Use of Liquid Culture with the ElecTIS Bioreactor for Faster Recovery of Blackberry (Rubus fruticosus L.) Shoots from Conservation at 4 °C
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions before the Transfer to ElecTIS Bioreactor
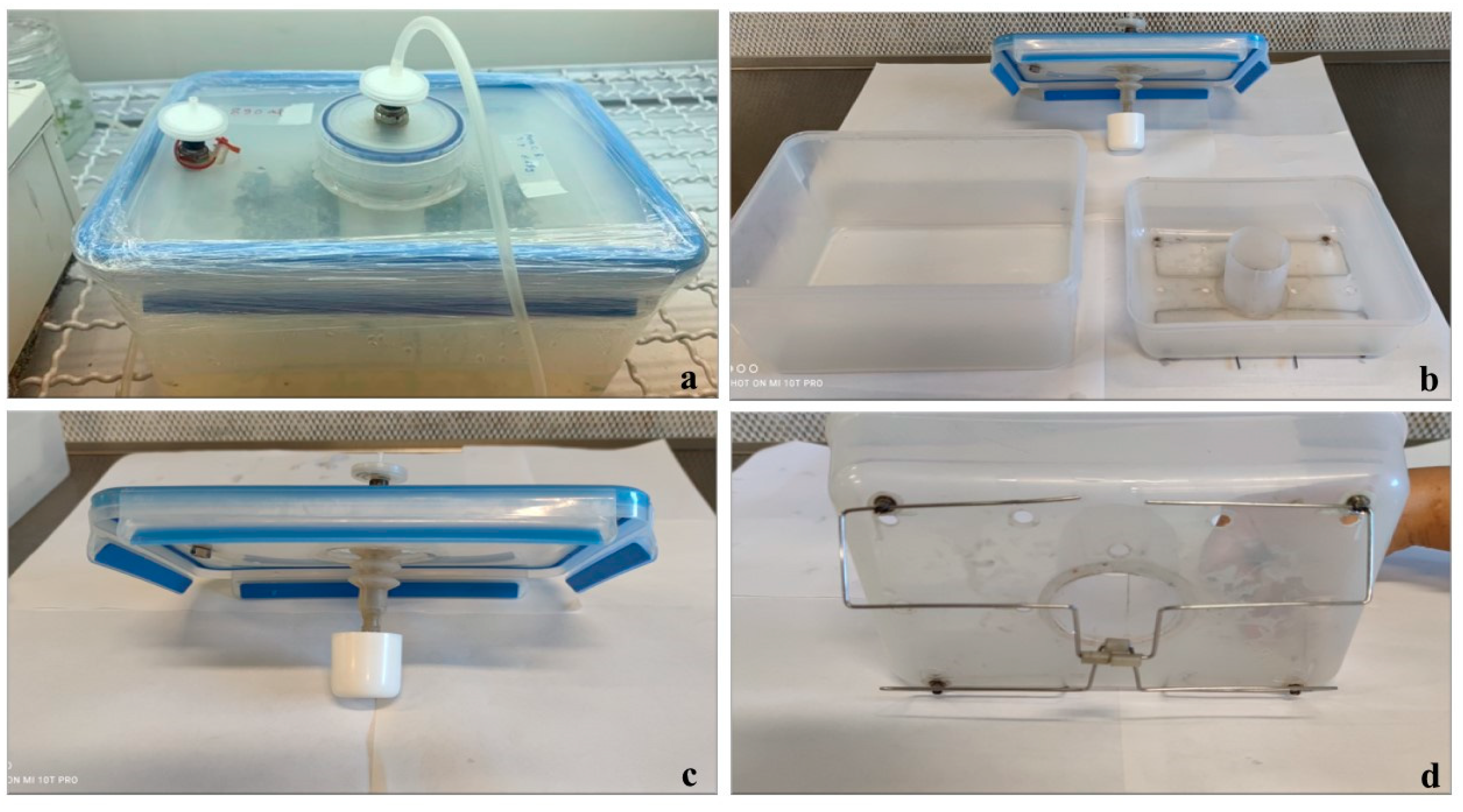
2.2. Description of the ElecTIS Bioreactor
2.3. Shoot Multiplication in Post-Conservation with ElecTIS Bioreactors and Gelled Medium
2.4. Rooting and Shooting of Clusters in ElecTIS Bioreactors and Gelled Medium and Transfer to Acclimatization
2.5. Data Collection
- Growth parameters
- Water content
- Relative Growth Rate
- Photosynthetic pigments
- Stomatal study
- Rooting and shoot development in clusters
2.6. Statistical Analysis
3. Results
3.1. Effect of Shoot Culture in ElecTIS and in Gelled Medium Systems on Vegetative Growth Parameters
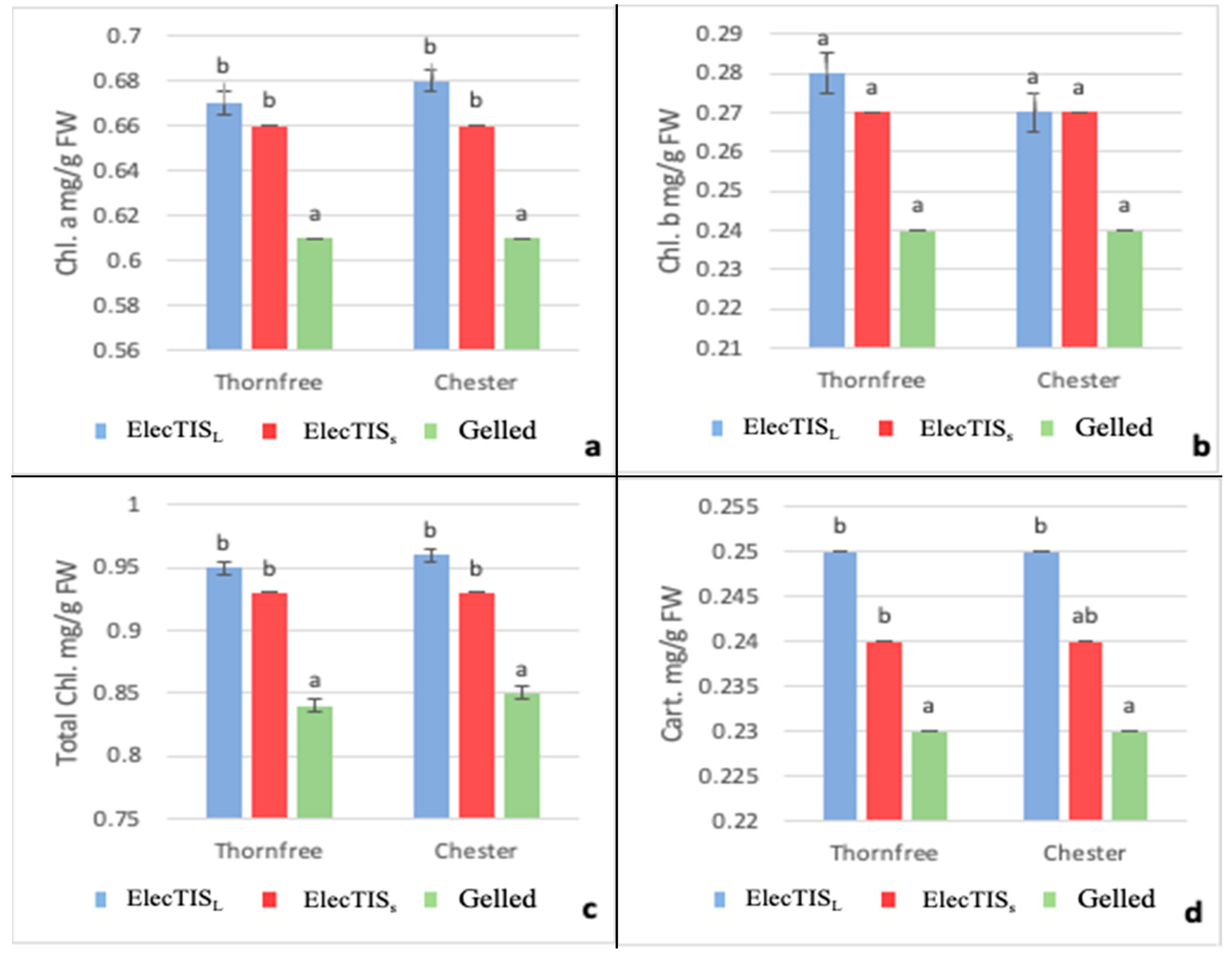
3.2. Effect of the Different Culture Systems on Chlorophyll Content
3.3. Effect of the Culture in the ElecTIS Bioreactors on Plant Rooting and Shooting before Acclimatization
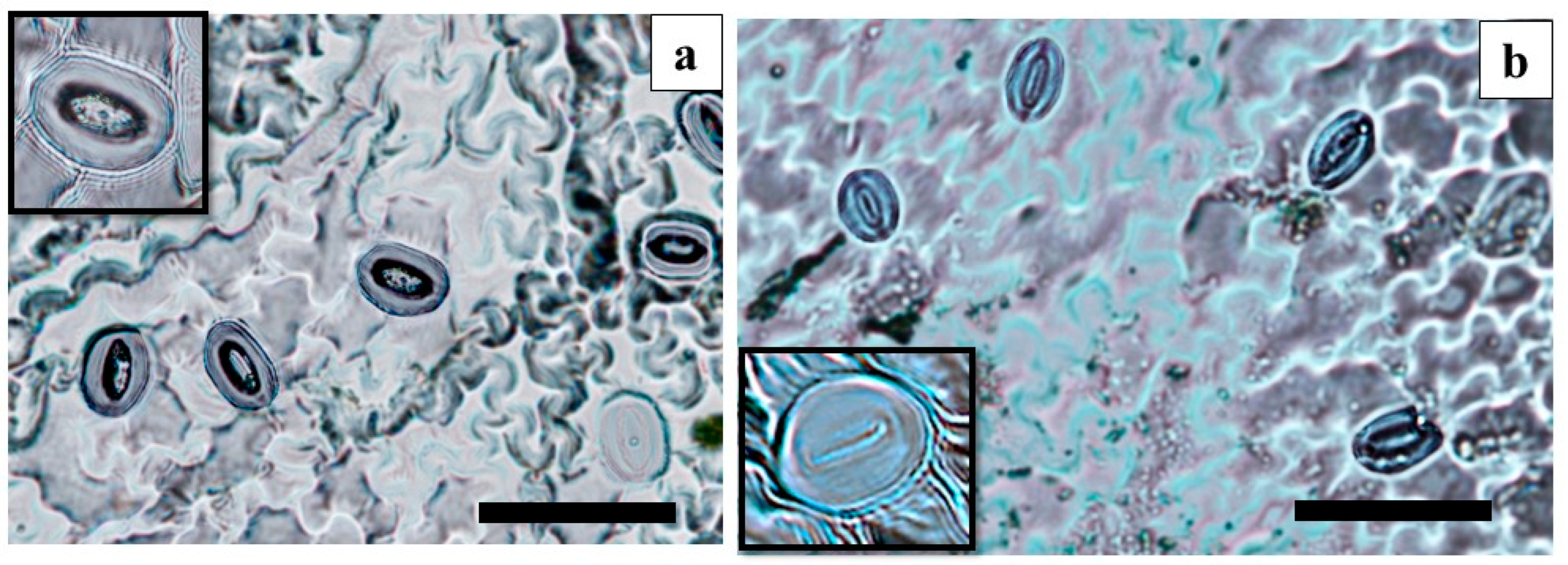
3.4. Analysis of Stomata in Leaves from ElecTIS Bioreactors and Gelled Medium
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Paoli, G.; Masini, M. Il laboratorio di micropropagazione commerciale: Organizzazione e tecnica per contenere i costi di produzione. Italus Hortus 2009, 16, 84–88, (with English abstract). [Google Scholar]
- Ziv, M. Simple bioreactors for mass propagation of plants. Plant Cell Tiss. Org. Cult. 2005, 81, 277–285. [Google Scholar] [CrossRef]
- Mehrotra, S.; Goel, M.K.; Kukreja, A.K.; Mishra, B.N. Efficiency of liquid culture systems over conventional micropropagation: A progress towards commercialization. Afr. J. Biotech. 2007, 6, 1484–1492. [Google Scholar]
- Carvalho, L.; Ozudogru, E.A.; Lambardi, M.; Paiva, L. Temporary immersion system for micropropagation of tree species: A bibliographic and systematic review. Not. Bot. Horti Agr. 2019, 47, 269–277. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tiss. Org. Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Ramirez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks. Vitr. Cell. Dev. Biol.-Plant 2016, 52, 154–160. [Google Scholar] [CrossRef]
- Lambardi, M. Micropropagazione in coltura liquida con sistema ad immersione temporanea. Riv. Fruttic. E Ortofloric. 2012, 12, 32–38, (with English abstract). [Google Scholar]
- Capuana, M.; Depaoli, C.; Ozudogru, E.A.; Lambardi, M. Una nuova proposta per la coltura liquida in immersione temporanea: Il bioreattore ‘ElecTIS’. Acta Italus Hortus 2018, 21, 98–100, (with English abstract). [Google Scholar]
- Takayama, S.; Akita, M. The types of bioreactors used for shoots and embryos. Plant Cell Tiss. Org. Cult. 1994, 39, 147–156. [Google Scholar] [CrossRef]
- Sota, V.; Benelli, C.; Çuko, B.; Papakosta, E.; Depaoli, C.; Lambardi, M.; Kongjika, E. Evaluation of ElecTIS bioreactor for the micropropagation of Malus sylvestris (L.) Mill., an important autochthonous species of Albania. Hortic. Sci. 2021, 48, 12–21. [Google Scholar] [CrossRef]
- Lambardi, M.; Ozudogru, E.A. Advances in the safe storage of micropropagated woody plants at low temperature. Acta Hortic. 2013, 988, 29–42. [Google Scholar] [CrossRef]
- Lambardi, M.; De Carlo, A. Application of tissue culture to the germplasm conservation of temperate broad-leaf trees. In Micropropagation of Woody Trees and Fruits; Jain, S.M., Ishii, K., Eds.; Kluwer Ac. Pub.: Dordrecht, The Netherlands, 2007; pp. 815–840. [Google Scholar]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox Walnut Rootstock. Hortscience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Murashige, G.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Radoglou, K.M.; Jarvis, P.G. Effects of CO2 enrichment on four poplar clones. II. Leaf surface properties. Ann. Bot. 1990, 65, 627–632. [Google Scholar] [CrossRef]
- Malone, S.R.; Mayeux, H.S.; Johnson, H.B.; Polley, H.W. Stomatal density and aperture length in four plant species grown across a subambient CO2 gradient. Am. J. Bot. 1993, 80, 1413–1418. [Google Scholar] [CrossRef]
- Maherali, H.; Reid, C.D.; Polley, H.W.; Johnson, H.B.; Jackson, R.B. Stomatal acclimation over a subambient to elevated CO2 gradient in a C3/C4 grassland. Plant Cell Environ. 2002, 25, 557–566. [Google Scholar] [CrossRef]
- SPSS. SPSS Base 16.0 User’s Guide; SPSS Inc.: Chicago, IL, USA, 2007; p. 551. [Google Scholar]
- Bello-Bello, J.J.; Cruz-Cruz, C.A.; Pérez-Guerra, J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). Vitr. Cell Dev. Biol.-Plant 2019, 55, 313–320. [Google Scholar] [CrossRef]
- Nasri, A.; Baklouti, E.; Romdhane, A.B.; Maalej, M.; Schumacher, H.M.; Drira, N.; Fki, L. Large-scale propagation of Myrobolan (Prunus cerasifera) in RITA® bioreactors and ISSR-based assessment of genetic conformity. Sci. Hortic. 2019, 245, 144–153. [Google Scholar] [CrossRef]
- Mancilla-Álvarez, E.; Pérez-Sato, J.A.; Núñez-Pastrana, R.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Comparison of Different Semi-Automated Bioreactors for In Vitro Propagation of Taro (Colocasia esculenta L. Schott). Plants 2021, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Arano-Avalos, S.; Gómez-Merino, F.C.; Mancilla-Álvarez, E.; Sánchez-Páez, R.; Bello-Bello, J.J. An efficient protocol for commercial micropropagation of malanga (Colocasia esculenta L. Schott) using temporary immersion. Sci. Hortic. 2020, 261, 108998. [Google Scholar] [CrossRef]
- Rosales, C.; Rica, I.T.D.C.; Brenes, J.; Salas, K.; Arce-Solano, S.; Abdelnour-Esquivel, A. Micropropagation of Stevia rebaudiana in temporary immersion systems as an alternative horticultural production method. Rev. Chapingo Ser. Hortic. 2018, 24, 69–84. [Google Scholar] [CrossRef]
- Da Silva, J.A.; Solis-Gracia, N.; Jifon, J.; Souza, S.C.; Mandadi, K.K. Use of bioreactors for large-scale multiplication of sugarcane (Saccharum spp.), energy cane (Saccharum spp.), and related species. Vitr. Cell Dev. Biol.-Plant 2020, 56, 366–376. [Google Scholar] [CrossRef]
- Gayon, J. History of the concept of allometry. Am Zool. 2000, 40, 748–758. [Google Scholar] [CrossRef]
- Martínez-Estrada, E.; Islas-Luna, B.; Pérez-Sato, J.A.; Bello-Bello, J.J. Temporary immersion improves in vitro multiplication and acclimatization of Anthurium andreanum Lind. Sci. Hortic. 2019, 249, 185–191. [Google Scholar] [CrossRef]
- Jova, M.C.; Kosky, R.G.; Cuellar, E.E. Effect of liquid media culture systems on yam plant growth (Dioscorea alata L. ‘Pacala Duclos’). Base 2011, 15, 515–521. [Google Scholar]
- Aragón, C.E.; Escalona, M.; Capote, I.; Pina, D.; Cejas, I.; Rodríguez, R.; Cañal, M.J.; Sandoval, J.; Roels, S.; De Bergh, P.; et al. Photosynthesis and carbon metabolism in plantain (Musa AAB) plantlets growing in temporary immersion bioreactors and during ex vitro acclimatization. Vitr. Cell Dev. Biol. Plant 2005, 41, 550–554. [Google Scholar] [CrossRef]
- Hwang, H.D.; Kwon, S.H.; Murthy, H.N.; Yun, S.W.; Pyo, S.S.; Park, S.Y. Temporary immersion bioreactor system as an efficient method for mass production of in vitro plants in horticulture and medicinal plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- Fan, L.M.; Zhao, Z.; Assmann, S.M. Guard cells: A dynamic signaling model. Curr. Opin. Plant Biol. 2004, 7, 537–546. [Google Scholar] [CrossRef]
- Büssis, D.; Von Groll, U.; Fisahn, J.; Altmann, T. Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Funct. Plant Biol. 2006, 33, 1037–1043. [Google Scholar] [CrossRef]
- Cochard, H.; Coll, L.; Le Roux, X.; Améglio, T. Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol. 2002, 128, 282–290. [Google Scholar] [CrossRef]
- Hazarika, B. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.H.; Shabala, S. Hypoxia sensing in plants: On a quest for ion channels as putative oxygen sensors. Plant Cell Physiol. 2017, 58, 1126–1142. [Google Scholar] [CrossRef]
- Schulze, E.D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Water Deficiency (Drought). In Plant Ecology; Schulze, E.-D., Beck, E., Buchmann, N., Clemens, S., Müller-Hohenstein, K., Scherer-Lorenzen, M., Eds.; Springer: Berlin, Germany, 2019; pp. 165–202. [Google Scholar]
- Aragón, C.E.; Sánchez, C.; Gonzalez-Olmedo, J.; Escalona, M.; Carvalho, L.; Amâncio, S. Comparison of plantain plantlets propagated in temporary immersion bioreactors and gelled medium during in vitro growth and acclimatization. Biol. Plant 2014, 58, 29–38. [Google Scholar] [CrossRef]
- Ahmadian, M.; Babaei, A.; Shokri, S.; Hessami, S. Micropropagation of carnation (Dianthus caryophyllus L.) in liquid medium by temporary immersion bioreactor in comparison with solid culture. J. Genet. Eng. Biotechnol. 2017, 15, 309–315. [Google Scholar] [CrossRef]
- Monja-Mio, K.M.; Olvera-Casanova, D.; Herrera-Herrera, G.; Herrera-Alamillo, M.Á.; Sánchez-Teyer, F.L.; Robert, M.L. Improving of rooting and ex vitro acclimatization phase of Agave tequilana by temporary immersion system (BioMINTTM). Vitr. Cell. Dev. Biol. Plant 2020, 56, 662–669. [Google Scholar] [CrossRef]
- Escalona, M.; Lorenzo, J.C.; González, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Tisserat, B.; Vandercook, C.E. Development of an automated plant culture system. Plant Cell Tiss. Org. Cult. 1985, 5, 107–117. [Google Scholar] [CrossRef]


| Cultivar/ Culture System | Growth Parameter | ||||
|---|---|---|---|---|---|
| Shoot Length (Average, cm) | Shoots/Cluster (Average, n°) | FW (Avg, g) | DW (Avg, g) | Water Content (%) | |
| Thornfree | |||||
| ElecTISL | 3.1 a | 31.4 a | 1.99 ± 0.2 | 0.17 ± 0.1 | 91.14 ± 4.1 |
| ElecTISS | 3.4 a | 31.5 a | 2.10 ± 0.2 | 0.16 ± 0.1 | 92.06 ± 5.0 |
| Gelled medium | 1.0 b | 6.6 b | 0.65 ± 0.1 | 0.06 ± 0.0 | 89.52 ± 4.6 |
| Chester | |||||
| ElecTISL | 3.7 a | 30.4 a | 1.99 ± 0.2 | 0.18 ± 0.0 | 90.96 ± 3.9 |
| ElecTISS | 3.4 a | 30.2 a | 2.27 ± 0.4 | 0.21 ± 0.1 | 90.39 ± 4.1 |
| Gelled medium | 0.9 b | 6.4 b | 0.64 ± 0.4 | 0.08 ± 0.0 | 86.61 ± 6.2 |
| Cultivar Subculture Culture System | Growth Parameter | ||
|---|---|---|---|
| Initial/Final Weight (g) | Increment of PM | RGR | |
| Thornfree | |||
| 1st subculture | |||
| ElecTISL | 4.6/72.9 | 15.8 a | 9.2 |
| ElecTISS | 2.7/57.3 | 21.2 a | 10.2 |
| Gelled medium | 1.2/12.2 | 10.2 b | 7.9 |
| 2nd subculture | |||
| ElecTISL | 5.1/75.4 | 14.8 a | 9.0 |
| ElecTISS | 5.3/55.2 | 10.4 a | 7.8 |
| Gelled medium | 2.3/11.4 | 5.0 b | 5.4 |
| 3rd subculture | |||
| ElecTISL | 5.1/71.6 | 14.0 a | 9.4 |
| ElecTISS | 4.3/54.2 | 12.6 ab | 9.1 |
| Gelled medium | 1.8/11.1 | 10.1 b | 6.5 |
| Average | |||
| ElecTISL | --- | 13.2 a | 8.7 |
| ElecTISS | --- | 14.7 a | 9.0 |
| Gelled medium | --- | 8.4 b | 6.6 |
| Chester | |||
| 1st subculture | |||
| ElecTISL | 6.7/88.6 | 13.2 a | 8.6 |
| ElecTISS | 4.5/51.0 | 11.3 ab | 8.1 |
| Gelled medium | 0.6/5.5 | 9.1 b | 7.4 |
| 2nd subculture | |||
| ElecTISL | 4.4/72.9 | 16.6 a | 9.3 |
| ElecTISS | 5.0/52.4 | 10.5 a | 7.8 |
| Gelled medium | 1.1/5.6 | 5.1 b | 5.5 |
| 3rd subculture | |||
| ElecTISL | 8.4/84.2 | 10.0 a | 8.2 |
| ElecTISS | 6.6/50.5 | 7.7 ab | 7.3 |
| Gelled medium | 1.0/5.5 | 5.5 b | 6.2 |
| Average | |||
| ElecTISL | --- | 13.3 a | 8.7 |
| ElecTISS | --- | 9.8 b | 7.7 |
| Gelled medium | --- | 6.6 c | 6.4 |
| Cultivar Culture System | Rooting and Shooting Parameters | |||
|---|---|---|---|---|
| Average No. of Roots per Cluster | Average No. of Roots per Cluster ≥ 0.5 cm | Average No. of Shoots per Cluster | Average Shoot Length (cm) | |
| Thornfree | ||||
| ElecTISL | 2.6 a | 1.7 a | 4.1 a | 3.2 a |
| ElecTISS | 2.5 a | 1.5 a | 4.3 a | 3.1 a |
| Gelled medium | 2.1 b | 0.3 b | 1.6 b | 2.2 b |
| Chester | ||||
| ElecTISL | 6.0 a | 4.1 a | 6.2 a | 4.4 a |
| ElecTISS | 5.8 ab | 3.8 a | 6.4 a | 4.3 a |
| Gelled medium | 5.5 b | 1.6 b | 1.4 b | 2.9 b |
| Cultivar Culture System | Stomata | ||||
|---|---|---|---|---|---|
| Density (mm2) | Length (µm) | Width (µm) | Open (%) | Closed (%) | |
| cv Thornfree | |||||
| ElecTIS | 246.5 a | 16.1 ± 0.77 | 11.9 ± 0.44 | 26.1 a | 73.9 a |
| Gelled medium | 201.5 b | 23.5 ± 0.68 | 16.0 ± 0.76 | 44.4 b | 55.6 b |
| cv Chester | |||||
| ElecTIS | 187.6 a | 23.4 ± 0.74 | 15.2 ± 0.75 | 26.6 a | 73.4 a |
| Gelled medium | 174.4 a | 20.4 ± 0.79 | 14.6 ± 0.44 | 50.5 b | 49.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elazab, D.; Capuana, M.; Ozudogru, E.A.; Anichini, M.; Lambardi, M. Use of Liquid Culture with the ElecTIS Bioreactor for Faster Recovery of Blackberry (Rubus fruticosus L.) Shoots from Conservation at 4 °C. Horticulturae 2023, 9, 680. https://doi.org/10.3390/horticulturae9060680
Elazab D, Capuana M, Ozudogru EA, Anichini M, Lambardi M. Use of Liquid Culture with the ElecTIS Bioreactor for Faster Recovery of Blackberry (Rubus fruticosus L.) Shoots from Conservation at 4 °C. Horticulturae. 2023; 9(6):680. https://doi.org/10.3390/horticulturae9060680
Chicago/Turabian StyleElazab, Doaa, Maurizio Capuana, Elif Aylin Ozudogru, Monica Anichini, and Maurizio Lambardi. 2023. "Use of Liquid Culture with the ElecTIS Bioreactor for Faster Recovery of Blackberry (Rubus fruticosus L.) Shoots from Conservation at 4 °C" Horticulturae 9, no. 6: 680. https://doi.org/10.3390/horticulturae9060680
APA StyleElazab, D., Capuana, M., Ozudogru, E. A., Anichini, M., & Lambardi, M. (2023). Use of Liquid Culture with the ElecTIS Bioreactor for Faster Recovery of Blackberry (Rubus fruticosus L.) Shoots from Conservation at 4 °C. Horticulturae, 9(6), 680. https://doi.org/10.3390/horticulturae9060680






