Pre-Harvest UVB Irradiation Enhances the Phenolic and Flavonoid Content, and Antioxidant Activity of Green- and Red-Leaf Lettuce Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Conditions
2.2. Determination of Chlorophyll and Carotenoid
2.3. Determination of Total Phenolic, Flavonoid Content, and Anthocyanin Relative Content
2.4. Determination of DPPH Radical Scavenging Capacity
2.5. Determination of MDA and H2O2 Content
2.6. Statistical Analysis
3. Results
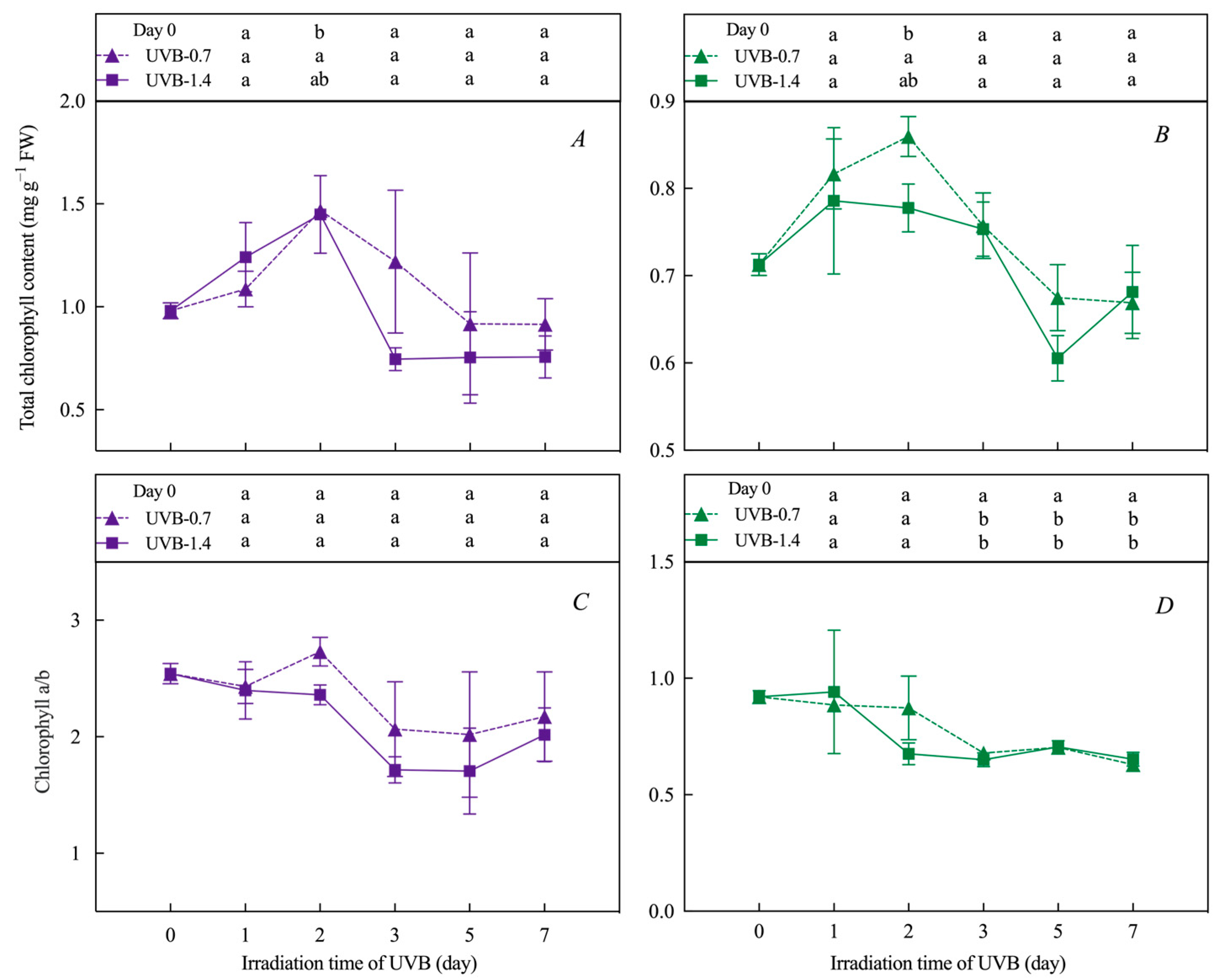
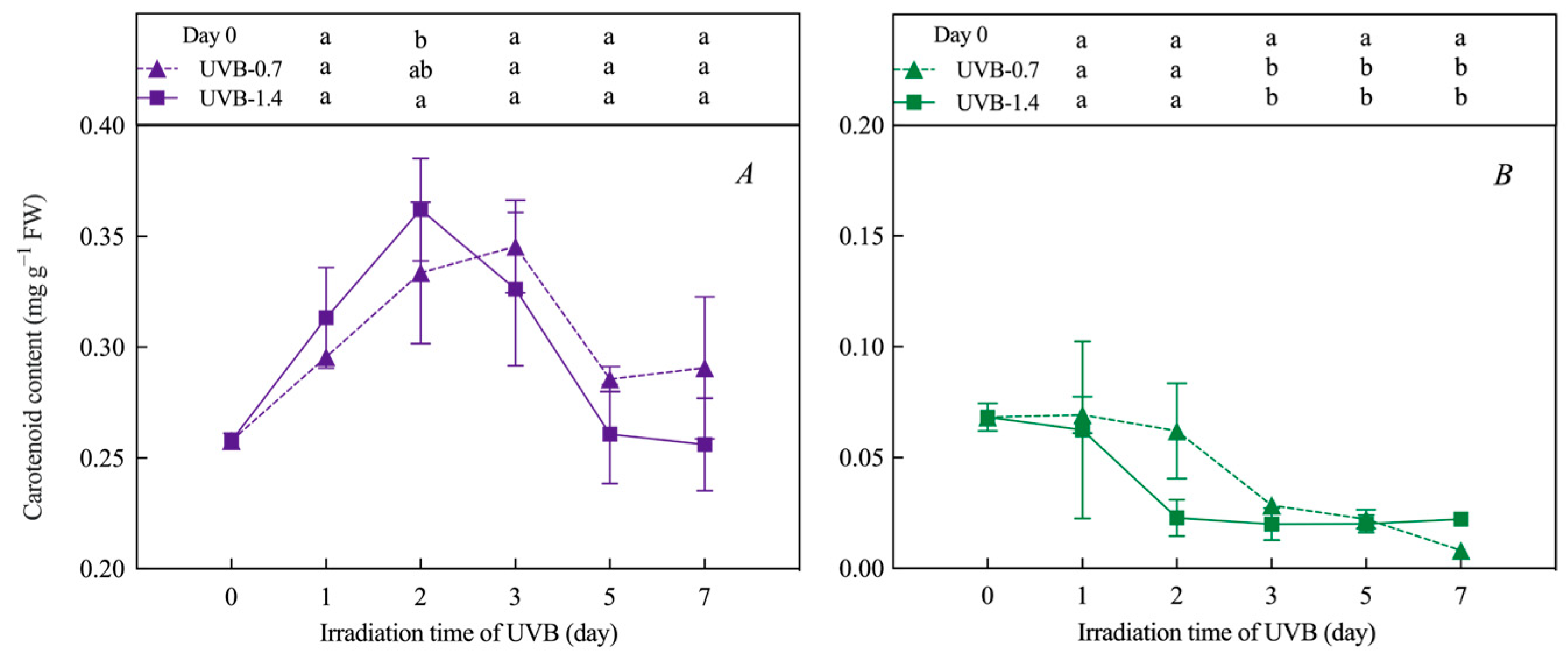
3.1. Effects of UVB Treatment on Chlorophylls’ and Carotenoids’ Content
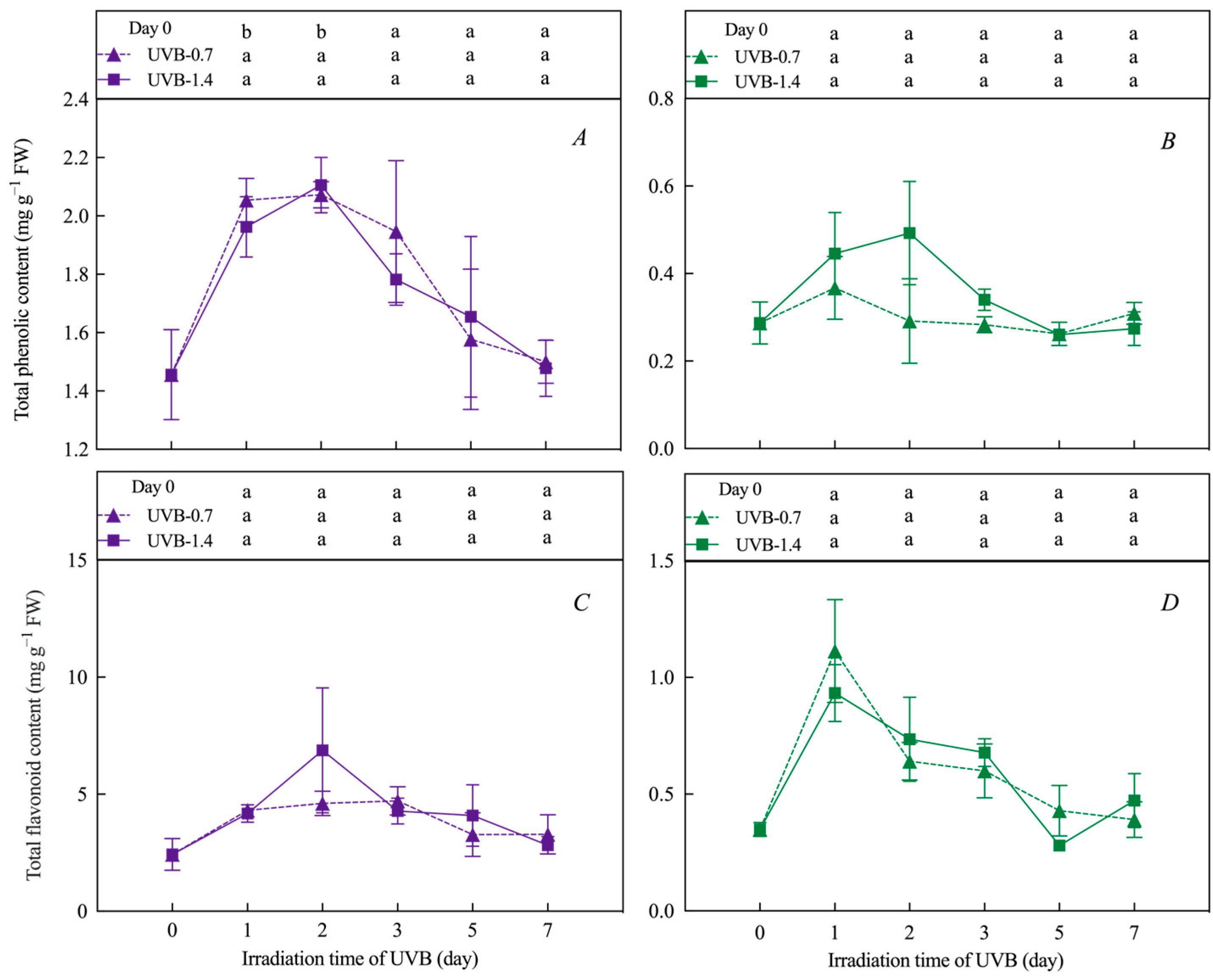
3.2. Effects of UVB Treatment on Total Phenolic, Flavonoid Content, and Anthocyanin Relative Content
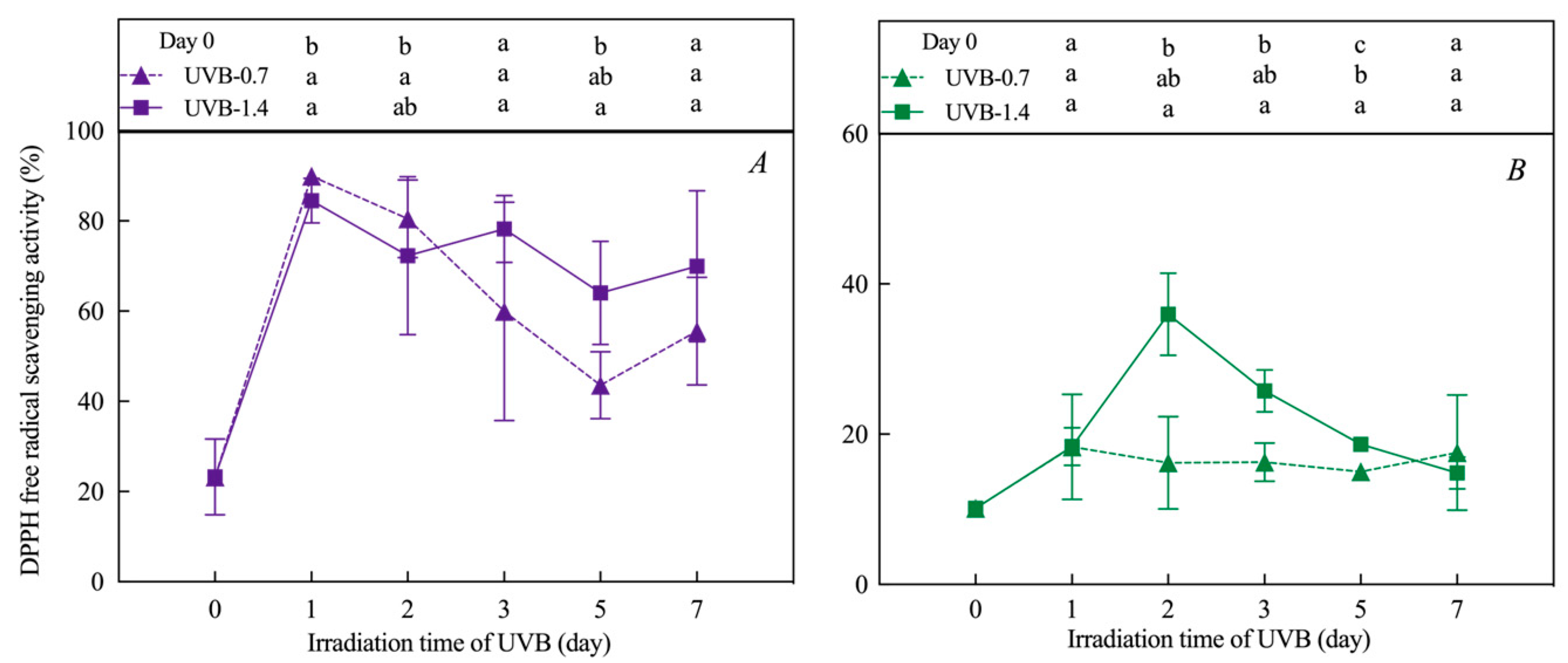
3.3. Effects of UVB Treatment on Antioxidant Capacity
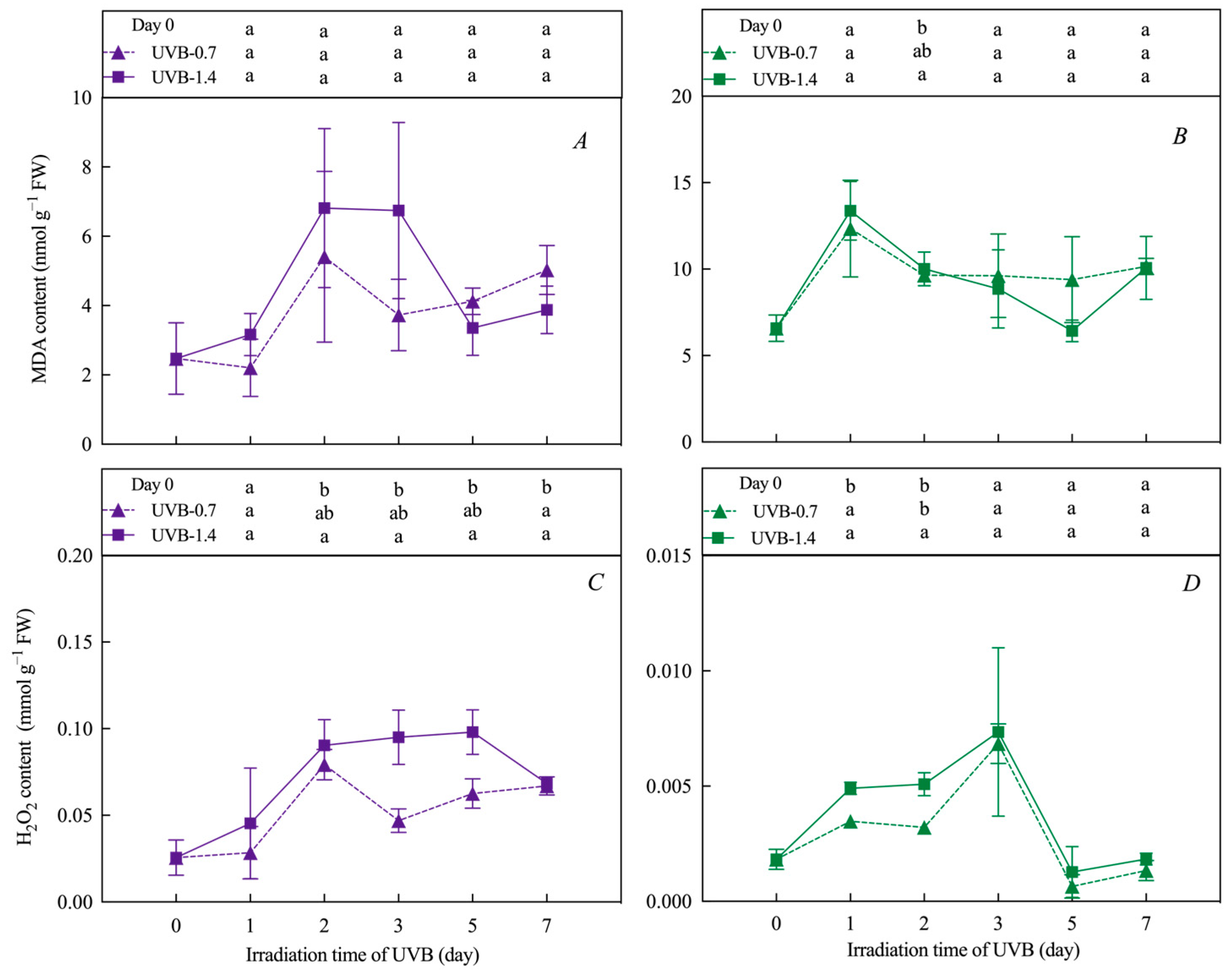
3.4. Effects of UVB Treatment on MDA and H2O2 Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, P.; Prasad, M.N.V. Environmental adaptations and stress tolerance of plants in the era of climate change. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; pp. 1–515. [Google Scholar] [CrossRef]
- McKenzie, R.L.; Björn, L.O.; Bais, A.; Ilyasd, M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. Photochem. Photobiol. Sci. 2003, 2, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Dzakovich, M.P.; Ferruzzi, M.G.; Mitchell, C.A. Manipulating Sensory and Phytochemical Profiles of Greenhouse Tomatoes Using Environmentally Relevant Doses of Ultraviolet Radiation. J. Agric. Food Chem. 2016, 64, 6801–6808. [Google Scholar] [CrossRef] [PubMed]
- Brandle, J.R.; Cappell, W.S.; Sisson, W.B.; Caldwell, M.M. Net photosynthesis, electron transports capacity and ultrastructure of Pisum sativum L. exposed to ultraviolet-B radiation 1. Plant Physiol. 1977, 60, 165–169. [Google Scholar] [CrossRef]
- Lou, Y.S.; Zhang, Z.; Wu, J. Crop growth, yield and quality as affected by ultraviolet-B (UV-B) radiation elevating. J. Agro-Environ. Sci. 2020, 39, 812–821. [Google Scholar]
- Interdonato, R.; Rosa, M.; Nieva, C.B.; González, J.A.; Hilal, M.; Prado, F.E. Effects of low UV-B doses on the accumulation of UV-B absorbing compounds and total phenolics and carbohydrate metabolism in the peel of harvested lemons. Environ. Exp. Bot. 2011, 70, 204–211. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet-B radiation: A review. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Liu, C.H.; Han, X.X.; Cai, L.Y.; Lu, X.Y.; Ying, T.J.; Jiang, Z.H. Postharvest UV-B irradiation maintains sensory qualities and enhances antioxidant capacity in tomato fruit during storage. Postharvest Biol. Technol. 2011, 59, 232–237. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Henbest, R.G.C.; Battey, N.H.; Hadley, P. The influence of ultraviolet radiation on growth, photosynthesis and phenolic levels of green and red lettuce: Potential for exploiting effects of ultraviolet radiation in a production system. Ann. Appl. Biol. 2010, 156, 357–366. [Google Scholar] [CrossRef]
- Caldwell, C.R.; Britz, S.J. Effect of supplemental ultraviolet radiation on the carotenoid and chlorophyll composition of greenhouse-grown leaf lettuce (Lactuca sativa L.) cultivars. J. Food. Compos. Anal. 2006, 19, 637–644. [Google Scholar] [CrossRef]
- Marin, A.; Ferreres, F.; Barbera, G.G.; Gil, M.I. Weather variability influences color and phenolic content of pigmented baby leaf lettuce throughout the season. J. Agric. Food Chem. 2015, 63, 1673–1681. [Google Scholar] [CrossRef]
- FAO. Available online: https://www.fao.org/faostat/en/#data (accessed on 16 October 2022).
- Dannehl, D.; Becker, C.; Suhl, J.; Josuttis, M.; Schmidt, U. Reuse of organomineral substrate waste from hydroponic systems as fertilizer in open-field production increases yields, flavonoid glycosides, and caffeic acid derivatives of red oak leaf lettuce (Lactuca sativa L.) much more than synthetic fertilizer. J. Agric. Food Chem. 2016, 64, 7068–7075. [Google Scholar] [CrossRef] [PubMed]
- García-Macías, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovrgrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem. 2007, 55, 10168–10172. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. Adv. Photosynth. Res. 1984, 2, 9–12. [Google Scholar] [CrossRef]
- Kaulmann, A.; Jonville, M.C.; Schneider, Y.J.; Hoffmann, L.; Bohn, T. Carotenoids, polyphenols and micronutrient profiles of Brassica oleraceae and plum varieties and their contribution to measures of total antioxidant capacity. Food Chem. 2014, 155, 240–250. [Google Scholar] [CrossRef]
- Mancinelli, A.L.; Rabino, I. Photoregulation of Anthocyanin Synthesis X. Dependence on Photosynthesis of High Irradiance Response Anthocyanin Synthesis in Brassica oleracea Leaf Disks and Spirodela polyrrhiza. Plant Cell Physiol. 1984, 25, 1153–1160. [Google Scholar] [CrossRef]
- Oueslati, S.; Trabelsi, N.; Boulaaba, M.; Legault, J.; Abdelly, C.; Ksouri, R. Evaluation of antioxidant activities of the edible and medicinal Suaeda species and related phenolic compounds. Ind. Crops. Prod. 2012, 36, 513–518. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of water stress on antioxidant systemsand oxidative parameters in fruits of tomato (Solanum Lycopersicon L.; cv Micro-tom). Physiol. Mol. Biol. Plants. 2013, 19, 363–378. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Global Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Zhan, L.; Hu, J.; Ai, Z.; Pang, L.; Li, Y.; Zhu, M. Light exposure during storage preserving soluble sugar and l-ascorbic acid content of minimally processed romaine lettuce (Lactuca sativa L. var. longifolia). Food Chem. 2013, 136, 273–278. [Google Scholar] [CrossRef]
- Aiamla-Or, S.; Kaewsuksaeng, S.; Shigyo, M.; Yamauchi, N. Impact of UV-B irradiation on chlorophyll degradation and chlorophyll-degrading enzyme activities in stored broccoli (Brassica oleracea L. Italica Group) florets. Food Chem. 2010, 120, 645–651. [Google Scholar] [CrossRef]
- Choi, B.Y.; Roh, K.S. UV-B radiation affects chlorophyll and activation of rubisco by rubisco activase in Canavalia ensiformis L. Leaves. J. Plant Biol. 2003, 46, 117–121. [Google Scholar] [CrossRef]
- Domonkos, I.; Kis, M.; Gombos, Z.; Ughy, B. Carotenoids, versatile components of oxygenic photosynthesis. Prog. Lipid Res. 2013, 52, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.M.; Teramura, A.H. The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol. 1993, 103, 741–752. [Google Scholar] [CrossRef]
- Becatti, E.; Petroni, K.; Giuntini, D.; Castagna, A.; Calvenzani, V.; Serra, G.; Mensuali-Sodi, A.; Tonelli, C.; Ranieri, A. Solar UV-B radiation influences carotenoid accumulation of tomato fruit through both ethylene-dependent and independent mechanisms. J. Agric. Food Chem. 2009, 57, 10979–10989. [Google Scholar] [CrossRef]
- Giovannucci, E. Tomatoes, Tomato-Based Products, Lycopene, and Cancer: Review of the Epidemiologic Literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Hectors, K.; O’Brien, N.M.; Guisez, Y.; Potters, G. Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Sci. 2008, 175, 449–458. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Jaiswal, D.; Pandey, A.; Agrawal, M.; Agrawal, S.B. Photosynthetic, Biochemical and Secondary Metabolite Changes in a Medicinal Plant Chlorophytum borivillianum (Safed musli) against Low and High Doses of UV-B Radiation. Photochem. Photobiol. 2023, 99, 45–56. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef]
- Takshak, S.; Agrawal, S.B. Defence strategies adopted by the medicinal plant Coleus forskohlii against supplemental ultraviolet-B radiation: Augmentation of secondary metabolites and antioxidants. Plant Physiol. Biochem. 2015, 97, 124–138. [Google Scholar] [CrossRef]
- Huchzermeyer, B.; Menghani, E.; Khardia, P.; Shilu, A. Metabolic Pathway of Natural Antioxidants, Antioxidant Enzymes and ROS Providence. Antioxidants 2022, 11, 761. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Schreiner, M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.P.; Natarajan, S.; Sullivan, J.H. Impact of solar ultraviolet-B radiation on the antioxidant defense system in soybean lines differing in flavonoid contents. Environ. Exp. Bot. 2008, 63, 39–48. [Google Scholar] [CrossRef]
- Laurie, C.L.; Clint, C.S. Arabidposis mutants lacking phenolic sunscreens exhibit enhanced Ultraviolet-B injury and oxidative damage. Plant Physiol. 1995, 109, 1159–1166. [Google Scholar]
- Ferreres, F.; Gil, M.I.; Castañer, M.; Tomás-Barberán, F.A. Phenolic Metabolites in Red Pigmented Lettuce (Lactuca sativa). Changes with Minimal Processing and Cold Storage. J. Agric. Food Chem. 1997, 45, 4249–4254. [Google Scholar] [CrossRef]
- Heijde, M.; Ulm, R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef]
- Lv, M.; Su, H.Y.; Li, M.L.; Yang, D.L.; Yao, R.Y.; Li, M.F.; Wei, J.H. Effect of UV-B radiation on growth, flavonoid and podophyllotoxin accumulation, and related gene expression in Sinopodophyllum hexandrum. Plant Biol. 2021, 23, 202–209. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Ferreira de Oliveira, J.M.P.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef]
- Singh, S.; Agrawal, S.B.; Agrawal, M. UVR8 mediated plant protective responses under low UV-B radiation leading to photosynthetic acclimation. J. Photoch. Photobio. B 2014, 137, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Behn, H.; Tittmann, S.; Walter, A.; Schurr, U.; Noga, G.; Ulbrich, A. UV-B transmittance of greenhouse covering materials affects growth and flavonoid content of lettuce seedlings. Eur. J. Hortic. Sci. 2010, 75, 259–268. [Google Scholar] [CrossRef]
- Mormile, P.; Rippa, M.; Graziani, G.; Ritieni, A. Use of greenhouse-covering films with tailored UV-B transmission dose for growing ‘medicines’ through plants: Rocket salad case. J. Sci. Food Agric. 2019, 99, 6931–6936. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Hogewoning, S.W.; van Ieperen, W. Responses of supplemental blue light on flowering and stem extension growth of cut chrysanthemum. Sci. Hortic. 2014, 165, 69–74. [Google Scholar] [CrossRef]
- Park, J.S.; Choung, M.G.; Kim, J.B.; Hahn, B.S.; Kim, J.B.; Bae, S.C.; Roh, K.H.; Kim, Y.H.; Cheon, C.I.; Sung, M.K.; et al. Genes up-regulated during red coloration in UV-B irradiated lettuce leaves. Plant Cell Rep. 2007, 26, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, I.; Huyskens-Keil, S.; Keller, A.; Ulrich, D.; Kroh, L.W.; Rohn, S. UV-B-induced changes of volatile metabolites and phenolic compounds in blueberries (Vaccinium corymbosum L.). Food Chem. 2011, 12, 60–64. [Google Scholar] [CrossRef]
- Harbaum-Piayda, B.; Palani, K.; Schwarz, K. Influence of postharvest UV-B treatment and fermentation on secondary plant compounds in white cabbage leaves. Food Chem. 2016, 197, 47–56. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef]


| UVB-0.7 | UVB-1.4 | ||||
|---|---|---|---|---|---|
| Variety | Day | Chlorophyll a mg g−1 | Chlorophyll b mg g−1 | Chlorophyll a mg g−1 | Chlorophyll b mg g−1 |
| Red-leaf lettuce | 0 | 0.70 ± 0.03 a | 0.28 ± 0.01 a | 0.70 ± 0.03 ab | 0.28 ± 0.01 ab |
| 1 | 0.77 ± 0.08 a | 0.32 ± 0.01 a | 0.88 ± 0.14 ab | 0.36 ± 0.04 ab | |
| 2 | 1.07 ± 0.01 a | 0.39 ± 0.02 a | 1.02 ± 0.14 a | 0.43 ± 0.05 a | |
| 3 | 0.84 ± 0.28 a | 0.38 ± 0.07 a | 0.47 ± 0.05 b | 0.27 ± 0.01 ab | |
| 5 | 0.62 ± 0.28 a | 0.29 ± 0.06 a | 0.49 ± 0.17 ab | 0.27 ± 0.05 b | |
| 7 | 0.63 ± 0.12 a | 0.29 ± 0.01 a | 0.51 ± 0.09 ab | 0.25 ± 0.02 b | |
| Green-leaf lettuce | 0 | 0.34 ± 0.01 ab | 0.37 ± 0.001 b | 0.34 ± 0.01 a | 0.37 ± 0.001 a |
| 1 | 0.38 ± 0.02 a | 0.43 ± 0.02 ab | 0.34 ± 0.02 a | 0.45 ± 0.08 a | |
| 2 | 0.35 ± 0.03 a | 0.41 ± 0.03 b | 0.31 ± 0.01 a | 0.47 ± 0.03 a | |
| 3 | 0.35 ± 0.01 ab | 0.51 ± 0.01 a | 0.30 ± 0.02 a | 0.46 ± 0.01 a | |
| 5 | 0.28 ± 0.01 b | 0.40 ± 0.03 b | 0.25 ± 0.01 a | 0.36 ± 0.02 a | |
| 7 | 0.26 ± 0.02 b | 0.41 ± 0.02 b | 0.30 ± 0.04 a | 0.46 ± 0.05 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; He, H.; Song, W.; Zheng, L. Pre-Harvest UVB Irradiation Enhances the Phenolic and Flavonoid Content, and Antioxidant Activity of Green- and Red-Leaf Lettuce Cultivars. Horticulturae 2023, 9, 695. https://doi.org/10.3390/horticulturae9060695
Zhang H, He H, Song W, Zheng L. Pre-Harvest UVB Irradiation Enhances the Phenolic and Flavonoid Content, and Antioxidant Activity of Green- and Red-Leaf Lettuce Cultivars. Horticulturae. 2023; 9(6):695. https://doi.org/10.3390/horticulturae9060695
Chicago/Turabian StyleZhang, Han, Huaming He, Weitang Song, and Liang Zheng. 2023. "Pre-Harvest UVB Irradiation Enhances the Phenolic and Flavonoid Content, and Antioxidant Activity of Green- and Red-Leaf Lettuce Cultivars" Horticulturae 9, no. 6: 695. https://doi.org/10.3390/horticulturae9060695
APA StyleZhang, H., He, H., Song, W., & Zheng, L. (2023). Pre-Harvest UVB Irradiation Enhances the Phenolic and Flavonoid Content, and Antioxidant Activity of Green- and Red-Leaf Lettuce Cultivars. Horticulturae, 9(6), 695. https://doi.org/10.3390/horticulturae9060695





