Study of Comparative Morphology of Eight Cultivated Genotypes of Olea europaea L
Abstract
1. Introduction
2. Results and Discussion
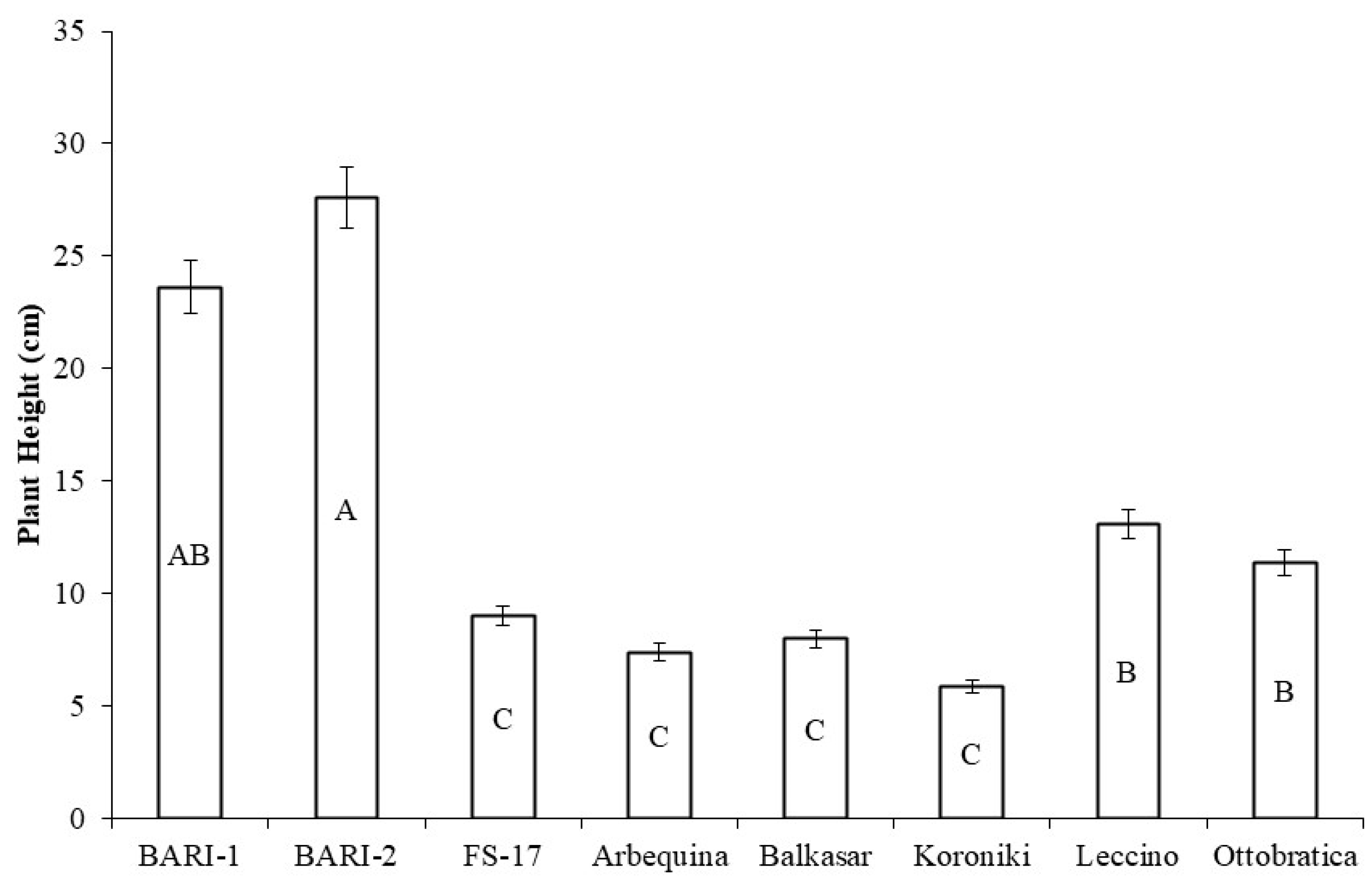
2.1. Plant Height
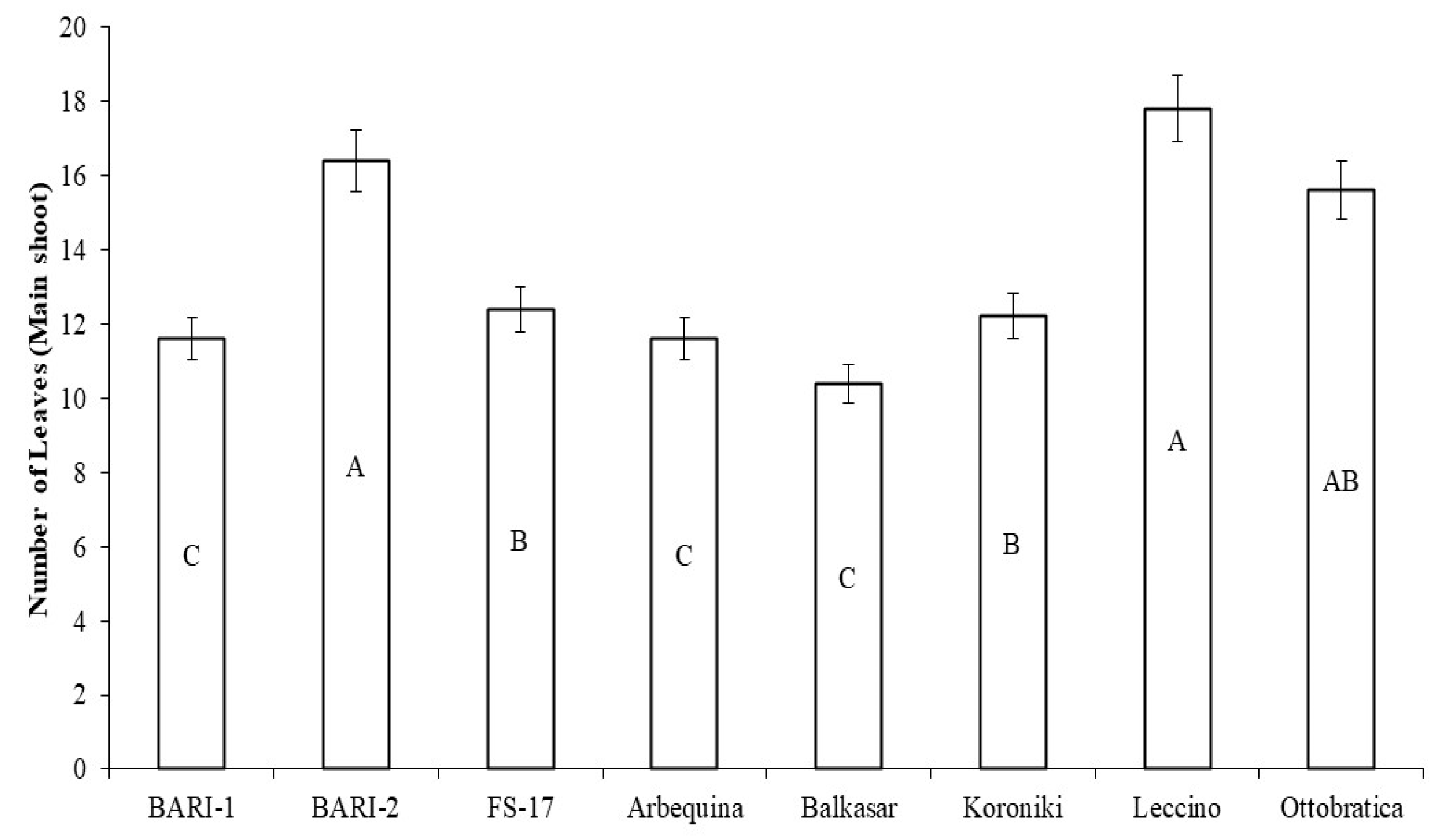
2.2. Number of Leaves on Main Shoot
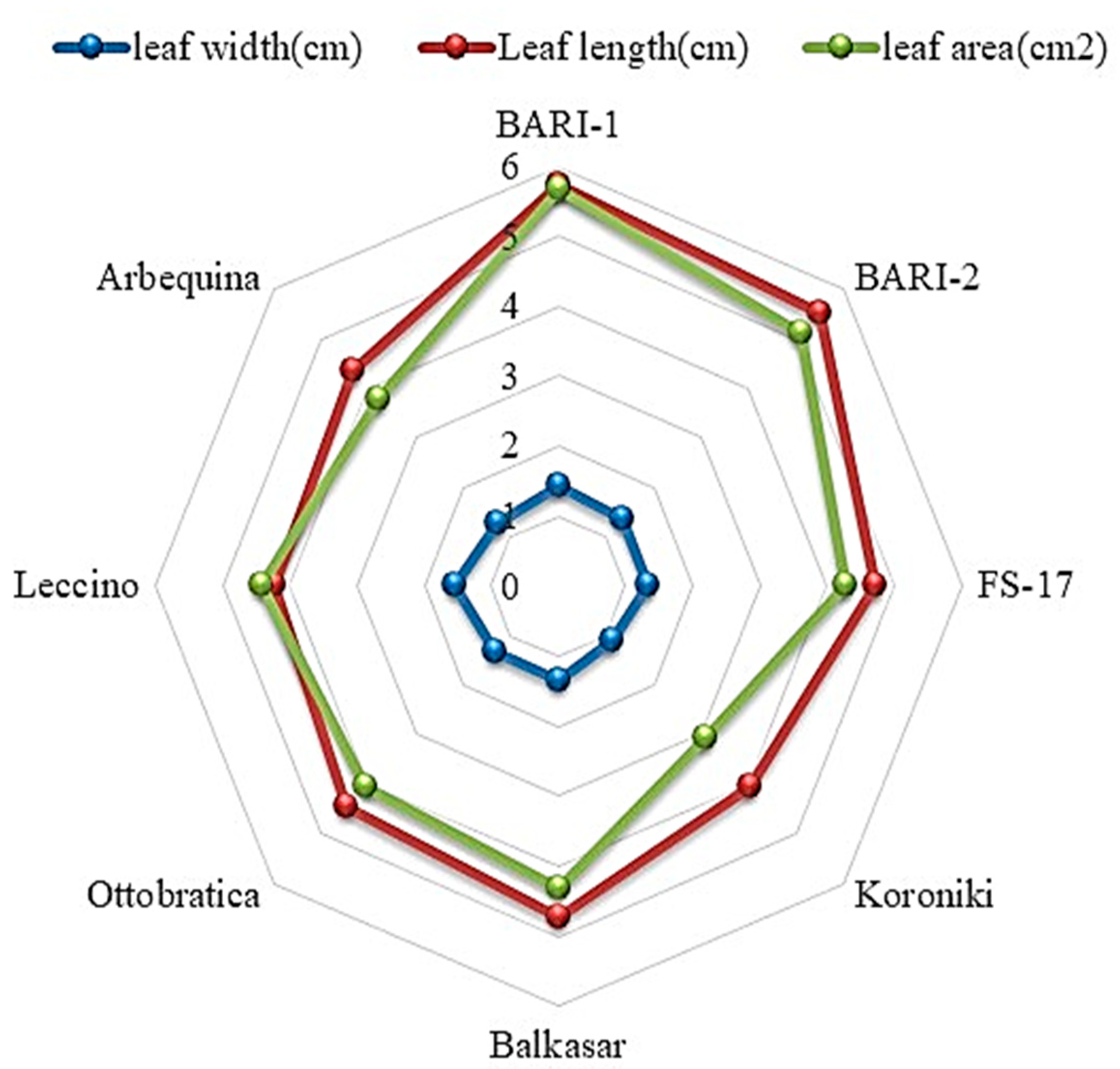
2.3. Leaf Length (cm), Width (cm), and Leaf Area (cm2)
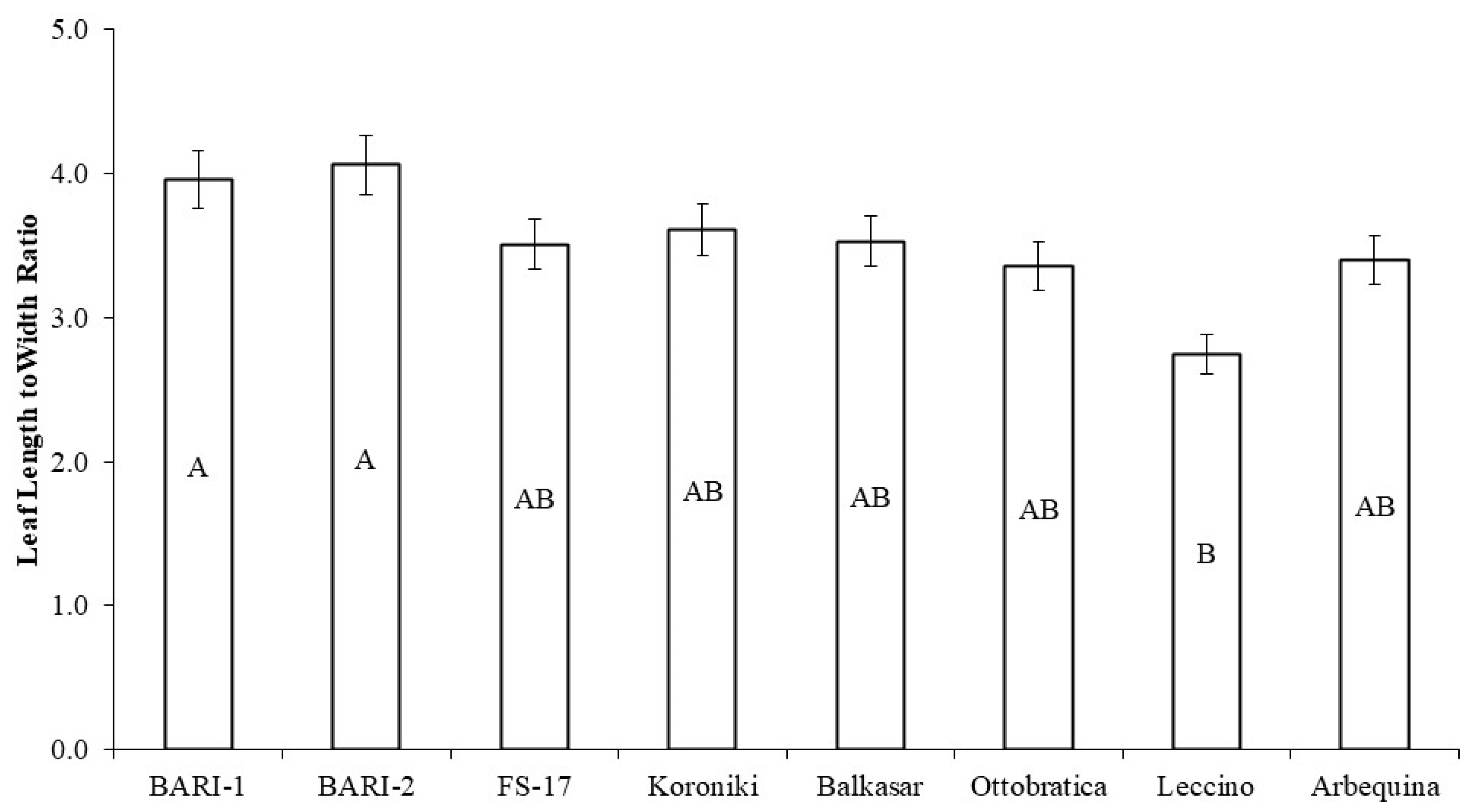
2.4. Leaf Length to Leaf Width Ratio
2.5. Leaf Base and Tip Shapes
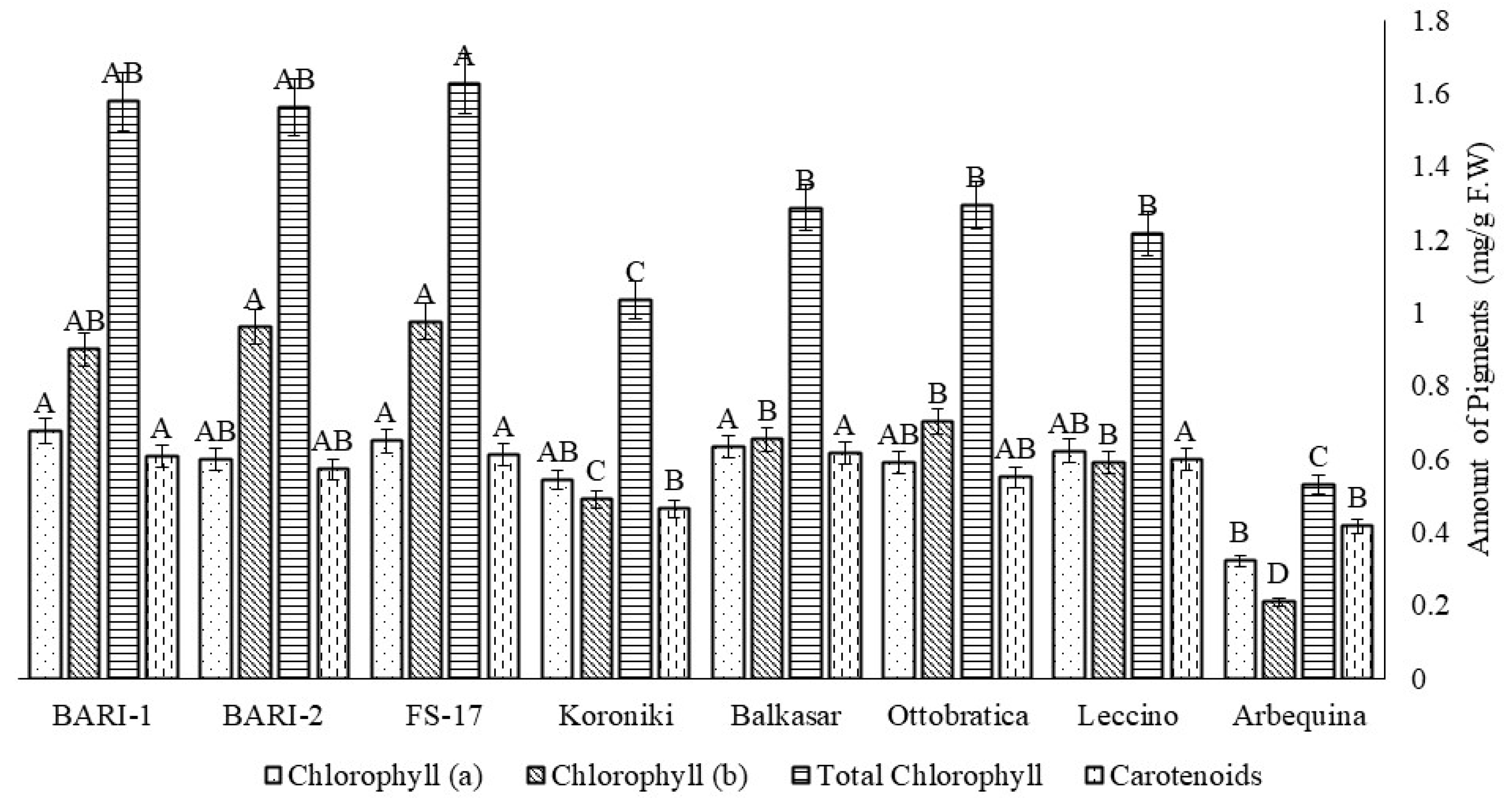
2.6. Chlorophyll a, b, and Carotenoids of Olive Cultivars
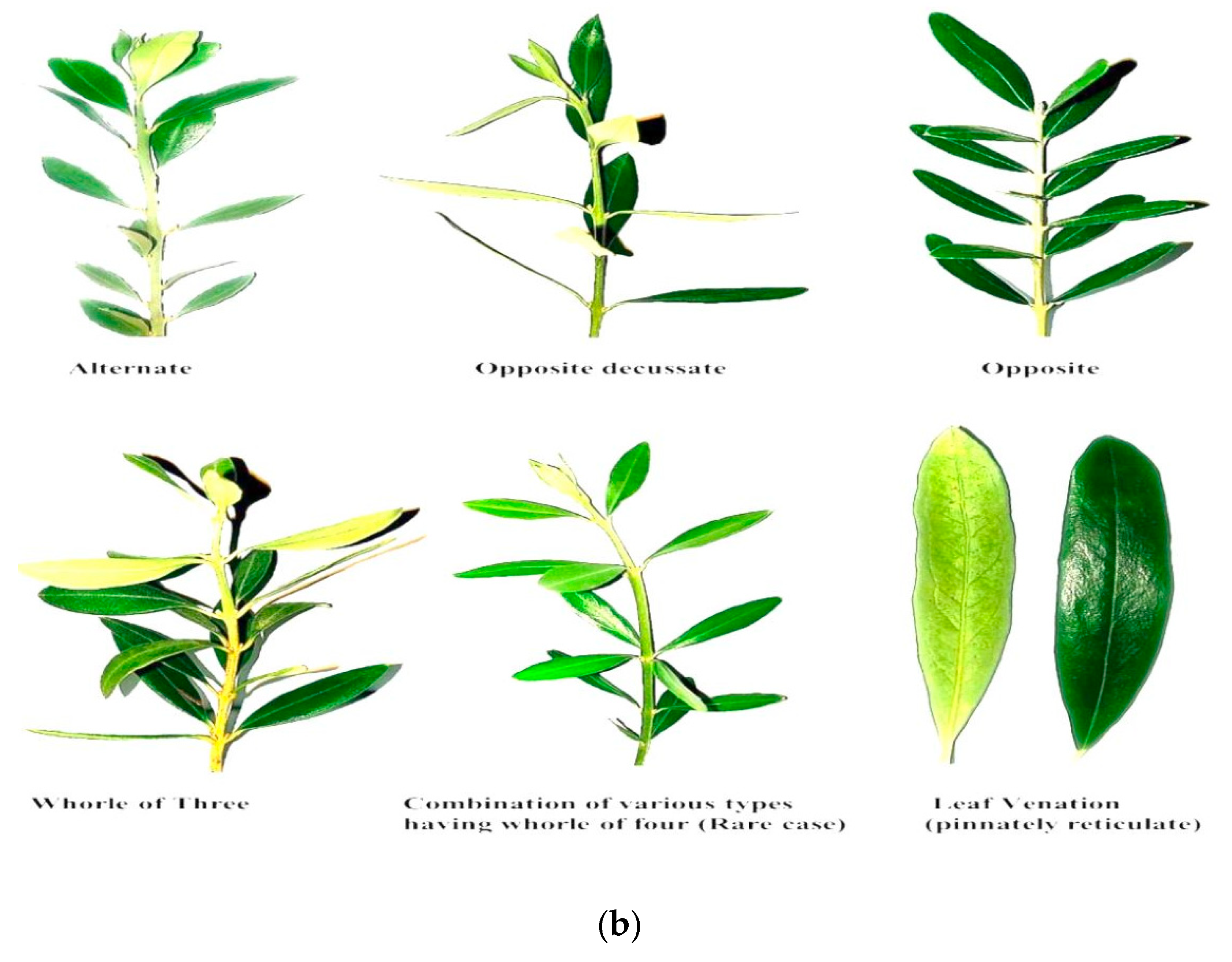
2.7. Leaf Arrangement, Color, and Venation of Olive Branches
3. Materials and Methods
3.1. Study Site
3.2. Climatic Conditions of the Study Area
3.3. Plant Material
3.4. Selected Morphological Parameters
3.5. Plant Height (cm) and Number of Leaves
3.6. Leaf Length (cm), Width (cm), and Leaf Area (cm2)
3.7. Leaf Angles (°)
3.8. Spectrophotometric Determination of Chlorophylls a, b and Carotenoids
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Therios, I. Olive Production; Gartaganis: Thessaloniki, Greece, 2005. [Google Scholar]
- Therios, I.N. Olives; CABI: Oxfordshire, UK, 2009. [Google Scholar]
- Cadogan, G. Palaces of Minoan Crete, Corrected ed.; Methuen: London, UK, 1980. [Google Scholar]
- Connor, D.J.; Fereres, E. The Physiology of Adaptation and Yield Expression in Olive. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 31, pp. 155–229. [Google Scholar] [CrossRef]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive Cultivation in the Southern Hemisphere: Flowering, Water Requirements and Oil Quality Responses to New Crop Environments. Front. Plant Sci. 2017, 8, 1830. [Google Scholar] [CrossRef]
- Fernández, J.E. Understanding olive adaptation to abiotic stresses as a tool to increase crop performance. Environ. Exp. Bot. 2014, 103, 158–179. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Fabbri, A.; Sebastiani, L. The Olive Tree Genome; Springer: Berlin/Heidelberg, Germany, 2016; pp. 13–26. [Google Scholar] [CrossRef]
- Chiappetta, A.; Muzzalupo, I. Botanical Description. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; Books on Demand: Paris, France, 2012; pp. 23–28. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive tree leaves—A source of valuable active compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Galli, G.; Caruso, D. Biological activities and metabolic fate of olive oil phenols. Eur. J. Lipid Sci. Technol. 2002, 104, 677–684. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in Olive and its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed]
- de Souza PA, L.; Marcadenti, A.; Portal, V.L. Effects of olive oil phenolic compounds on inflammation in the prevention and treatment of coronary artery disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Contreras, M.; Lama-Muñoz, A.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 314, 126218. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2021, 11, 56. [Google Scholar] [CrossRef]
- Barranco, D.; Rallo, L. Las variedades de olivo cultivadas en España. Olivae 1985, 9, 16–22. [Google Scholar]
- Antonio, C.; Graziano, C.C. Morphological evaluation of olive germplasm present in Tuscany region. Euphytica 1999, 109, 173–181. [Google Scholar] [CrossRef]
- Trujillo, I.; Ojeda, M.A.; Urdiroz, N.M.; Potter, D.; Barranco, D.; Rallo, L.; Diez, C.M. Identification of the Worldwide Olive Germplasm Bank of Córdoba (Spain) using SSR and morphological markers. Tree Genet. Genomes 2013, 10, 141–155. [Google Scholar] [CrossRef]
- Siakou, M.; Bruggeman, A.; Eliades, M.; Djuma, H.; Kyriacou, M.C.; Moriana, A. Phenology, Morphology and Physiology Responses of Deficit Irrigated “Koroneiki” Olive Trees as Affected by Environmental Conditions and Alternate Bearing. Agronomy 2022, 12, 879. [Google Scholar] [CrossRef]
- Khadivi, A.; Mirheidari, F.; Moradi, Y.; Paryan, S. Identification of the promising olive (Olea europaea L.) cultivars based on morphological and pomological characters. Food Sci. Nutr. 2022, 10, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Barranco, D.; Rallo, L. Olive Cultivars in Spain. Horttechnology 2000, 10, 107–110. [Google Scholar] [CrossRef]
- Del Río, C.; Caballero, J. Preliminary agronomical characterization of 131 cultivars introduced in the olive germplasm bank of cordoba in march 1987. Acta Hortic. 1994, 356, 110–115. [Google Scholar] [CrossRef]
- Bartolini, G.; Messeri, C.; Carignani, G.; Prevost, G. Olive Germplasm: Cultivars and World-Wide Collections; FAO, Plant Production and Protection Division: Rome, Italy, 1998. [Google Scholar]
- De Carvalho-Sobrinho, J.G.; De Queiroz, L.P. Morphological cladistic analysis of Pseudobombax Dugand (Malvaceae, Bombacoideae) and allied genera. Braz. J. Bot. 2011, 34, 197–209. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Grati-Kamoun, N.; Attia, H. Physico-chemical change and heat stability of extra virgin olive oils flavoured by selected Tunisian aromatic plants. Food Chem. Toxicol. 2009, 47, 2613–2619. [Google Scholar] [CrossRef]
- Youssef, O.; Guido, F.; Daoud, D.; Mokhtar, Z. Effect of cultivar on minor components in Tunisia olive fruits cultivated in microclimate. J. Hortic. For. 2011, 3, 13–20. [Google Scholar]
- Asadiar, L.S.; Rahmani, F.; Siami, A. Assessment of genetic diversity in the Russian olive (Elaeagnus angustifolia) based on ISSR genetic markers. Rev. Cinêcia Agron. 2013, 44, 310–316. [Google Scholar] [CrossRef]
- Azmat, M.A.; Khan, A.A.; Khan, I.A.; Buerkert, A.; Wiehle, M. Morphology, biochemistry, and management of Russian olive (Elaeagnus angustifolia L.) accessions in Gilgit-Baltistan, northern Rural Develop. Trop. Subtrop. Pakistan. J. Agri. 2020, 121, 151–160. [Google Scholar]
- Zaher, H.; Boulouha, B.; Baaziz, M.; Sikaoui, L.; Gaboun, F.; Udupa, S.M. Morphological and genetic diversity in olive (Olea europaea subsp. europaea L.) clones and varieties. Plant Omics 2011, 4, 370–376. [Google Scholar]
- Milotic, A.; Setic, E.; Persuric, D.; Poljuha, D.; Sladonja, B.; Brscic, K. Identification and characterization of autochthonous olive varieties in Istria (Croatia). Ann. Ser. Hist. Nat. 2005, 7, 251–256. [Google Scholar]
- Mehr, R.S.A.; Maassoumi, A.A.; Saidi, A.; Osaloo, S.K.; Nohooji, M.G. Morphological cladistic analysis of some bifurcate hairy sections of Astragalus (Fabaceae) in Iran. Turk. J. Bot. 2012, 36, 434–442. [Google Scholar] [CrossRef]
- Abdali, N.; Ataei, S.; Keihan, A.; Motaghi, L.; Naghavi, M.; Kazemitabar, S.K.; Hosseini-Mazinani, M. Comparative study of the morphological and molecular characteristics of 10 Iranian olive (Olea europaea L.) cultivars. Ann. Biol. Res. 2014, 5, 76–83. [Google Scholar]
- Al-Ruqaie, I.; Al-Khalifah, N.S.; Shanavaskhan, A.E. Morphological cladistic analysis of eight popular Olive (Olea europaea L.) cultivars grown in Saudi Arabia using Numerical Taxonomic System for personal computer to detect phyletic relationship and their proximate fruit composition. Saudi J. Biol. Sci. 2016, 23, 115–121. [Google Scholar] [CrossRef]
- Tombesi, S.; Tombesi, A.; Molfese, M.; Cipolletti, M.; Visco, T. Evaluation of four cultivars regarding their suitability in high-intensity olive orchards. Acta Hortic. 2011, 924, 321–326. [Google Scholar] [CrossRef]
- Google Maps. 2022. Available online: https://www.google.com/maps/@29.3796775,71.7537149,14z (accessed on 10 January 2022).
- Pakistan Topographic Map, Elevation, Relief. 2022. Available online: https://en-us.topographic-map.com/maps/tlia/Pakistan/ (accessed on 10 January 2022).
- Meteoblue. Observed Historical Climate & Weather Data for Bahawalpur. 2022. Available online: https://www.meteoblue.com/en/weather/historyclimate/climateobserved/bahawalpur_pakistan_1183880 (accessed on 10 January 2022).
- Atlas, G.S. Solar Radiation Map Bahawalpur, Punjab, Pakiatan 29.425843°, 071.783981°; Synergis: Washington, DC, USA, 2021; Available online: https://globalsolaratlas.info/map?c=29.384269,71.759262,11&s=29.425843,71.783981&m=site (accessed on 10 January 2021).
- Sukhera, M.; Pasha, M. Solar radiation maps for Pakistan. Sol. Wind. Technol. 1987, 4, 229–238. [Google Scholar] [CrossRef]
- Cima, R.; Urbano, F. Selection of Suitable Areas for Olive Growing in Pakistan Selection of Suitable Areas; Nova Arti Grafiche Signa: Firenze, Italy, 2008. [Google Scholar]
- Opti-Sciences, I. AM 300 Field Portable Leaf Area Meter—Opti-Sciences. 2018. Available online: https://www.yumpu.com/en/document/read/40524439/am-300-field-portable-leaf-area-meter-opti-sciences (accessed on 10 June 2018).
- Rasband, W.S. ImageJ, U.S.; National Institutes of Health: Bethesda, MA, USA, 2018. Available online: https://imagej.nih.gov/ij/ (accessed on 10 July 2018).
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Davies, B. Carotenoids. In Chemistry and Biochemistry of Plant Pigments, 2nd ed.; Goodwin, T.W., Ed.; Academic Press: London, UK, 1976; pp. 38–165. [Google Scholar]
- Statistix8.1. User’s Manual; Analytical Software: Tallahassee, FL, USA, 2003. [Google Scholar]


| Sr. No | Olive Cultivar | Leaf Base | Leaf Apex | Leaf Margin |
|---|---|---|---|---|
| 1 | Bari Zaitoon-1 | Equilateral | Apiculate | Entire |
| 2 | Bari Zaitoon-2 | Acute | Acute | Entire |
| 3 | FS-17 | Cuneate | Cuspidate | Entire |
| 4 | Koroneiki | Equilateral | Apiculate | Entire |
| 5 | Balkasar | Cuneate | Rounded | Entire |
| 6 | Ottobratica | Acute | Cuspidate | Entire |
| 7 | Leccino | Cuneate | Apiculate | Entire |
| 8 | Arbequina | Cuneate | Acute | Entire |
| Sr. No | Cultivar | Origin | Purpose |
|---|---|---|---|
| 1 | Bari Zaitoon-1 (Coratina selection) | Pakistan | Dual (Table & Oil) |
| 2 | Bari Zaitoon-2 (Frantoio selection) | Pakistan | Dual |
| 3 | Favolosa (FS-17) | Italy | Dual |
| 4 | Koroneiki | Greece | Oil |
| 5 | Balkasar | Pakistan | Dual |
| 6 | Ottobratica | Italy | Oil |
| 7 | Leccino | Italy | Oil |
| 8 | Arbequina | Spain | Oil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwar, G.; Anwar, T.; Chaudhary, M.S.; Jamil, M.; Kamal, A.; Mustafa, A.E.-Z.M.A.; Al-Ghamdi, A.A.; Ullah, F.; Zaman, W. Study of Comparative Morphology of Eight Cultivated Genotypes of Olea europaea L. Horticulturae 2023, 9, 696. https://doi.org/10.3390/horticulturae9060696
Sarwar G, Anwar T, Chaudhary MS, Jamil M, Kamal A, Mustafa AE-ZMA, Al-Ghamdi AA, Ullah F, Zaman W. Study of Comparative Morphology of Eight Cultivated Genotypes of Olea europaea L. Horticulturae. 2023; 9(6):696. https://doi.org/10.3390/horticulturae9060696
Chicago/Turabian StyleSarwar, Ghulam, Tauseef Anwar, Muhammad Shafique Chaudhary, Moazzam Jamil, Asif Kamal, Abd El-Zaher M. A. Mustafa, Abdullah Ahmed Al-Ghamdi, Fazal Ullah, and Wajid Zaman. 2023. "Study of Comparative Morphology of Eight Cultivated Genotypes of Olea europaea L" Horticulturae 9, no. 6: 696. https://doi.org/10.3390/horticulturae9060696
APA StyleSarwar, G., Anwar, T., Chaudhary, M. S., Jamil, M., Kamal, A., Mustafa, A. E.-Z. M. A., Al-Ghamdi, A. A., Ullah, F., & Zaman, W. (2023). Study of Comparative Morphology of Eight Cultivated Genotypes of Olea europaea L. Horticulturae, 9(6), 696. https://doi.org/10.3390/horticulturae9060696








