme Activity and Fruit Yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Farhoudi, R.; Tafti, M.M. Effect of salt stress on seedlings growth and ions homeostasis of soybean (Glycine max) cultivars. Adv. Environ. Biol. 2011, 5, 2522–2526. [Google Scholar]
- Zhani, K.; Elouer, M.A.; Aloui, H.; Hannachi, C. Selection of a salt tolerant Tunisian cultivar of chili pepper (Capsicum frutescens). EurAsian J. BioSci. 2012, 6, 47–59. [Google Scholar] [CrossRef]
- Kanwal, H.; Ashraf, M.; Hameed, M. Water relations and ionic composition in the seedlings of some newly developed and candidate cultivars of wheat (Triticum aestivum L.) under saline conditions. Pak. J. Bot. 2013, 45, 1221–1227. [Google Scholar]
- Ghezal, N.; Rinez, I.; Sbai, H.; Saad, I.; Farooqd, M.; Rinez, A.; Zribi, I.; Haouala, R. Improvement of Pisum sativum salt stress tolerance by bio-priming their seeds using Typha angustifolia leaves aqueous extract. S. Afr. J. Bot. 2016, 105, 240–250. [Google Scholar] [CrossRef]
- Omar, A.; Zayed, B.; Abdel Salam, A.; Hafez, Y.M.; Abdelaal, K. Folic acid as foliar application can improve growth and yield characters of rice plants under irrigation with drainage water. Fresenius Environ. Bull. 2020, 29, 9420–9428. [Google Scholar]
- Smith, A.M.; Duan, H.W.; Mohs, A.M.; Nie, S.M. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Song, H. Calcium protects Trifolium repens L. plants against cadmium stress. Plant Cell Rep. 2009, 28, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latef, A.H.A.; Chaoxing, H. Does inoculation with Glomus mosseae improve salt tolerance in pepper plants? J. Plant Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Yildirim, E.; Ekinci, M.; Turan, M.; Dursun, A.; Parlakova, F.; Kul, R. Exogenously applied glycine betaine regulates some chemical characteristics and antioxidative defense system in lettuce under salt stress. Hortic. Environ. Biotechnol. 2016, 57, 225–231. [Google Scholar] [CrossRef]
- Agathokleous, E.; Zhou, B.; Xu, J.; Ioannou, A.; Feng, Z.; Saitanis, C.J.; Frei, M.; Calabrese, E.J.; Fotopoulos, V. Exogenous application of melatonin to plants, algae, and harvested products to sustain agricultural productivity and enhance nutritional and nutraceutical value: A meta-analysis. Environ. Res. 2021, 200, 111746. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.A.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–729. [Google Scholar] [CrossRef]
- Chen, Y.; Li, R.; Ge, J.; Liu, J.; Wang, W.; Xu, M.; Zhang, R.; Hussain, S.; Wei, H.; Dai, Q. Exogenous melatonin confers enhanced salinity tolerance in rice by blocking the ROS burst and improving Na+/K+ homeostasis. Environ. Exp. Bot. 2021, 189, 104530. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Chen, L.; Ding, C.; Tang, S.; Jiang, Y.; Liu, Z.; Li, G. Melatonin enhances Na+/K+ homeostasis in rice seedlings under salt stress through increasing the root H+-pump activity and Na+/K+ transporters sensitivity to ROS/RNS. Environ. Exp. Bot. 2021, 182, 104328. [Google Scholar] [CrossRef]
- Damodaran, T.; Rai, R.; Jha, S.; Kannan, R.; Pandey, B.; Sah, V.; Mishra, V.; Sharma, D. Rhizosphere and endophytic bacteria for induction of salt tolerance in gladiolus grown in sodic soils. J. Plant Interact. 2014, 9, 577–584. [Google Scholar] [CrossRef]
- Mukarram, M.; Petrik, P.; Mushtaq, Z.; Khan, M.; Gulfishan, M.; Lux, A. Silicon nanoparticles in higher plants: Uptake, action, stress tolerance, and crosstalk with phytohormones, antioxidants, and other signaling molecules. Environ. Pollut. 2022, 310, 119855. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Gomaa, A.M.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 173, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Kamiab, F. Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J. Plant Nutr. 2020, 43, 1468–1484. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, L.; Wang, X.; Wang, Z.; Zhang, H.; Chen, J.; Liu, X.; Wang, Y.; Li, C. Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.). Plants 2021, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Shahin, A.; Esmaeil, R.A.; Badr, M.; Abdelaal, K.; Hassan, F.A.S.; Hafez, Y.M. Phenotypic characterization of race-specific and slow rusting resistance to stem rust disease in promising wheat genotypes. Fresenius Environ. Bull. 2021, 30, 6223–6236. [Google Scholar]
- Abdelaal, K.; Omara, I.R.; Hafez, Y.M.; Esmail, S.M.; EL Sabagh, A. Anatomical, biochemical and physiological changes in some Egyptian wheat cultivars inoculated with Puccinia graminis f. sp. tritici. Fresenius Environ. Bull. 2018, 27, 296–305. [Google Scholar]
- Esmail, S.M.; Omara, R.I.; Abdelaal, K.; Hafez, M. Histological and biochemical aspects of compatible and incompatible wheat-Puccinia striiformis interactions. Physiol. Mol. Plant Pathol. 2019, 106, 120–128. [Google Scholar] [CrossRef]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.M.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.; El-Esawi, M.; El Nahhas, N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Abdelaal, K. Investigation of susceptibility and resistance mechanisms of some Egyptian wheat cultivars (Triticum aestivum L.) inoculated with Blumeria graminis f.sp. tritici using certain biochemical, molecular characterization and SEM. J. Plant Prot. Pathol. Mansoura Univ. 2015, 6, 431–454. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chen, Q.; Liu, Q.; Zhang, W.H.; Ding, R.X. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef]
- Verslues, P.E. Time to grow: Factors that control plant growth during mild to moderate drought stress. Plant Cell Environ. 2017, 40, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Medina-Rico, A.; Delgado-Cano, A. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Ghaffaria, H.; Tadayona, M.R.; Bahadora, M.; Razmjoo, J. Investigation of the proline role in controlling traits related to sugar and root yield of sugar beet under water deficit conditions. Agric. Water Manag. 2021, 243, 106448. [Google Scholar] [CrossRef]
- Wutipraditkul, N.; Wongwean, P.; Buaboocha, T. Alleviation of salt-induced oxidative stress in rice seedlings by proline and/or glycine betaine. Biol. Plant. 2015, 59, 547–553. [Google Scholar] [CrossRef]
- Sharma, S.; Villamor, J.G.; Verslues, P.E. Essential role of tissue specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef]
- Abd El-Hamed, W.F.; Yousef, N.S.; Mazrou, Y.; Elkholy, W.; El-Refaiy, A.I.; Elfeky, F.A.; Albadrani, M.; El-Tokhy, A.I.; Abdelaal, K. Anticryptosporidium Efficacy of Olea europaea and Ficus carica Leaves Extract in Immunocompromised Mice Associated with Biochemical Characters and Antioxidative System. Cells 2021, 10, 2419. [Google Scholar] [CrossRef]
- Ferchichi, S.; Hessini, K.; Dell’Aversana, E.; D’Amelia, L.; Woodrow, P.; Ciarmiello, L.F.; Fuggi, A.; Carillo, P. Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct. Plant Biol. 2018, 45, 1096–1099. [Google Scholar] [CrossRef]
- El-Shawa, G.M.R.; Alharbi, K.; AlKahtani, M.; AlHusnain, L.; Attia, K.A.; Abdelaal, K. Improving the Quality and Production of Philodendron Plants Using Nanoparticles and Humic Acid. Horticulturae 2022, 8, 678. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shammari, W.B.; Altamimi, H.R.; Abdelaal, K. Improvement in Physiobiochemical and Yield Characteristics of Pea Plants with Nano Silica and Melatonin under Salinity Stress Conditions. Horticulturae 2023, 9, 711. https://doi.org/10.3390/horticulturae9060711
Al-Shammari WB, Altamimi HR, Abdelaal K. Improvement in Physiobiochemical and Yield Characteristics of Pea Plants with Nano Silica and Melatonin under Salinity Stress Conditions. Horticulturae. 2023; 9(6):711. https://doi.org/10.3390/horticulturae9060711
Chicago/Turabian StyleAl-Shammari, Wasimah B., Haya R. Altamimi, and Khaled Abdelaal. 2023. "Improvement in Physiobiochemical and Yield Characteristics of Pea Plants with Nano Silica and Melatonin under Salinity Stress Conditions" Horticulturae 9, no. 6: 711. https://doi.org/10.3390/horticulturae9060711
APA StyleAl-Shammari, W. B., Altamimi, H. R., & Abdelaal, K. (2023). Improvement in Physiobiochemical and Yield Characteristics of Pea Plants with Nano Silica and Melatonin under Salinity Stress Conditions. Horticulturae, 9(6), 711. https://doi.org/10.3390/horticulturae9060711





