Abstract
Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) is an important economic pest and has a worldwide distribution. In Chile, this species has been reported over a large geographical area and is associated with tomato production. Although several plants have been described as hosts of the whitefly, this insect’s behavior against multiple hosts is still unclear. Therefore, the objective of our work was to identify the host plants, behaviors, preferences, performance, and choices of T. vaporariorum. First, over one year, we monitored nine production sites where tomato is the principal crop and identified 50 host plants belonging to 27 families, mostly Asteraceae and Solanaceae. Among the plants, those that were most infested by greenhouse whiteflies comprised Solanum lycopersicum, Phaseolus vulgaris, Cucurbita maxima, Malva sylvestris, Bidens aurea, and Sonchus oleraceus. In laboratory tests, greenhouse whiteflies showed a greater preference for S. oleraceus, S. lycopersicum, and P. vulgaris. The highest population growth rate was observed for B. aurea, followed by S. lycopersicum and S. oleraceus. Significant differences were found in the pairwise choice test, showing a greater preference for C. maxima and S. lycopersicum. Although this pest tends to choose tomatoes, this crop was not always the first choice in terms of preference and performance. This information is necessary for the development of integrated whitefly management programs that include adjacent habitats.
1. Introduction
Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae), known as greenhouse whitefly (hereafter whitefly), is an insect that has been highlighted as the cause of significant economic damage to several horticultural and ornamental crops and is one the most harmful greenhouse pests worldwide [1,2,3,4]. Trialeurodes vaporariorum originated in North America, likely the U.S.A or México, but is now widely distributed throughout almost all continents from the Mediterranean to tropical regions [5,6,7,8,9].
Whiteflies are piercing–sucking hemimetabolous insects whose life cycle (eggs, four nymphal stages, and adults) takes 25–30 days at 21 °C or 22–25 days at 24 °C [10,11,12,13]. Several studies have shown that the duration of the whitefly lifecycle depends on the host plant [14,15,16,17]. Greenhouse crops are considered the best habitat for Trialeurodes vaporariorum, providing the best conditions for its development in a short time [11] and achieving up to 15 generations per year [18,19].
Both adults and nymphs feed on plant sap, consequently weakening the plant and affecting its photosynthesis rate and respiration in addition to dehydrating it and causing chlorosis [4,20], which results in lower yield and plant quality [21]. The insect also excretes large quantities of honeydew, which favors the development of certain fungi such as “sooty mold” (Cladosporium); this fungus reduces photosynthesis and, at the same time, affects plant vigor and fruit quality [4,10,12,18,22]. Another indirect detrimental effect of whiteflies stems from their role as vectors for certain viruses, thus causing a variety of diseases and deformations [23,24,25]
Trialeurodes vaporariorum polyphagous habits include several genera and families of plants that humans use as sources of fruits and vegetables or as ornamentals, in addition to weeds [6,18,26]. At present, the crops most attacked by this species are tomato, sweet pepper, lettuce, melon, watermelon, beans, pumpkin, and others. About 249 host plant species have been reported, which are distributed in 80 families, among which Asteraceae represent the highest register with 44 species, followed by Solanaceae (22) and Malvaceae (18) [27,28]. In Finland, Ovčarenko et al. [29] identified five new host plant species outside greenhouses containing whiteflies once the main crops were harvested. In this case, plants surrounding the production systems acted as natural reservoirs for the insect, allowing its survival until a new crop appeared both inside and outside the greenhouses [30].
Trialeurodes vaporariorum uses visual stimuli while searching for hosts and prefers yellow objects shaped similarly to leaves [31,32,33,34]. However, the establishment of these insects is determined by the physical and chemical properties of the host plant (leaf texture and density of trichomes), which can hinder the arrival, mobility, feeding, and oviposition of this species [35,36,37,38]. Furthermore, the presence of secondary metabolites and the nutritional content of the plant determine the development and performance of future progeny [1,39].
The principal economic impact of the whitefly was observed on tomato crops [20] , strongly decreasing yields. This pest is usually controlled using pesticides such as carbamates and neonicotinoids, which may have a negative impact on the environment [40,41,42]. Trialeurodes vaporariorum has not been successfully controlled via conventional management systems (anti-aphid nets, insecticides, or biological control), thus causing its expansion into new territories [1,4,5,18,26,40]. At present, biological controls and physical natural barriers have been used in other countries [2,43,44]; however, to manage this pest efficiently, it is necessary to recognize the principal host plants associated with the targeted crops in order to develop an integrated management program. The principal goal of this work was to identify the host plants and facilitate whitefly monitoring and counting both inside and outside of greenhouses, as well as determine the behaviors, preferences, performance, and choices of T. vaporariorum in intensive production areas of Solanum lycopersicum.
2. Materials and Methods
2.1. Sampling Area for Monitoring
The study was conducted in a zone with a Mediterranean climate in the Valparaíso Region of Chile (latitude: 32°52′ S; longitude 71°15′ W) (Table 1), covering a 30,200 ha area featuring the intensive production of Solanum lycopersicum and Trialeurodes vaporariorum infestation. The sites chosen for sampling are areas that currently intensively cultivate tomatoes; however, as a control, we also included two areas within the production zone without tomato cultivation.

Table 1.
Location of the sites under study and the type of cultivation carried out.
2.2. Vegetative Material for Tests
Based on the results obtained for the identification of the hosts of T. vaporariorum (Table 2), six plant species were selected for this study. Three species corresponded to economically important crops—Solanum lycopersicum L. cv. “Patron” (tomato), Phaseolus vulgaris L. (bean), and Cucurbita maxima Duch. (squash); the other three corresponded to frequently observed weeds in the area: Malva sylvestris L. (mallow), Bidens aurea (Aiton) Sherff (Arizona beggarticks), and Sonchus oleraceus L. (thistle).

Table 2.
Alternative hosts of Trialeurodes vaporariorum in an area with a high production of tomatoes in Chile.
For the olfactometer tests, Allium sativum (garlic) and Ocimum basilicum (basil) were also used, as they have been widely studied as whitefly repellents [44]. These plants were included as a negative control.
One-month-old tomatoes, beans, and squash were obtained from commercial nurseries. In the case of weeds, seedlings with two true leaves and similar sizes were collected from a whitefly-free area. In both cases, the plants were washed in sodium hypochlorite (1%) solution and distilled water. All host plants were transferred into 2.5 L plastic pots containing a sterilized leaf mulch substrate and maintained in a 30 m2 polyethylene greenhouse with natural ventilation for 3 months prior to use.
2.3. Insect Rearing
For the rearing of whiteflies, adults were collected from six commercial tomato greenhouses. The insects were mixed and reared in a polycarbonate greenhouse and maintained for at least five generations, and they grew on tomato genotypes cv. Luciana, 7742, and Mistral. No preadaptation conditions of the whitefly according to Ovčarenko et al. [29]. To avoid possible selection bias, the variety of tomatoes was changed in the test. The whitefly progeny was used in the subsequent experiments of this study.
2.4. Experimental Procedure
2.4.1. Whitefly Population Dynamics in the Quillota Province
To assess the population sizes of T. vaporariorum, nine tomato farms were monitored: Five farms produced tomatoes in greenhouses, and another four did not use greenhouses (Table 1). In farms with greenhouses, two rows of tomatoes were selected in one greenhouse, with one located 3 m from the principal entrance and the other in the middle of the room. For each selected row, one 15 × 25 cm yellow adhesive trap was placed at the beginning, one was placed in the middle, and one was placed at the end, for a total of three traps. Outside the greenhouses, six more traps were placed around the greenhouse based on the four cardinal points (with a total of 12 traps per site). The traps were located at a distance of 10 m relative to the greenhouses. In farms without greenhouses, six sampling points per farm were selected based on the four cardinal points and the presence of alternative hosts of the pest. On each site, we maintained a minimum distance of 20 m between the installed yellow traps. At all farms, traps were placed at a height of 0.5 m above the ground for 24 h. All trapped T. vaporariorum individuals were counted under a stereoscopic microscope (Zeiss Stemi T4 Model). Whiteflies were monitored every 15 days over a period of 12 months at all evaluated sites.
2.4.2. Determination of Host Plants
Host plant determination at every site was carried out every 15 days over one year using 100 m transects located 10 m from greenhouses. Each plant found along the transect was scanned for 1 min to assess the presence of whiteflies. When whiteflies were detected, the plant was sampled and stored in a plastic bag until its determination in the laboratory, for which we followed the taxonomic keys in Espinoza [45], Bayer [46], and Espinoza [47]. The vegetative material was classified as a host plant when adults and immature stages of T. vaporariorum were found on it.
All observed T. vaporariorum individuals were subjected to a preliminary evaluation in the field, in which we evaluated the wing position and body color against a field key [48]. Afterward, stereomicroscopic observations of adults and immature stages were carried out according to Carapia-Ruiz and Castillo-Gutierrez [49], paying special attention to the compound-eye structure (separation of the upper and lower ommatidia), the shape of the wings, and egg mass disposition on leaves (circular or semi-circular).
2.4.3. Multiple-Choice Test
Multiple-choice tests were conducted according to Huang et al. [50]. Three plants of each selected host species (see Section 2.2) were randomly distributed in a 48 m2 greenhouse under controlled conditions (L: D 16: 8; 60% RH) with 30 cm of distance between the plants. For insects, the species’ sex was determined by observing the clearly defined supra-genital plate in females [49]. Fifty female adults were placed in a Petri dish located in the greenhouse’s center for their release, allowing free movement of insects. Seven days after release, adult and immature insects were counted. In the greenhouse, leaves were selected from the top, middle, and bottom of each plant, and the adult stages of the whitefly were counted directly on the abaxial side of each leaf. For the immature stage (eggs), the same leaves were removed and maintained at 0 °C until counting. The total leaf area was measured using millimeter paper to determine the number of individuals per cm2 on each host.
2.4.4. Host Plant Effect on Population Growth
The performance test was accomplished using a leaf from the apical zone isolated with a fine mesh (10 cm2) and subsequently inoculated with five adult females. Fifteen replicates of each selected host plant were used in a greenhouse under controlled conditions (L: D 16: 8; 60% RH). Ten days after inoculation, the total eggs and nymphs were counted in the laboratory, and the insects’ performance on each host species was calculated as the population growth rate (PGR)(1) [51]:
where N1 corresponds to the initial population of adult whiteflies, N2 is the final population (eggs, nymphs, and adults), and (t2 − t1) is the trial’s duration expressed in days (10 days).
PGR = (ln N2 − ln N1) × (t2 − t1)−1,
2.4.5. Paired Choice Test
A paired test was performed to contrast the host species. Leaves of similar size were obtained from the apical zone of each host and placed in a transparent plastic chamber (500 mL capacity and 15 cm diameter). Leaves were placed 5 cm apart. The leaves were moistened with wet absorbent cotton on the stem. The chamber was inoculated with 10 adults of T. vaporariorum located in the middle of the arena and maintained under controlled conditions with a temperature range between 15 ± 2 °C at night and 25 ± 2 °C during the day for 24 h. A total of 15 combinations were used, considering 15 replicates for each case. The number of adults on each host was recorded at 1, 2, 3, 6, 12, and 24 h after inoculation.
2.4.6. Olfactometer Test
The response of T. vaporariorum to plant volatiles and visual stimuli (2.5 cm diameter perforated yellow circle) was studied using a Y-tube olfactometer made from a Y-glass tube (2.5 cm in diameter) and two glass containers (30 cm in length and 16 cm in diameter) with an airflow of 0.5 L/min that passed through an activated charcoal filter and humidifier with PTFE connections (Soviquim Ltd.a, Santiago, Chile). All tests were performed between 11 a.m. and 4 p.m. under laboratory conditions (22 °C, 60 R.H.). Thirty insects with valid responses were considered for each trial of the different tests made in the olfactometer. A valid response was considered when the insect made the host choice within the first 15 min of each test. Each stimulus was placed in the chamber 30 min before the assay, and the tube was rotated after each test and cleaned after five repetitions to avoid contamination. The insects used were discarded after each test to avoid pseudoreplication.
In relation to assays, four responses were considered. The first group was used to study whitefly responses to plant volatiles (Malva sylvestris, Bidens aurea, Sonchus oleraceus, and Solanum lycopersicum L. cv. “Patron” as host plants and Allium sativum and Ocimun basilicum as negative controls) and a blank chamber. The second assay assessed responses to plant volatiles (Malva sylvestris, Bidens aurea, and Sonchus oleraceus) versus volatile tomato compounds. For the third group, we included a yellow circle on the top of the arm in the Y-tube as a visual stimulus and evaluated the responses against plant volatiles (Malva sylvestris, Bidens aurea, Sonchus oleraceus, and Solanum lycopersicum L. cv. “Patron”). It should be noted that the yellow circle was cut from a yellow sticky trap and punched to avoid altering the airflow. Finally, the last group was formed to assess the preference of T. vaporariorum relative to plant volatiles in addition to visual stimuli and volatile tomato compounds.
2.5. Statistical Analysis
For the multiple-choice test, a generalized linear model was applied by assuming a Poisson distribution. The best model was chosen by the Akaike information criterion, and ANOVA type II was carried out using the car package (Fox et al., 2016). Pairwise multiple comparisons were developed with Tukey’s test with the “one-step” method using the multcomp package (Hothorn et al., 2016). In the performance test, since the data followed a normal distribution, one-way ANOVA was performed, and Tukey’s HSD was used for multiple comparisons of different hosts. In the paired choice tests, Wilk’s Lambda (GLM algorithms) was used via ANOVA with repeated measures. Bonferroni tests were used for multiple comparisons. All statistical analyses were performed with R 3.6.5 statistical software (R Core Team 2019). In the olfactometer test, a chi-squared test was performed using Minitab 16.
3. Results
3.1. Whitefly Population Dynamics
The largest adult populations of Trialeurodes vaporariorum captured in the yellow traps were found in areas producing tomato greenhouses. During the months of April and November, we recorded an average of more than 10,000 individuals captured inside the greenhouses (Figure 1a). The adult population of insects outside the greenhouses was higher in the months of March to May (Figure 1b). Productive areas without the presence of greenhouses also had whitefly populations, although in a lower proportion, and their increases were mainly due to the presence of tomatoes outside (Figure 2). Finally, it was observed that, in non-productive areas with a large amount of native vegetation, the whitefly population was almost nil due to the distance from the tomato production areas, the geographical position, and the scarcity of hosts (Figure 3).
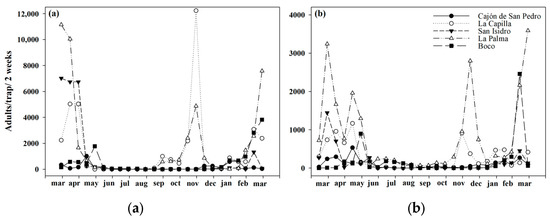
Figure 1.
Adult population of Trialeurodes vaporariorum during the year (a) in greenhouse tomatoes and (b) outside greenhouse tomatoes.
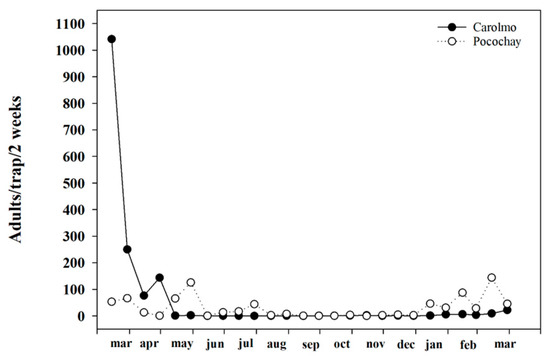
Figure 2.
Adult population of Trialeurodes vaporariorum tomato outdoor crops during the year.
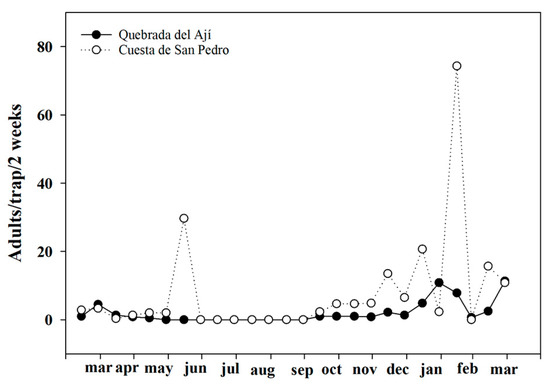
Figure 3.
Adult population of Trialeurodes vaporariorum in non-productive areas with a large amount of native vegetation.
3.2. Host Plants
Fifty different host plant species of Trialeurodes vaporariorum were identified, seventeen of which corresponded to crops and ornamental plants and thirty-three were weeds. These host plants belonged to 27 families, mainly Asteraceae and Solanaceae, with nine and four species, respectively (Table 2).
3.3. Multiple-Choice Test
Populations of T. vaporariorum analyzed in the multiple-choice test (Figure 4) showed significant differences among different hosts (GLM, χ2 = 21.445; df = 5; p = 0.001). The largest total population of adults and immature stages was observed in thistle, tomato, and bean, without significant differences between them. No differences were observed regarding the adult insect populations between host species (GLM, χ2 = 0.38416; df = 5; p = 0.99575). The number of eggs laid by adults varied significantly among different host plants, between zero and four eggs per cm2 (GLM, χ2 = 21.499, df = 5, p = 0.0007), and the highest populations were observed in thistles, tomatoes, and beans.
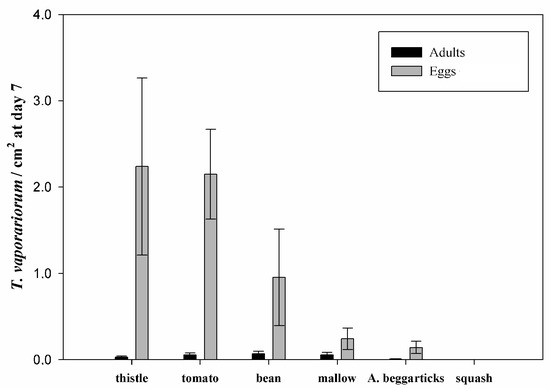
Figure 4.
Adults and eggs of Trialeurodes vaporariorum per cm2 of the foliar area (mean ± standard error) from 3 replicates for different host plants at day 7. Thistle = Sonchus oleraceus; tomato = Solanum lycopersicum; bean = Phaseolus vulgaris; mallow = Malva sylvestris; Arizona beggarticks = Bidens aurea; squash = Cucurbita maxima.
3.4. Performance Test
For the performance test, the population growth rate (PGR) showed significant differences between the host plants (F = 25.82, df = 5, p < 0.001) (Figure 5). Arizona beggarticks (0.418 ± 0.012) showed the best performance, followed by tomatoes (0.315 ± 0.014). Furthermore, beans (0.164 ± 0.031) registered the lowest population rates.
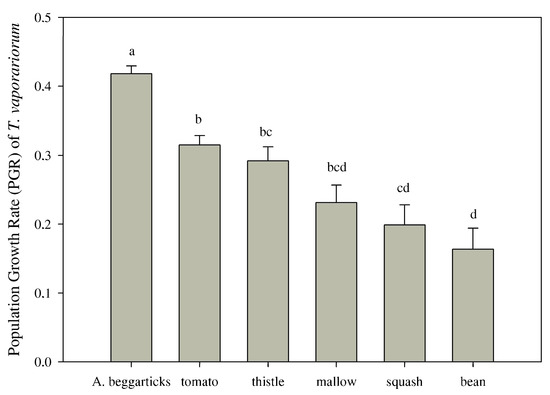
Figure 5.
Performance expressed as the population growth rate (PGR, mean ± standard error) of Trialeurodes vaporariorum from 15 replicates for the different host species. Letters indicate significant differences based on a multiple comparison Tukey HSD. Thistle = Sonchus oleraceus; tomato = Solanum lycopersicum; bean = Phaseolus vulgaris; mallow = Malva sylvestris; Arizona beggarticks = Bidens aurea; squash = Cucurbita maxima.
3.5. Paired Choice Test
The preferences of greenhouse whiteflies for each host were analyzed at different hours, showing significant differences in the paired-choice test (Table A1) where major preferences were registered for squash, tomatoes, and beans, while non-preference was observed in Arizona beggarticks (Figure 6). During the first hours (hours 1, 2, and 3), there was generally no clear choice in terms of the host plant. In most cases, principal differences were observed after hour 12.
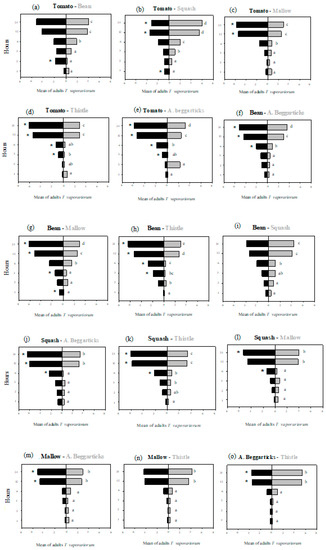
Figure 6.
Paired choice tests with 15 paired combinations of host plants for Trialeurodes vaporariorum and the preferences of the insects at 1, 2, 3, 6, 12, and 24 h (Table A2). A multivariate Wilk’s Lambda test was used to determine significant differences, and a Bonferroni adjustment was used to test multiple comparisons. Letters show significant differences among hours, and an asterisk (*) indicates significant differences between host plants. Thistle = Sonchus oleraceus; tomato = Solanum lycopersicum; bean = Phaseolus vulgaris; mallow = Malva sylvestris; Arizona beggarticks = Bidens aurea; squash = Cucurbita maxima.
3.6. Olfactometer Test
The positional influence of the Y-tube olfactometer was evaluated by comparing blank versus blank stimuli with non-significant differences (X2 = 0.533, df = 1; p = 0.465). When comparing blank vs. visual stimuli, we did not find significant differences either (X2 = 2.133, df = 1; p = 0.1441).
When comparing the preferences between tomatoes with a visual stimulus, the trials showed that T. vaporariorum preferred tomato (X2 = 19.2, df = 1; p < 0.0001). All host plants evaluated in this study were contrasted with a blank, with mallow and tomato showing significant differences (X2 = 8.533, df = 1, p = 0.0035; and X2 = 16.133, df = 1, p < 0.0001, respectively; Figure 7).
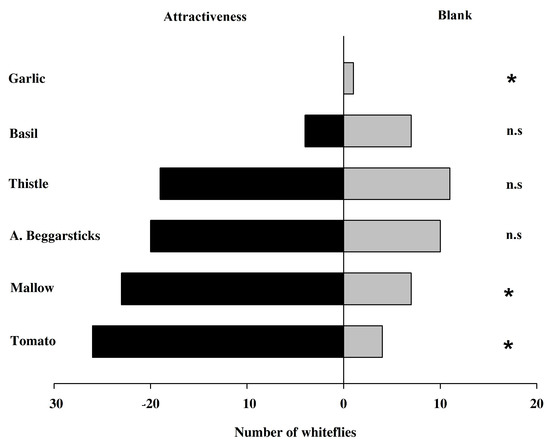
Figure 7.
Number of greenhouse whiteflies Trialeurodes vaporariorum in Y olfactometric assays contrasted with blank controls (no stimuli, odor, and/or color). * indicates significant differences with a X2 test; n.s., no significant differences.
Tomatoes were contrasted with other host plants, showing significant differences (Figure 8a). The same response was observed when the tomato was contrasted with the host plant in addition to visual stimuli (Figure 8b). The test statistics are shown in Table 3.
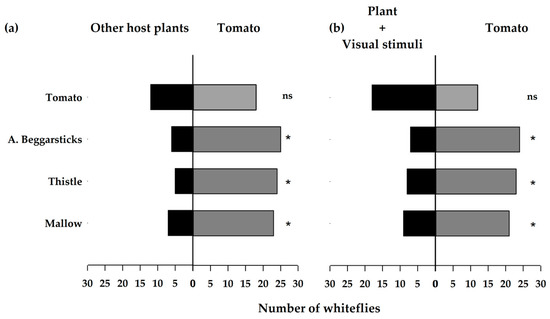
Figure 8.
Number of greenhouse whiteflies Trialeurodes vaporariorum in Y olfactometric assays contrasting (a) other host plants against tomato; (b) other host plants + visual stimuli (yellow color) against tomatoes. * indicates significant differences with an X2 test; n.s., no significant differences.

Table 3.
X2 test statistics in olfactometry assays for Trialeurodes vaporariorum contrasting other host plants versus tomato; other host plants + visual stimuli (yellow color) versus tomato.
4. Discussion
We found 50 hosts of Trialeurodes vaporariorum associated with principal crops in the study zone, documenting that the whitefly has a broad geographic distribution in Chile. This insect has been found in a transect of 3000 km from north to south in this country where a Mediterranean climate predominates [52]. This area is characterized by a large surface featuring possible host crops such as tomatoes, peppers, cucurbits, beans, lettuce, ornamental plants, etc. Before this study, 27 weeds had been recognized in Quillota Valley as whitefly hosts [11,53]. We were able to identify 22 new weed hosts in the study area. Therefore, the number of host plants could be higher in the entire country.
Our study showed that inside the greenhouse populations of T. vaporariorum exceeded 2000 adults/trap. These high populations were observed in February, April, May, and November during a period of one year. On the other hand, populations in outdoor crops were lower, and this increment was reflected between March and May. In areas without tomato cultivation but that are within the production zone, the whitefly population was low despite the presence of host weeds. The increase in greenhouse tomato crops, the large number of hosts, and the rapid population growth rate with 11 to 15 generations per year have allowed this insect to become a primary pest [18,54,55]. In addition, T. vaporariorum is a generalist insect and can extend to different hosts [5], and its resistance to many types of insecticides [54,56] has facilitated the regular appearance of this pest in greenhouse production systems. Our study confirmed the above information since high populations were found in greenhouse tomato production. Populations were considered high when more than 500 adults per trap were counted in yellow traps for at least 3 months during a period of one year. In areas without tomato cultivation but within the production zone, the whitefly population was low despite the presence of host weeds.
In this context, it was interesting to analyze insect–plant interactions while considering crop and weed hosts due to their importance as reservoirs for insects during diapauses and/or the absence of the principal host crops in the greenhouses. Furthermore, the preference for oviposition depends on the host plant [57], which provides the best conditions for feeding and subsequent offspring [58,59] according to the preference–performance hypothesis [60,61]. In our case, in the multiple-choice test, Trialeurodes vaporariorum showed the best oviposition in Sonchus oleraceus, with a null result in C. maxima. Our results agree with those of Lu et al. [57] but differ from those of López et al. [62], who indicated that the host plant did not influence the ability to generate offspring. In addition, this ability could depend on other factors such as the age of the plant, the position of the leaves within the foliage [63], or the quality of the foliage and its chemical composition [64]. However, these parameters were not measured in our study.
Our results from the performance test conducted under controlled conditions showed that weeds such as Bidens aurea exhibited higher populations than commercial crops. However, this behavior was not observed under field conditions in the study area; this is in contrast to Ovčarenko et al. [29], who argued that whiteflies prefer weeds surrounding crops rather than the crops inside greenhouses. The factors that could affect the performance of the insect depend on the physical and chemical properties of the plant, such as trichome density [36,38], secondary metabolites [39], and thick cuticles [1]. Bruce et al. [65] indicated that volatile organic compounds released by plants likely act as semiochemicals, which are important in enabling insects to recognize host plants from a distance. This evidence suggests that different concentrations or quality levels of volatile compounds present in the leaves of plants could affect the selection by whitefly adults [66].
This pest has also been described as a generalist insect that lives in different habitats, showing a remarkable capacity to change behavior and physiology [67] according to the volatile organic compounds (VOCs) emitted by host plants [68]. Our study showed that host selection by whiteflies is preferentially mediated by olfactory stimuli rather than by visual stimuli.
A controversial point is related to the preference and landing of Trialeurodes vaporariorum for/on its host plant, as different studies have indicated that these factors depend on visual and/or olfactive signals [68,69,70,71]. Additionally, plant volatiles may cause a visual response in the insect, compelling it to select its host plant [71]. Insect–plant interactions have been widely described, where chemical and physical stimuli from the plant (volatile compounds, texture, shape, and color of leaf) and specialized insect structures (antennas, oral apparatus, eyes, and thorax) affect the insect’s choice of a host [31,72]. This preference is higher when host plants have a lower amount of glandular trichomes because the whitefly’s stylet can easily penetrate the leaf’s surface [31,36,37,69,70]. Moreover, the nutritional content and presence of secondary metabolites in the plant affect the preference of the pest [1,39].
The paired choice test showed that the preference order was squash > tomato > bean > mallow > thistle > A. beggarticks. Similar results were reported by López et al. [62], who conducted a choice trial with the same crop species and found that the number of adults of greenhouse whiteflies per plant in squash was significantly higher than that in beans and tomatoes. In the multiple choice and performance test, squash was not a good host because it showed a scarcity of offspring in this species. This could indicate that the whitefly chooses its host in the first instance according to the volatiles it emits; however, this is not necessarily the host where the insect will leave its offspring. The whitefly has the capacity to choose a correct host plant by means of the following cues: green color of the visual spectrum [71], olfactory signals [72], and volatile concentrations [73]. The insect can be dissuaded by the presence of secondary metabolites [39] or avoid plants in the presence of predators [74].
The identification of whitefly hosts associated with tomato crops, the number of insects inside and outside the greenhouse, and the choice and performance of the insect are variables that should be taken into account when developing integrated pest management plans since knowledge of these variables determines the intensity and frequency of management and control activities that growers should carry out [11]. Secondary hosts play an important role in the survival of the pest when the principal host is not present [2]. Although it is not necessary to eliminate secondary hosts completely, adequate agronomic management should be carried out, and the development of other plants that act as reservoirs of biological controllers should be favored in order to facilitate more natural regulation of the pest [12]. On the other hand, understanding the population fluctuations of the pest during cultivation will enable the development of preventive actions to avoid the emergence of high populations of whitefly, thereby avoiding the overuse of chemical insecticides that increase production costs and may cause damage to the environment [70].
5. Conclusions
The presence of host plants outside greenhouses plays a key role in attracting T. vaporariorum. Therefore, this insect can use weeds as a food reservoir and for shelter and maintenance of its young when crops are not present in the fields. According to our results, whiteflies generally prefer crops to weeds; however, their oviposition and yield are carried out indistinctly. This behavior exhibited by the whitefly gives the insect invasive characteristics, making this pest difficult to control. Therefore, the presence of potential hosts outside the greenhouse, the chemical stimuli generated by host plants, the monitoring of pests, and the behavior of insects are important variables to consider in order to achieve the adequate sustainable management of whiteflies and control populations of this pest in different crops.
Author Contributions
Conceptualization, M.V.A., J.A.V. and M.F.F.; methodology, M.V.A., J.A.V. and M.F.F.; software, M.V.A. and J.A.V.; validation, M.V.A., J.A.V. and M.F.F.; formal analysis, M.V.A., J.A.V. and M.F.F.; investigation, E.C. and S.A.B.; resources, J.A.V.; data curation, J.A.V., E.C. and S.A.B.; writing—original draft preparation, M.V.A., J.A.V., M.F.F., E.C. and S.A.B.; writing—review and editing, M.V.A., J.A.V. and M.F.F.; visualization, J.A.V. and M.F.F.; supervision, M.V.A. and J.A.V.; project administration, M.V.A., J.A.V. and M.F.F.; funding acquisition, M.V.A., J.A.V. and M.F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Inserción-Comisión Nacional de Investigación Científica y Tecnológica CONICYT 781204020 and Fondecyt de Iniciación 11130433 of J.A.V.; Proyecto Interno Asociativo PIA 37468/2015 PUCV of M.F.F. and Iniciativa Científica Milenio (ICM) NC120027; Fortalecimiento de Centro Regionales ANID R22A0002; and Centro Ceres of M.V.A.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We thank Centro Regional de Investigación e Innovación para la Sostenibilidad de la Agricultura y los Territorios Rurales, Ceres, Chile for their technical and funding support.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Combinations of paired choice and the behaviour of the whitefly at different evaluated hours—multivariate test–Lambda de Wilks.
Table A1.
Combinations of paired choice and the behaviour of the whitefly at different evaluated hours—multivariate test–Lambda de Wilks.
| Host | Source of Variation | F | df | p |
|---|---|---|---|---|
| tomato/bean | hour | 31.038 | 5 | 0.0001 |
| hour × host host | 4.460 3.015 | 5 1 | 0.005 0.093 | |
| tomato/squash | hour | 24.219 | 5 | 0.0001 |
| hour × host host | 4.238 3.739 | 5 1 | 0.007 0.0630 | |
| tomato/mallow | hour | 49.895 | 5 | 0.0001 |
| hour × host host | 9.932 22.475 | 5 1 | 0.0001 0.0001 | |
| tomato/thistle | hour | 65.875 | 5 | 0.0001 |
| hour × host host | 8.243 36.193 | 5 1 | 0.0001 0.0001 | |
| tomato/A. beggarticks | hour | 194.068 | 5 | 0.0001 |
| hour × host host | 17.013 50.561 | 5 1 | 0.0001 0.0001 | |
| bean/A. beggarticks | hour | 21.810 | 5 | 0.0001 |
| hour × host host | 0.835 12.684 | 5 1 | 0.538 0.001 | |
| bean/mallow | hour | 38.885 | 5 | 0.0001 |
| hour × host host | 4.129 17.359 | 5 1 | 0.008 0.0001 | |
| bean/thistle | hour | 100.700 | 5 | 0.0001 |
| hour × host host | 17.743 52.714 | 5 1 | 0.0001 0.0001 | |
| bean/squash | hour | 29.044 | 5 | 0.0001 |
| hour × host host | 0.770 0.001 | 5 1 | 0.5800 0.980 | |
| squash/A. beggarticks | hour | 27.281 | 5 | 0.0001 |
| hour × host host | 4.656 17.942 | 5 1 | 0.004 0.0001 | |
| squash/thistle | hour | 52.324 | 5 | 0.0001 |
| hour × host host | 5.225 15.554 | 5 1 | 0.002 0.0001 | |
| squash/mallow | hour | 90.455 | 5 | 0.0001 |
| hour × host host | 4.976 3.796 | 5 1 | 0.003 0.061 | |
| mallow/thistle | hour | 43.549 | 5 | 0.0001 |
| hour × host host | 0.500 0.318 | 5 1 | 0.773 0.577 | |
| mallow/A. beggarticks | hour | 29.205 | 5 | 0.0001 |
| hour × host host | 4.661 10.338 | 5 1 | 0.0004 0.003 | |
| A. beggarticks/thistle | hour | 29.336 | 5 | 0.0001 |
| hour × host host | 1.945 2.253 | 5 1 | 0.124 0.121 |

Table A2.
Mean and SD for paired choice and the behaviour of the whitefly at different evaluated hours. Multivariate test–Lambda de Wilks.
Table A2.
Mean and SD for paired choice and the behaviour of the whitefly at different evaluated hours. Multivariate test–Lambda de Wilks.
| Host Plant | Statistics | Hour 1 | Hour 2 | Hour 3 | Hour 6 | Hour 12 | Hour 24 |
|---|---|---|---|---|---|---|---|
| tomato/bean | Mean | 0.40/0.53 | 1.87/0.27 | 1.67/0.93 | 2.13/1.93 | 4.20/3.80 | 5.20/4.07 |
| SD | 0.63/0.74 | 1.81/0.59 | 1.49/1.28 | 1.77/1.44 | 2.11/1.57 | 1.69/1.67 | |
| tomato/squash | Mean | 0.80/0.13 | 0.87/0.53 | 1.07/1.06 | 1.93/2.07 | 3.53/5.53 | 3.20/6.01 |
| SD | 0.94/0.52 | 0.99/0.64 | 0.98/0.98 | 0.79/1.67 | 2.36/2.23 | 2.11/1.89 | |
| tomato/mallow | Mean | 0.33/0.73 | 0.20/0.33 | 0.60/0.67 | 1.53/0.80 | 5.47/2.53 | 5.80/2.93 |
| SD | 0.49/0.70 | 0.56/0.62 | 0.99/0.82 | 1.72/0.86 | 1.55/1.13 | 1.32/1.62 | |
| tomato/thistle | Mean | 0.20/0.07 | 0.27/0.20 | 0.93/0.13 | 1.33/0.27 | 5.47/2.87 | 6.13/2.93 |
| SD | 0.41/0.26 | 0.46/0.41 | 1.03/0.35 | 1.45/0.59 | 1.64/1.41 | 1.55/1.03 | |
| tomato/A. beggarticks | Mean | 0.33/0.13 | 0.40/0.27 | 0.87/0.13 | 1.93/0.13 | 5.40/2.47 | 5.87/3.20 |
| SD | 0.62/0.35 | 0.74/0.59 | 1.13/0.35 | 1.62/0.35 | 1.24/1.41 | 0.92/0.86 | |
| bean/A. beggarticks | Mean | 0.60/0.27 | 1.07/0.40 | 1.27/0.47 | 2.07/0.87 | 4.40/2.93 | 5.20/3.60 |
| SD | 0.91/0.46 | 1.33/0.63 | 1.53/0.64 | 1.28/1.13 | 1.88/1.79 | 1.89/1.76 | |
| bean/mallow | Mean | 0.67/0.13 | 1.07/0.87 | 1.47/0.60 | 2.47/1.53 | 5.13/2.53 | 6.20/2.93 |
| SD | 0.82/0.52 | 1.03/0.74 | 1.30/0.74 | 1.88/1.36 | 2.17/1.36 | 1.89/1.49 | |
| bean/thistle | Mean | 0.13/0.07 | 1.00/0.40 | 1.93/0.20 | 2.80/0.33 | 5.27/2.73 | 6.40/3.00 |
| SD | 0.35/0.26 | 0.93/0.74 | 1.28/0.41 | 1.86/0.49 | 1.33/1.58 | 1.24/1.13 | |
| bean/squash | Mean | 0.53/0.40 | 0.87/0.80 | 1.20/1.13 | 2.07/1.13 | 3.40/4.13 | 3.87/4.40 |
| SD | 0.83/0.74 | 1.13/1.08 | 1.26/1.25 | 1.49/0.99 | 2.59/2.28 | 2.36/2.16 | |
| squash/A. beggarticks | Mean | 0.80/0.40 | 0.93/0.47 | 1.20/0.53 | 2.33/0.27 | 6.00/3.13 | 6.47/3.33 |
| SD | 1.32/0.83 | 1.33/1.06 | 1.08/0.99 | 1.76/0.46 | 2.14/1.55 | 2.07/2.02 | |
| squash/thistle | Mean | 0.33/0.20 | 0.80/0.53 | 1.20/0.53 | 2.13/0.73 | 5.87/3.27 | 6.07/3.40 |
| SD | 0.72/0.56 | 1.15/0.74 | 1.52/0.83 | 1.77/1.03 | 1.81/1.62 | 1.71/1.40 | |
| squash/mallow | Mean | 0.13/0.53 | 0.53/0.60 | 0.80/0.73 | 1.40/0.33 | 4.60/3.87 | 5.33/4.00 |
| SD | 0.35/0.83 | 1.13/0.63 | 0.86/1.09 | 1.35/0.49 | 0.91/1.30 | 1.23/1.60 | |
| mallow/thistle | Mean | 0.20/0.27 | 0.07/0.33 | 0.33/0.33 | 0.87/0.73 | 4.27/3.67 | 4.40/4.20 |
| SD | 0.56/0.46 | 0.26/0.62 | 0.62/0.62 | 1.18/0.79 | 1.39/1.45 | 1.50/1.52 | |
| mallow/A. beggarticks | Mean | 0.20/0.47 | 0.27/0.40 | 0.60/0.27 | 4.73/2.80 | 4.73/2.80 | 5.13/3.00 |
| SD | 0.41/0.83 | 0.46/0.74 | 0.74/0.79 | 0.88/1.08 | 1.49/1.32 | 1.73/1.31 | |
| A. beggarticks/thistle | Mean | 0.33/0.13 | 0.33/0.13 | 0.27/0.20 | 0.87/1.00 | 3.53/5.33 | 3.60/5.47 |
| SD | 0.49/0.35 | 0.72/0.35 | 0.46/0.41 | 1.25/1.41 | 2.20/2.38 | 2.13/2.26 |
References
- Inbar, M.; Gerling, D. Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Ann. Rev. Entomol. 2008, 53, 431–448. [Google Scholar] [CrossRef]
- López, S.N.; Riquelme, M.B.; Botto, E. Integration of biological and chemical control of the whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Rev. Colomb. Entomol. 2010, 36, 190–194. [Google Scholar] [CrossRef]
- Pan, P.L.; Xu, Q.; Qin, Y.C. Circadian rhythm and spatial distribution of mixed populations of two whitefly species on cucumber in greenhouses. Afr. Entomol. 2015, 23, 306–313. [Google Scholar] [CrossRef]
- Perring, T.M.; Stansly, P.A.; Liu, T.X.; Smith, H.A.; Andreason, S.A. Whiteflies: Biology, ecology, and management. In Sustainable Management of Arthropod Pests of Tomato; Academic Press: Cambridge, MA, USA, 2018; pp. 73–110. [Google Scholar] [CrossRef]
- Byrne, D.N.; Bellows, T.S., Jr. Whitefly Biology. Annu. Rev. Entomol. 1991, 36, 431–457. [Google Scholar] [CrossRef]
- Carapia-Ruiz, V.E. Taxonomía y diagnosis. In Moscas Blancas: Temas Selectos sobre su Manejo; Mundi Prensa México: Mexico City, Mexico, 2008; pp. 7–18. [Google Scholar]
- Laurenção, A.; Alves, A.; Fugi, C.; Matos, E.S. Outbreaks of Trialeurodes vaporariorum (West.) (Hemiptera: Aleyrodidae) under field conditions in the state of São Paulo, Brazil. Neotrop. Entomol. 2008, 37, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Anis, S.B.; Farooqi, M.K.; Rehmat, T.; Fatma, J. Aphelinidae parasitoids (Hymenoptera; Aphelinidae) of whiteflies (Homoptera: Aleyrodidae) from India. Biol. Med. 2011, 3, 222–231. [Google Scholar]
- Trialeurodes vaporariorum in Invasive Species Compendium. Available online: www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.54660 (accessed on 13 April 2022).
- Perea, E.I.; Rojas, E.; Villalobos, A. Diagnóstico de Trialeurodes vaporariorum (Homoptera: Aleyrodidae) en tabaco y fríjol de García Rovira (Santander). Rev. Colomb. Entomol. 2003, 29, 7–10. [Google Scholar] [CrossRef]
- Estay, P. Control biológico de las plagas claves del tomate. Mosquita blanca de los invernaderos. Rev. Tierra Adentro. 2007, 76, 36–39. [Google Scholar]
- Escalona, V.; Alvarado, P.; Monardes, H.; Urbina, C.; Martin, A. Manual de Cultivo de Tomate (Lycopersicon esculentum Mill.); Nodo Hortícola VI Región; Facultad de Ciencias Agronómicas, Universidad de Chile e Innova Chile Corfo: Santiago, Chile, 2009. [Google Scholar]
- Capinera, J.L. Greenhouse whitefly, Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae). In Encyclopedia of Entomology, 2nd ed.; Capinera, J.L., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2008; Volume 4346, pp. 1835–1840. [Google Scholar]
- Zachrisson, B.; Poveda, J. Las moscas blancas de Panamá. In Las Moscas Blancas (Homoptera: Aleyrodidae) en América Central y el Caribe; Serie Técnica. Informe Técnico; Hilje, L., Arboleda, O., Eds.; CATIE: Turrialba, Costa Rica, 1993; pp. 64–66. [Google Scholar]
- Morillo, F.E.; Marcano, R. Estudio del desarrollo de la mosca blanca en diferentes genotipos de tomate. Agron. Trop. 1997, 47, 271–286. [Google Scholar]
- Kumari, A.; Kaushik, N. Oviposition deterrents in herbivorous insects and their potential use in integrated pest management. Indian J. Exp.-Biol. 2016, 54, 163–174. [Google Scholar]
- Tomar, S.; Sharma, S.; Malik, K. Life parameters of whitefly (Bemisia tabaci, Genn.) on different host plants. Ind. J. Sc. Res. 2017, 16, 34–37. [Google Scholar]
- Ortega, L.D. Bioecología de las moscas blancas. In Moscas Blancas: Temas Selectos sobre su Manejo; Ortega, L., Ed.; Colegio Postgraduados—Mundi Prensa: México City, Mexico, 2008; pp. 1–6. [Google Scholar]
- Sood, A.K.; Sood, S.; Devi, A. Morphometrics and annual life cycle of greenhouse whitefly Trialeurodes vaporariorum (Westwood)] in Himachal pradesh. Him. J. Agric. Res. 2014, 40, 50–57. [Google Scholar]
- Escaff, M.; Gil, P.; Ferreyra, R.; Estay, P.; Bruna, A.; Maldonado, P.; Barrera, C. Cultivo del Tomate bajo Invernadero [en Línea]; Boletín INIA—Instituto de Investigaciones Agropecuarias: Quillota, Chile, 2005; Volume 128, Available online: https://hdl.handle.net/20.500.14001/7051 (accessed on 10 October 2022).
- Leskey, T.C.; Short, B.D.; Emery, M.; Evans, B.; Janisiewicz, W.; Takeda, F. Effect of UV-C irradiation on greenhouse whitefly, Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Fla. Entomol. 2021, 104, 148–150. [Google Scholar] [CrossRef]
- Lemos, J.P.; Sousa, É.A. The effects of sooty mold on photosynthesis and mesophyll structure of mahogany (Swietenia macrophylla King., Meliaceae). Bragantia 2006, 65, 11–17. [Google Scholar] [CrossRef]
- Duffus, J.E.; Liu, H.Y.; Wisler, G.C. Tomato infectious chlorosis virus—A new clostero-like virus transmitted by Trialeurodes vaporariorum. Eur. J. Plant Pathol. 1996, 102, 219–226. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; López-Moya, J.J.; Aranda, M.A. Whitefly-transmitted RNA viruses that affect intensive vegetable production. Ann. App. Biol. 2014, 165, 155–171. [Google Scholar] [CrossRef]
- Walia, Y.; Dhir, S.; Zaidi, A.A.; Hallan, V. Apple scar skin viroid naked RNA is actively transmitted by the whitefly Trialeurodes vaporariorum. RNA Biol. 2015, 12, 1131–1138. [Google Scholar] [CrossRef]
- Pym, A.; Singh, K.S.; Nordgren, Å.; Davies, T.G.; Zimmer, C.T.; Elias, J.; Bass, C. Host plant adaptation in the polyphagous whitefly, Trialeurodes vaporariorum, is associated with transcriptional plasticity and altered sensitivity to insecticides. BMC Genom. 2019, 20, 996. [Google Scholar] [CrossRef]
- Roditakis, N.E. Host plants of greenhouse whitefly Trialeurodes vaporariorum Westwood (Homoptera: Aleyrodidae) in Crete. Attractiveness and impact on whitefly life stages. Agric. Ecosyst. Environ. 1990, 31, 217–224. [Google Scholar] [CrossRef]
- Mound, L.; Halsey, S.H. Whitefly of the World. A Systematic Catalogue of the Aleyrodidae (Homoptera) with Host Plant and Natural Enemy Data; John Wiley and Sons: Hoboken, NJ, USA, 1977; Volume 10, pp. 1–340. [Google Scholar] [CrossRef]
- Ovčarenko, I.; Lindström, L.; Saikkonen, K.; Jauhiainen, L.; Kaseva, J.; Vanninen, I. Preconditioning of the generalist herbivore Trialeurodes vaporariorum to greenhouse monocultures and its subsequent performance on wild polycultures. Entomol. Exp. App. 2016, 159, 1–16. [Google Scholar] [CrossRef]
- Mainali, B.P.; Lim, U.T. Use of flower model trap to reduce the infestation of greenhouse whitefly on tomato. J. Asia Pac. Entomol. 2008, 11, 65–68. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Noldus, L.P. Whitefly plant relationship: Behavioral and ecological aspects. In Whiteflies: Their Bionomics, Pest Status and Management; Gerling, D., Ed.; Intercept, Andover: Hants, UK, 1990; pp. 47–89. [Google Scholar]
- Singer, M.C. Reducing ambiguity in describing plant–insect interactions: “preference”, “acceptability” and “electivity”. Ecol. Lett. 2000, 3, 159–162. [Google Scholar] [CrossRef]
- Sampson, C.; Covaci, A.D.; Hamilton, J.G.C.; Hassan, N.; Al-Zaidi, S.; Kirk, W.D.J. Reduced translucency and the addition of black patterns increase the catch of the greenhouse whitefly, Trialeurodes vaporariorum, on yellow sticky traps. PLoS ONE 2018, 13, e0193064. [Google Scholar] [CrossRef] [PubMed]
- Moreau, T.L.; Isman, M. Trapping whiteflies? A comparison of greenhouse whitefly (Trialeurodes vaporariorum) responses to trap crops and yellow sticky traps. Pest Manag. Sci. 2011, 67, 408–413. [Google Scholar] [CrossRef]
- Butler, G.D.; Henneberry, T.J. Bemisia tabaci: Effect of cotton leaf pubescence on abundance. Southwest Entomol. 1984, 9, 91–94. [Google Scholar]
- Avery, P.B.; Kumar, V.; Simmonds, M.S.J.; Faull, J. Influence of leaf trichome type and density on the host plant selection by the greenhouse whitefly, Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). App. Entomol. Zool. 2014, 50, 79–87. [Google Scholar] [CrossRef]
- Bergau, N.; Bennewitz, S.; Syrowatka, F.; Hause, G.; Tissier, A. The development of type VI glandular trichomes in the cultivated tomato Solanum lycopersicum and a related wild species S. habrochaites. BMC Plant Biol. 2015, 15, 289. [Google Scholar] [CrossRef]
- Mymko, D.; Avila-Sakar, G. The influence of leaf ontogenic stage and plant reproductive phenology on trichome density and constitutive resistance in six tomato varieties. Arthropod Plant Interact. 2019, 13, 797–803. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. A greenhouse without pesticides: Fact or fantasy? Crop Prot. 2000, 19, 375–384. [Google Scholar] [CrossRef]
- Bale, J.S.; van Lenteren, J.C.; Bigler, F. Biological control and sustainable food production. Phil. Trans. R. Soc. 2008, 363, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.J.; Oletting, R.D.; Bethke, J.A.; Green, C.; Chamberlin, J. Understanding the dynamics of neonicotinoid activity in the management of Bemisia tabaci whiteflies on poinsettias. Crop Prot. 2010, 29, 260–266. [Google Scholar] [CrossRef]
- Zang, L.S.; Liu, T.X. Host-feeding of three parasitoid species on Bemisia tabaci biotype B and implications for whitefly biological control. Entomol. Exp. App. 2008, 127, 55–63. [Google Scholar] [CrossRef]
- Tasli, M.; Yoldas, Z.; Öztekin, G.B.; Tüzel, Y. Effects of some repellent plants on greenhouse whitefly Trialeurodes vaporariorum (Westw.) in greenhouse tomato production. Acta Hortic. 2017, 1164, 407–412. [Google Scholar] [CrossRef]
- Espinoza, N. Malezas Presentes en Chile; Monografías INIA: Temuco, Chile, 1996; p. 483. [Google Scholar]
- Manual de Reconocimiento y Manejo de Malezas. 2012. Available online: http://cropscience.bayer.com.ar/upload/PDF/Manejointegradodemalezas.pdf (accessed on 3 October 2022).
- Espinoza, N. Guía Visual para Identificar Malezas en Terreno, 1st ed.; Trama Impresores: Temuco, Chile, 2017; 266p. [Google Scholar]
- Caballero, R. Clave de campo para inmaduros de mosca blanca de Centroamérica (Homoptera: Aleyrodidae). CEIBA 1994, 35, 45–51. [Google Scholar]
- Carapia-Ruiz, V.E.; Castillo-Gutiérrez, A. Morphological comparision between Trialeurodes vaporariorum (Westwood) and Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Acta Zool. Mex. 2013, 29, 178–193. [Google Scholar] [CrossRef]
- Huang, T.I.; Reed, D.A.; Perring, T.M.; Palumbo, J.C. Host selection behavior of Bagrada hilaris (Hemiptera: Pentatomidae) on commercial cruciferous host plants. Crop Prot. 2014, 59, 7–13. [Google Scholar] [CrossRef]
- Gotelli, N.J.A. Primer of Ecology, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Archivo Entomológico SAG. Available online: https://microimagenes.sag.gob.cl/default.asp?idgal=265 (accessed on 12 January 2022).
- Torres, P.A. (Ed.) Manual de Cultivo del Tomate Bajo Invernadero; Boletín INIA—Instituto de Investigaciones Agropecuarias: La Cruz, Chile, 2017; Volume 377. [Google Scholar]
- Vargas, R.; Alvear, A. Determinación de la susceptibilidad en tres poblaciones de Trialeurodes vaporariorum Wetswood (Hemiptera: Aleyrodidae) a metilo y buprofezin. Agric. Tec. 2000, 60, 341–349. [Google Scholar]
- Nasruddin, A.; Jumardi, J.; Melina, M. Population dynamics of Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) and its populations on different planting dates and host plant species. Ann. Agric. Sci. 2021, 66, 109–114. [Google Scholar] [CrossRef]
- Kapantaidaki, D.E.; Sadikoglou, E.; Tsakireli, D.; Kampanis, V.; Stavrakaki, M.; Schorn, C.; Ilias, A. Insecticide resistance in Trialeurodes vaporariorum populations and novel diagnostics for Kdr mutations. Pest Manag. Sci. 2018, 74, 59–69. [Google Scholar] [CrossRef]
- Fu, B.; Li, Q.; Qiu, H.; Tang, L.; Zeng, D.; Liu, K.; Gao, Y. Oviposition, feeding preference, and biological performance of Thrips hawaiiensis of four host plants with and without supplemental foods. Arthr. Plant Interact. 2018, 13, 441–452. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
- Lorenzo, M.E.; Grille, G.; Basso, C.; Bonato, O. Host preferences and biotic potential of Trialeurodes vaporariorum and Bemisia tabaci (Hemiptera: Aleyrodidae) in tomato and pepper. Arthr. Plant Interact. 2016, 10, 293–301. [Google Scholar] [CrossRef]
- Jaenike, J. On optimal oviposition behaviour in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.N. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. App. 1988, 47, 3–14. [Google Scholar] [CrossRef]
- López, S.N.; Viscarret, M.M.; Botto, E.N. Selección de la planta hospedera y ciclo de desarrollo de Trialeurodes vaporariorum (Westwood) (Homoptera: Aleyrodidae) sobre zapallito (Cucurbita maxima Duch.; Cucurbitales: Cucurbitacea) y tomate (Lycopersicum esculentum Mill.; Tubiflorales: Solanacea). Bol. Sanid. Veg. Plagas 1999, 25, 21–29. [Google Scholar]
- Lima, L.C.; Campos, A.R. Fatores que afetam a oviposição de Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae) em pimentão. Neotrop. Entomol. 2008, 37, 180–184. [Google Scholar] [CrossRef]
- Hewa, L.C.; Darshanee, H.L.C.; Ren, H.; Ahmed, N.; Zhang, Z.F.; Liu, Y.H.; Liu, T.X. Volatile-mediated attraction of greenhouse whitefly Trialeurodes vaporariorum to tomato and eggplant. Front. Plant Sci. 2017, 8, 1285. [Google Scholar]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Tsueda, H.; Tsuduki, T.; Tsuchida, K. Factors that affect the selection of tomato leaflets by two whiteflies, Trialeurodes vaporariorum and Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Entomol. Zool. 2014, 49, 561–570. [Google Scholar] [CrossRef]
- Finlay-Doney, M.; Walter, G.H. The conceptual and practical implications of interpreting diet breadth mechanistically in generalist predatory insects. Biol. J. Linnean Soc. 2012, 107, 737–763. [Google Scholar] [CrossRef]
- Arimura, G.; Shiojiri, K.; Karban, R. Acquired immunity to herbivory and allelopathy caused by airborne plant emissions. Phytochemistry 2010, 71, 1642–1649. [Google Scholar] [CrossRef]
- González-Klenner, F.; Albornoz, M.V.; Ávila Sakar, G.; Verdugo, J. Tomato defense against whiteflies under drought stress: Non-additive effects and cultivar-specific responses. Plants 2022, 11, 1049. [Google Scholar] [CrossRef]
- Ramachandran, S.; Renault, S.; Markham, J.; Verdugo, J.; Albornoz, M.V.; Avila-Sakar, G. Lower nitrogen availability enhances resistance to whiteflies in tomato. Plants 2020, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.M.H.; Lee, G.S.; Lee, S.; Lee, K.Y. Acquisition of tomato yellow leaf curl virus enhances attraction of Bemisia tabaci to green light emitting diodes. J. Asia Pac. Entomol. 2014, 17, 79–82. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schütz, S.; de Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef]
- Togni, P.H.B.; Laumann, R.A.; Medeiros, M.A.; Sujii, E.R. Odour masking of tomato volatiles by coriander volatiles in host plant selection of Bemisia tabaci biotype B. Entomol. Exp. Appl. 2010, 136, 164–173. [Google Scholar] [CrossRef]
- Nomikou, M.; Janssen, A.; Sabelis, M.W. Herbivore host plant selection: Whitefly learns to avoid host plants that harbour predators of her offspring. Oecologia 2003, 136, 484–488. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).