Developing Paraphalaenopsis labukensis (Shim, A. Lamb & C.L. Chan), an Orchid Endemic to Sabah, Borneo, Asymbiotic Seed Germination and In Vitro Seedling Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Hand Pollination Technique
2.3. Capsule Formation and Development
2.4. Surface Sterilisation
2.5. Tetrazolium Viability Test (TZ)
2.6. Culture Initiation, Seed Germination on Different Basal Media
2.7. Protocorm Proliferation and Development
2.8. Statistical Analysis
3. Results
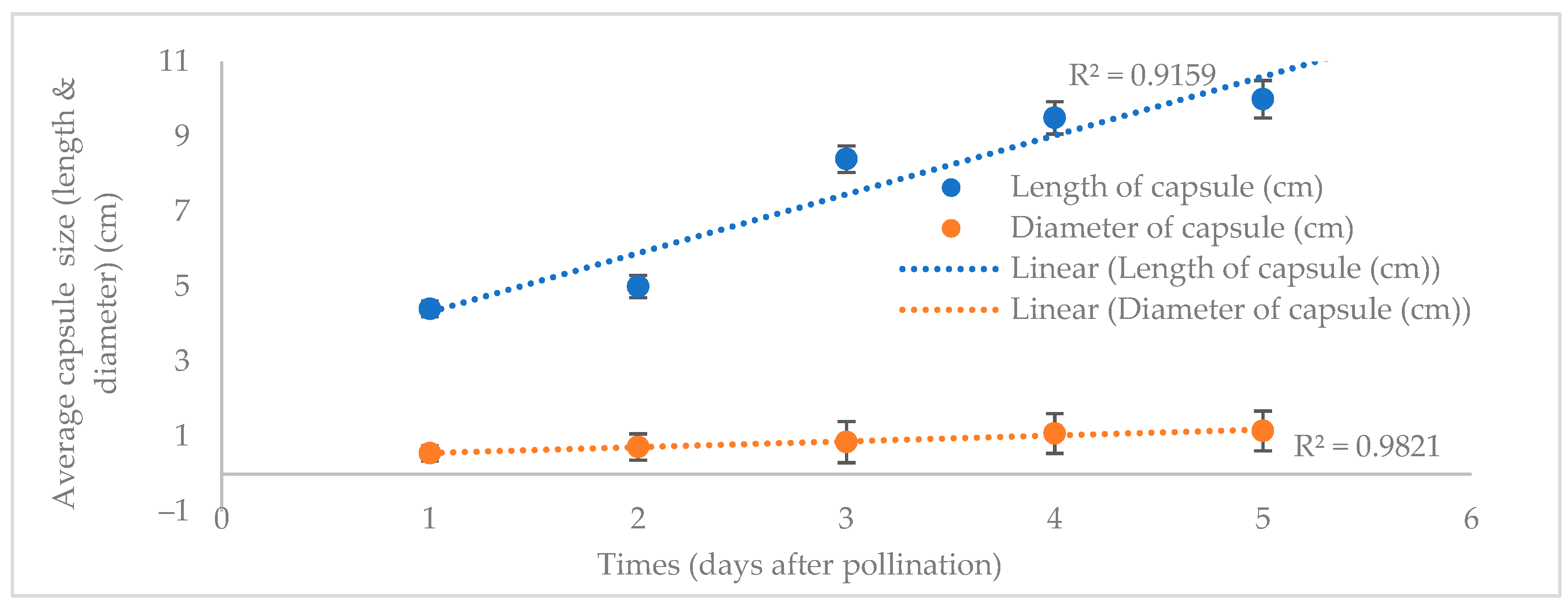
3.1. Morphology Indicator for Capsule Maturity
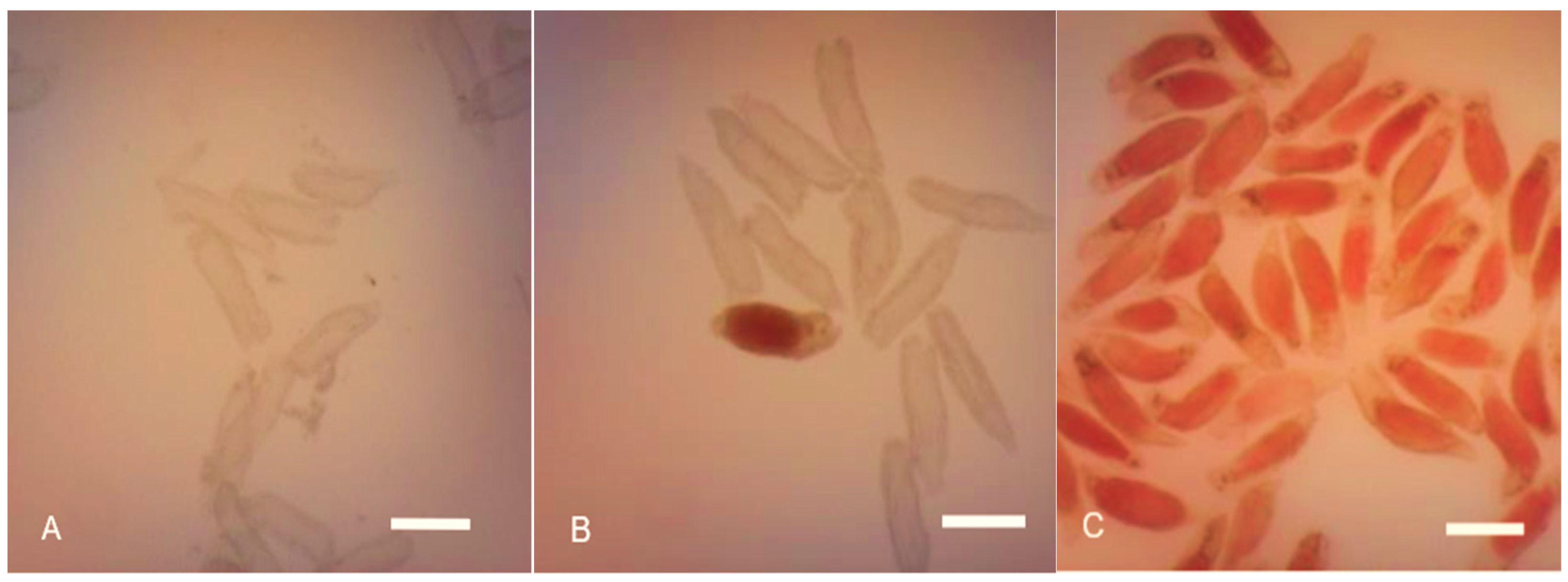
3.2. Seed Viability
3.3. The Effects of Basal Media on In Vitro Seed Germination
3.4. Observation of Protocorm Developmental Stages
3.5. The Effects of KC Basal Medium Supplemented with Organic Additive on Protocorm Development and Proliferation
3.6. The Effect of KC Basal Media Supplementd with Single PGRs on Protocorm Development and Proliferation under 16 h Photoperiod at 25 ± 2 °C after 150 Days of Culture
3.7. The Effects of KC Basal Medium Supplemented with Single PGRs on Seedling Formation
3.8. The Effects of KC Basal Medium Supplemented with PGRs Combinations on Seedling Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utami, E.S.W.; Hariyanto, S. In vitro seed germination and seedling development of a rare Indonesian native orchid Phalaenopsis amboinensis J.J. Sm. Scietifica 2019, 2019, 8105138. [Google Scholar]
- Juiling, S.; Leon, S.K.; Jumian, J.; Tsen, S.; Lee, Y.L.; Khoo, E.; Sugau, J.B.; Nilus, R.; Pereira, J.T.; Damit, A. Conservation assessment and spatial distribution of endemic orchids in Sabah. Borneo. Nat. Conserv. Res. 2020, 5, 136–144. [Google Scholar] [CrossRef]
- Wood, J.; Beaman, J.; Beaman, R. The Plants of Mount Kinabalu 2. Orchids; Royal Botanic Garden: Richmond, UK, 1993. [Google Scholar]
- Chan, C.L.; Lamb, A.; Shim, P.S.; Wood, J.J. Orchids of Borneo: Introduction and Selection of Species; Print and Co. Sdn Bhd: Kuala Lumpur, Malaysia, 1994; p. 402. [Google Scholar]
- Swarts, N.D.; Dixon, K.W. Perspectives on orchid conservation in botanic gardens. Trends Plant Sci. 2009, 14, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Lamb, A. The conservation of orchids in Sabah (Malaysian Borneo). In Tropical Botanic Gardens: Their Roles in Conservation and Development; Heywood, V.H., Wyse Jackson, P.S., Eds.; Academic Press: London, UK, 1991; pp. 308–327. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2020-I. 2020. Available online: https://www.iucnredlist.org/ (accessed on 3 March 2023).
- Basker, S.; Bai, V.N. In vitro propagation of an epiphytic and rare orchid Eria bambusifolia Lindl. Res. Biotechnol. 2010, 1, 15–20. [Google Scholar]
- Chen, Y.; Goodale, U.; Fan, X.; Gao, J. Asymbiotic seed germination and in vitro seedling development of Paphiopedilum spicerianum: An orchid with an extremely small population in China. Glob. Ecol. Conserv. 2015, 3, 367–378. [Google Scholar] [CrossRef]
- Johnson, T.R.; Kane, M.E. Asymbiotic germination of ornamental Vanda: In vitro germination and development of three hybrids. Plant Cell Tissue Organ Cult. 2007, 91, 251–261. [Google Scholar] [CrossRef]
- Kahraman, M.U.; Cullum, F.J. Asymbiotic germination and seedling development of terrestrial orchid Bletilla striata using in vitro and ex vitro Cultures. Hortic. Stud. 2010, 38, 1–14. [Google Scholar] [CrossRef]
- Ferreira, W.D.M.; Oliveira, A.M.D.; Viana, J.C.; Suzuki, R.M.; Oliveira, J.R.G.D. Asymbiotic germination, initial development in vitro and acclimatisation of Cyrtopodium paludicolum Hoehne, a Brazilian Savanna orchid species. Rodriguésia 2022, 73, 1–14. [Google Scholar] [CrossRef]
- Barrientos, B.A.B.; Fang, J.Y. Influence of photoperiod and culture medium on the speed of asymbiotic seed germination and seedling development in Spathoglottis plicata. HortScience 2019, 54, 1570–1575. [Google Scholar] [CrossRef]
- Yang, N.; Wang, D.; Gao, Y.; Hu, E.; Yu, X.; Peng, S.; Ji, J.; Zhang, M.S. An efficient micropropagation protocol, chemical components, and hypoglycemic activity for Cremastra appendiculata (D. Don) Makino pseudobulbs. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 213–224. [Google Scholar] [CrossRef]
- Yao, L.; Huang, J.; Zhang, S. An Improved Protocol for Asymbiotic seed germination and seedling development of Paphiopedilum tigrinum. Horticulturae 2021, 7, 298. [Google Scholar] [CrossRef]
- Dutra, D.; Johnson, T.R.; Kauth, P.J.; Stewart, S.L.; Kane, M.E.; Richardson, L. Asymbiotic seed germination, in vitro seedling development, and greenhouse acclimatisation of the threatened terrestrial orchid Bletia purpurea. Plant Cell Tissue Organ Cult. 2008, 94, 11–21. [Google Scholar] [CrossRef]
- Parthibhan, S.; Benjamin, J.F.; Muthukumar, M.; Ahamed, N.; Sherif, T.; Senthi Kumar, M.V.R. Influence of nutritional media and photoperiods on in vitro asymbiotic seed germination and seedling development of Dendrobium aqueum Lindley. Afr. J. Plant Sci. 2012, 6, 383–393. [Google Scholar] [CrossRef]
- Paul, S.; Kumaria, S.; Tandon, P. An effective nutrient medium for asymbiotic seed germination and large-scale in vitro regeneration of Dendrobium hookerianum, a threatened orchid of northeast India. AoB Plants 2012, 2012, plr032. [Google Scholar] [CrossRef]
- Jawan, R.; Gansau, J.A.; Abdullah, J.O. In vitro culture of Borneo’s endemic orchid, Vanda dearei. Asia-Pac. J. Mol. Biol. Biotechnol. 2010, 18, 203–207. [Google Scholar]
- Islam, M.; Akter, M.; Prodhan, A. Effect of potato extract on in vitro seed germination and seedling growth of local Vanda roxburgii orchid. J. Bangladesh Agric. Univ. 2012, 9, 211–215. [Google Scholar] [CrossRef]
- Dwiyani, R.; Yuswanti, H.; Darmawati, I.A.P.; Suada, K.; Mayadewi, N.N.A. In vitro germination and its subsequent growth of an orchid of Vanda tricolor Lindl. var. suavis from Bali on complex additives enriched medium. J. Agric. Sci. 2015, 37, 144–150. [Google Scholar] [CrossRef]
- Jadhav, S.G.; Chavan, D.A.; Waghmare, Y.M. Effect of plant spacing, growth regulator and nutrient management on yield, quality and economics of Bt cotton. J. Cotton Res. Dev. 2015, 29, 48–52. [Google Scholar]
- Bhattarai, P. Effects of plant growth regulators on growth and yield of pre-basic seed potato production under glasshouse condition. SAARC J. Agric. 2017, 15, 149–160. [Google Scholar] [CrossRef]
- Chin, C.K.; Lee, Z.H.; Mubbarakh, S.A.; Antony, J.J.J.; Chew, B.L.; Subramaniam, S. Effects of plant growth regulators and activated charcoal on somaclonal variations of protocorm-like bodies (PLBs) of Dendrobium Sabin Blue orchid. Biocatal. Agric. Biotechnol. 2019, 22, 101426. [Google Scholar] [CrossRef]
- Hossain, M.M. Asymbiotic seed germination and in vitro seedling development of Epidendrum ibaguense Kunth. (Orchidaceae). Afr. J. Biotechnol. 2008, 7, 3614–3619. Available online: http://www.academicjournals.org/AJB (accessed on 1 September 2019).
- Mahendran, G.; Muniappan, V.; Ashwini, M.; Muthukumar, T.; Narmatha Bai, V. Asymbiotic seed germination of Cymbidium bicolor Lindl. (Orchidaceae) and the influence of mycorrhizal fungus on seedling development. Acta Physiol. Plant 2013, 35, 829–840. [Google Scholar] [CrossRef]
- Roy, A.R.; Patel, R.S.; Patel, V.V.; Sajeev, S.; Deka, B.C. Asymbiotic seed germination, mass propagation and seedling development of Vanda coerulea Griff ex.Lindl. (Blue Vanda): An in vitro protocol for an endangered orchid. Sci. Hortic. 2011, 128, 325–331. [Google Scholar] [CrossRef]
- Gallo, F.; Souza, L.; Milaneze, M.; Almeida, O. Seed structure and in vitro seedling development of certain Laeliinae species (Orchidaceae). Rev. Mex. Biodivers. 2016, 87, 68–73. [Google Scholar] [CrossRef]
- Gansau, J.A.; Indan, H.; Abdullah, S.N.; David, D.; Marbawi, H.; Jawan, R. Effects of organic additives and plant growth regulators on protocorm development of Dendrobium lowii. Trans. Sci. Technol. 2016, 3, 462–468. [Google Scholar]
- Reddy, J.; Niveshika; Shaju, A.; Jose, A.; Betty, A.; Yarmichon, H. Plant growth regulators used for in vitro micropropagation of orchids: A research review. Int. J. Biol. Res. 2021, 8, 37–42. [Google Scholar]
- Amiri, S.; Mohammadi, R. Establishment of an efficient in vitro propagation protocol for Sumac (Rhus coriaria L.) and confirmation of the genetic homogeneity. Sci Rep. 2021, 11, 173. [Google Scholar] [CrossRef]
- Salsabila, S.S.; Fatimah, K.; Noorhazira, S.; Halimatun, T.S.T.A.B.; Aurifullah, M.; Suhana, Z. Effect of coconut water and peptone in micropropagation of Phalaeonopsis amabilis (L.) Blume Orchid. IOP Conf. Ser. Earth Environ. Sci. 2022, 1102, 012002. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Chan, M.-T.; Sanjaya; Chai, M.-L.; Tanaka, M. Priming abiotic factors for optimal hybrid Cymbidium (Orchidaceae) PLB and callus induction, plantlet formation, and their subsequent cytogenetic stability analysis. Sci. Hortic. 2006, 109, 368–378. [Google Scholar] [CrossRef]
- Kauth, P.J.; Johnson, T.R.; Stewart, S.L.; Kane, M.E. A classroom exercise in hand pollination and in vitro asymbiotic orchid seed germination. Plant Cell Tissue Organ Cult. 2008, 93, 223–230. [Google Scholar] [CrossRef]
- Hosomi, S.T.; Santos, R.B.; Custódio, C.C.; Seaton, P.T.; Marx, P.R.; Machado-Neto, N.B. Pre-conditioning Cattleya seeds to improve the efficacy of the tetrazolium test for viability Seed. Seed Sci. Technol. 2011, 39, 178–189. [Google Scholar] [CrossRef]
- Alomia, Y.A.; Mosquera-E, A.T.; Flanagan, N.S.; Otero, J.T. Seed viability and symbiotic seed germination in Vanilla spp. (Orchidaceae). Res. J. Seed Sci. 2017, 10, 43–52. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Bot. Gaz. 1962, 15, 473–477. [Google Scholar]
- Knudson, L. A new nutrient solution for orchid seed germination. Bot. Gaz. 1946, 15, 214–217. [Google Scholar]
- Vacin, F.; Went, F.W. Some pH changes in nutrient solutions. Bot. Gaz. 1949, 110, 605–613. [Google Scholar] [CrossRef]
- Harrison, C.R.; Arditti, J. Physiological changes during the germination of Cattleya aurantiaca (Orchidaceae). Bot. Gaz. 1978, 139, 180–189. [Google Scholar] [CrossRef]
- Balilashaki, K.; Ghehsareh, M. Micropropagation of Phalaenopsis amabilis var. Manila by leaves obtained from in vitro culturing the nodes of flower stalks. Not. Sci. Biol. 2016, 8, 164–169. [Google Scholar] [CrossRef]
- Edens-Meier, R.; Arduser, M.; Westhus, E.; Bernhardt, P. Pollination ecology of Cypripedium regina Walter (Orchidaceae): Size matters. Telopea 2010, 13, 327–340. [Google Scholar] [CrossRef]
- Muñoz, M.; Jiménez, V. Capsule development, in vitro germination and plantlet acclimatization in Phragmipedium humboldtii, P. longifolium and P. pearcei. Lankesteriana 2008, 8, 23–31. [Google Scholar] [CrossRef]
- Mohammad, N.N.; Rusdi, N.A. Scanning electron microscopy analysis of early floral development in Renanthera bella J.J. Wood, an Endemic orchid from Sabah. Pertanika J. Trop. Agric. Sci. 2020, 43, 77–389. [Google Scholar]
- Koene, F.M.; Amano, É.; Smidt, E.D.C.; Ribas, L.L.F. Asymbiotic germination and morphological studies of seeds of Atlantic Rainforest micro-orchids (Pleurothallidinae). PLoS ONE 2020, 15, e0243297. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Mercado, S.A. Germinación asimbiótica de semillas y desarrollo in vitro de plántulas de Cattleya mendelii Dombrain (Orchidaceae). Acta Agron 2012, 61, 69–78. [Google Scholar]
- Mayo-Mosqueda, A.; Maceda-López, L.F.; Andrade-Canto, S.B.; Noguera-Savelli, E.; Caamal-Velázquez, H.; Cano-Sosa, J.D.S.; Alatorre-Cobos, F. Efficient protocol for in vitro propagation of Laelia rubescens Lindl. from asymbiotic seed germination. S. Afr. J. Bot. 2020, 133, 264–272. [Google Scholar] [CrossRef]
- Lo, S.F.; Nalawade, S.M.; Kuo, C.L.; Chen, C.L.; Tsay, H.S. Asymbiotic germination of immature seeds, plantlet development and ex vitro establishment of plants of Dendrobium tosaense makino—A medicinally important orchid. In Vitro Cell. Dev. Biol.-Plant 2004, 40, 528–535. [Google Scholar] [CrossRef]
- Doria, J. Generalidades sobre las semillas: Su producción, conservación y almacenamiento. Cultiv. Trop. 2010, 31, 74–85. [Google Scholar]
- Mercado, S.A.S.; Caleño, J.D.Q.; Rozo, L.Y.M. Improvement of the methodology of the tetrazolium test using different pretreatments in seeds of the genus Epidendrum (Orchidaceae). J. Seed Sci. 2020, 42, e202042013. [Google Scholar] [CrossRef]
- Hosomi, S.T.; Souza, T.B.; Custódio, C.C.; Machado-Neto, N.B. Refining the tetrazolium test for evaluation of Cattleya labiata and C. tigrina seeds viability. Aust. J. Crop Sci. 2017, 11, 1320–1326. [Google Scholar] [CrossRef]
- Hirano, T.; Godo, T.; Mii, M.; Ishikawa, K. Cryopreservation of immature seeds of Bletilla striata by vitrification. Plant Cell Rep. 2005, 23, 534–539. [Google Scholar] [CrossRef]
- Yamazaki, J.; Miyoshi, K. In vitro asymbiotic germination of immature seed and formation of protocorm by Cephalanthera falcata (Orchidaceae). Ann. Bot. 2006, 98, 1197–1206. [Google Scholar] [CrossRef]
- Filho, M.J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Singh, F. Differential staining of orchid seeds for viability testing. Am. Orchid. Soc. Bull. 1981, 50, 416–418. [Google Scholar]
- Lauzer, D.; St-Arnaud, M.; Barabeâ, D. Tetrazolium staining and in vitro germination of mature seeds of Cypripedium acaule (Orchidaceae). Lindleyana 1994, 9, 197–204. [Google Scholar]
- Vujanovic, V.; St-Arnaud, M.; Barabé, D.; Thibeault, G. Viability testing of orchid seed and the promotion of colouration and germination. Ann. Bot. 2000, 86, 79–86. [Google Scholar] [CrossRef]
- Arditti, J.; Ernst, R. Physiology of germinating orchid seeds. In Orchid Biology, Reviews and Perspectives III; Arditti, J., Ed.; Cornell University Press: Ithaca, NY, USA, 1984; pp. 177–222. [Google Scholar]
- Zeigler, E.; Grivet, C.; Assmann, S.M.; Deitzer, G.F.; Hannegan, M.W. Stomatal limitation of carbon gain in Paphiopedilum sp. (Orchidaceae) and its reversal by blue light. Plant Physiol. 1985, 77, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Van Waes, J.M.; Debergh, P.C. In vitro germination of some Western European orchids. Physiol. Plant. 1986, 67, 253–261. [Google Scholar] [CrossRef]
- Bhowmik, T.K.; Rahman, M.M. Effect of different basal media and PGRs on in vitro seed germination and seedling development of medicinally important orchid Cymbidium aloifolium. J. Pharmacogn. Phytochem. 2017, 6, 167–172. [Google Scholar]
- Stewart, J. Orchid propagation by tissue culture techniques-past, present and future. In Modern Methods in Orchid Conservation: The Role of Physiology, Ecology and Management; Pritchard, H.W., Ed.; Cambridge University Press: Cambridge, UK, 1989; pp. 87–100. [Google Scholar]
- Suzuki, R.M.; Moreira, V.C.; Pescador, R.; Melo Ferreira, W. Asymbiotic seed germination and in vitro seedling development of the threatened orchid Hoffmannseggella cinnabarina. In Vitro Cell. Dev. Biol.-Plant 2012, 48, 500–551. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, K.L.; Teixeira Da Silva, J.; Zhang, J.X.; Chen, Z.L.; Xia, N.H.; Duan, J. Asymbiotic seed germination, seedling development and reintroduction of Paphiopedilum wardii Sumerh., an endangered terrestrial orchid. Sci. Hortic. 2012, 138, 198–209. [Google Scholar] [CrossRef]
- Nambiar, N.; Tee, C.S.; Maziah, M. Effects of organic additives and different carbohydrate sources on proliferation of protocorm like bodies in Dendrobium Alya Pink. Plant Omics 2012, 5, 10–18. [Google Scholar]
- Teixeira Da Silva, J.A. Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, Vol I–IV.; Global Science Books: London, UK, 2006; p. 2506. [Google Scholar]
- Gnasekaran, P.; Poobathy, R.; Mahmood, M.; Samian, M.R.; Subramaniam, S. Effects of complex organic additives on improving the growth of PLBs of Vanda Kasem’s delight. Aust. J. Crop Sci. 2012, 6, 1245–1248. [Google Scholar]
- Tawara, S.; Suraninpong, P.; Chanprame, S. Germination and Regeneration of Cymbidium findlaysonianum Lindl. on a medium supplemented with some organic sources. Walailak J. Sci. Technol. 2008, 5, 125–135. [Google Scholar]
- Van Winkle, S.C.; Pullman, G.S. Achieving desired plant growth regulator levels in liquid plant tissue culture media that include activated carbon. Plant Cell Rep. 2005, 22, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Banerjee, N. Induction of callus and plant regeneration from shoot tip explant of Dendrobium fimbriatum. Lindl. Var Octum. Sci. Hortic. 2003, 97, 333–340. [Google Scholar] [CrossRef]
- De Paw, M.A.; Remphrey, W.R.; Palmer, C.E. The cytokinin preference for in vitro germination and protocorm growth of Cypripedium candidum. Ann. Bot. 1995, 75, 267–275. [Google Scholar] [CrossRef]
- Parthibhan, S.; Rao, M.V.; Kumar, T.S. In vitro regeneration from protocorms in Dendrobium aqueum Lindley-An imperiled orchid. J. Genet. Eng. Biotechnol. 2015, 13, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sumontip, B.; Warisa, P. Agrobacterium-mediated transformation of Dendrobium chrysotoxum Lindl. Afr. J. Biotechnol. 2012, 11, 2472–2476. [Google Scholar] [CrossRef]
- Khoddamzadeh, A.A.; Sinniah, U.R.; Kadir, M.A.; Kadzimin, S.B.; Mahmood, M.; Sreeramanan, S. In vitro induction and proliferation of protocorm-like bodies (PLBs) from leaf segments of Phalaenopsis bellina (Rchb.f.) Christenson. Plant Growth Regul. 2011, 65, 381–387. [Google Scholar] [CrossRef]
- Sujjartitthurakarn, P.; Kanchanapoom, K. Efficient direct protocorm-like bodies induction of dwarf Dendrobium using Thidiazuron. Not. Sci. Biol. 2011, 3, 88–92. [Google Scholar] [CrossRef]
- Hossain, M.M. In vitro Embryo Morphogenesis and Micropropagation of Dendrobium aggregatum Roxb. Plant Tissue Cult. Biotechnol. 2013, 23, 241–249. [Google Scholar] [CrossRef]
- Pradha, S.; Pant, B. In vitro seed germination in Cymbidium elegans Lindl. and Dendrobium densiflorum Lindl. ex Wall. (Orchidaceae). Botanica Orientalis. J. Plant Sci. 2010, 6, 100–102. [Google Scholar] [CrossRef]
- Chen, J.T.; Chang, W.C. Effects of auxins and cytokinins on direct somatic embryogenesis on leaf explants of Oncidium “Gower Ramsey”. Plant Growth Regul. 2001, 34, 29–232. [Google Scholar] [CrossRef]
- Tao, J.; Yu, L.; Kong, F.; Zhao, D. Effects of plant growth regulators on in vitro propagation of Cymbidium faberi Rolfe. Afr. J. Biotech. 2011, 10, 15639–15646. [Google Scholar] [CrossRef]
- Devina, D.; Jualang, A.G.; Janna, O.A. Effect of NAA and BAP on protocorm proliferation of Borneo scented orchid, Vanda helvola. Asia-Pac. J. Mol. Biol. Biotechnol. 2010, 16, 221–224. [Google Scholar]
- Kaur, S.; Bhutani, K.K. In vitro conservation and asymbiotic propagation of Coelogyne flaccida (Lindl.): A threatened orchid. Plant Biosyst. 2014, 148, 935–944. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sharma, M.; Teixeira Da Silva, J.; Pathak, P. Seed germination and tissue culture of Cymbidium giganteum Wall. ex Lindl. Sci. Hortic. 2010, 123, 479–487. [Google Scholar] [CrossRef]
- Mahendran, G.; Bai, V.N. Mass propagation of Satyrium nepalense D. Don. A medicinal orchid via seed culture. Sci. Hortic. 2009, 119, 203–207. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumaria, S.; Tandon, P. High frequency regeneration protocol for Dendrobium nobile: A model tissue culture approach for propagation of medicinally important orchid species. S. Afr. J. Bot. 2016, 104, 232–243. [Google Scholar] [CrossRef]
- Long, B.; Niemiera, A.X.; Cheng, Z.Y.; Long, C.L. In vitro propagation of four threatened Paphiopedilum species (Orchidaceae). Plant Cell Tissue Organ Cult. 2010, 101, 151–162. [Google Scholar] [CrossRef]
- Wotavová-Novotná, K.; Vejsadová, H.; Kindlmann, P. Effects of sugars and growth regulators on in vitro growth of Dactylorhiza species. Biol. Plant. 2007, 51, 198–200. [Google Scholar] [CrossRef]
- Paudel, M.R.; Pant, B. A reliable protocol for micropropagation of Esmeralda Clarkei Rchb.f. (Orchidaceae). Asia-Pac. J. Mol. Biol. Biotechnol. 2013, 21, 114–120. [Google Scholar]
- Santana-Buzzy, N.; Rojas-Herrera, R.; Galaz-Ávalos, R.M.; Ku-Cauich, J.R.; Mijangos-Cortés, J.; Gutiérrez-Pacheco, L.C.; Canto, A.; Quiroz-Figueroa, F.; Loyola-Vargas, V.M. Advances in coffee tissue culture and its practical applications. In Vitro Cell. Dev. Biol. 2007, 43, 507–520. [Google Scholar] [CrossRef]
- Bektaş, E.; Cüce, M.; Sökmen, A. In vitro germination, protocorm formation, and plantlet development of Orchis coriophora (Orchidaceae), A naturally growing orchid species in Turkey. Turk. J. Bot. 2013, 37, 336–342. [Google Scholar] [CrossRef]
- Asa, M.; Kaviani, B. In vitro propagation of orchid Phalaenopsis amabilis (L.) Blume var. Jawa. Iran. J. Plant Physiol. 2020, 10, 3113–3123. [Google Scholar] [CrossRef]
- Decruse, S.W.; Gangaprasad, A.; Seeni, S.; Menon, V.S. Micropropagation and ecorestoration of Vanda spathulata, an exquisite orchid. Plant Cell Tissue Organ Cult. 2003, 41, 924–927. [Google Scholar] [CrossRef]
- Seeni, S.; Latha, P.G. In vitro multiplication and ecorehabilitation of the endangered Blue Vanda. Plant Cell Tissue Organ Cult. 2000, 61, 1–8. [Google Scholar] [CrossRef]
- Takamura, T.; Tanaka, M. Callus formation and plant regeneration from callus through somatic embryo structures in Cymbidium orchid. Plant Sci. 2004, 166, 1443–1449. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Teixeira Da Silva, J.A.; Nataraja, K.; Mulgund, G.S. Shoot tip transverse thin cell layers and 24-epibrassinolide in the micropropagation of Cymbidium bicolor Lindl. Floric. Ornam. Biotech 2008, 2, 44–48. [Google Scholar]
- Baker, A.; Kaviani, B.; Nematzadeh, G.; Negahdar, N. Micropropagation of Orchis catasetum–a rare and endangered orchid. Acta Sci. Pol. Hortorum Cultus. 2014, 13, 197–205. [Google Scholar]



| Stage | Description |
|---|---|
| 1 | Original seeds and seeds without embryo |
| 2 | Swollen seeds and seeds with broken seed coats |
| 3 | Protocorms formed |
| 4 | Seedlings with a single leaf |
| 5 | Plantlets with two leaves |
| 6 | Roots formed |
| Capsule Maturity Level (Days after Pollination) (DAP) | Percentage of Seed Viability (%) |
|---|---|
| 60 | No response |
| 90 | 43.85 ± 1.20% |
| 120 | 88.42 ± 2.15% |
| Basal Medium | Days to Germinate | Germination Percentage (%) (Mean ± SD) | ||
|---|---|---|---|---|
| 50 DAC | 100 DAC | 150 DAC | ||
| Knudson C | 34 | 42.90 ± 3.98 a | 93.61 ± 12.50 a | 98.77 ± 15.02 a |
| Murashige and Skoog | 55 | 32.65 ± 5.13 b | 88.04 ± 9.94 b | 92.80 ± 14.71 b |
| Vacin and Went | 231 | 29.22 ± 1.19 c | 73.46 ± 7.48 c | 75.66 ± 11.42 c |
| Natural Additives (%) | Percentage of Protocorm Proliferation (% ± SD) | Mean Number of New Protocorm (±SD) | Percentage of Necrosis (% ± SD) | |
|---|---|---|---|---|
| Coconut water (CW) | 5 | 2.25 ± 1.26 b | 0.75 ± 0.56 b | 10.00 ± 0.47 a |
| 10 | 12.75 ± 1.71 a | 6.50 ± 1.26 a | 5.00 ± 0.88 b | |
| 15 | 1.25 ± 0.50 b | 0.00 ± 0.00 c | 20.00 ± 0.3 a | |
| 20 | 1.00 ± 0.52 b | 0.50 ± 0.12 b | 35.00 ± 0.49 a | |
| Banana homogenate (BH) | 5 | 15.75 ± 0.96 a | 9.50 ± 1.92 a | 5.00 ± 0.2 b |
| 10 | 3.25 ± 1.26 b | 1.21 ± 0.58 ab | 10.00 ± 0.30 a | |
| 15 | 3.50 ± 1.00 b | 1.00 ± 0.43 ab | 10.00 ± 0.47 a | |
| 20 | 2.50 ± 0.58 b | 0.25 ± 0.50 b | 10.00 ± 0.47 a | |
| Plant Growth Regulators (mg/L) | Percentage of Protocorm Proliferation (% ± SD) | Mean Number of New Protocorm (±SD) | Percentage of Necrosis (% ± SD) | |
|---|---|---|---|---|
| BAP | NAA | |||
| 0.5 | - | 7.50 ± 0.12 b | 3.00 ± 0.82 b | 30.00 ± 0.38 ab |
| 1.0 | - | 10.00 ± 0.36 b | 2.33 ± 0.50 b | 8.00 ± 0.44 b |
| 1.5 | - | 15.00 ± 0.58 b | 2.58 ± 1.15 b | 5.00 ± 0.50 c |
| 2.0 | - | 5.00 ± 0.10 c | 2.00 ± 0.50 b | 35.00 ± 1.00 a |
| - | 0.5 | 17.25 ± 0.96 a | 3.50 ± 0.57 b | 10.00 ± 1.43 b |
| - | 1.0 | 15.75 ± 0.58 b | 2.75 ± 0.50 b | 5.00 ± 0.44 c |
| - | 1.5 | 16.50 ± 0.50 b | 3.00 ± 0.58 b | 13.33 ± 0.85 b |
| - | 2.0 | 11.00 ± 0.82 b | 2.50 ± 0.60 b | 11.00 ± 1.21 b |
| Plant Growth Regulators (mg/L) | Percentage Number of Protocorm with Leaf (% ± SD) | Leaf Numbers (±SD) | Percentage Number of Protocorm with Root (% ± SD) | Root Number (±SD) | Length (mm ± SD) | ||
|---|---|---|---|---|---|---|---|
| BAP | NAA | Leaf | Root | ||||
| 0.5 | - | 5.65 ± 0.82 c | 2.25 ± 0.96 c | 5.65 ± 1.00 d | 3.25 ± 0.82 d | 0.68 ± 0.23 b | 0.34 ± 0.50 b |
| 1.0 | - | 8.50 ± 0.96 c | 1.50 ± 0.58 c | 5.00 ± 0.37 d | 2.65 ± 0.50 d | 0.71 ± 0.11 b | 0.41 ± 0.37 b |
| 1.5 | - | 5.00 ± 0.57 c | 1.25 ± 0.96 c | 3.33 ± 0.21 e | 2.50 ± 0.57 de | 0.72 ± 0.26 a | 0.41 ± 0.90 ab |
| 2.0 | - | 5.00 ± 0.57 c | 1.00 ± 0.20 c | 3.00 ± 0.43 e | 2.00 ± 0.50 e | 0.54 ± 0.13 c | 0.36 ± 0.21 b |
| - | 0.5 | 13.75 ± 0.50 a | 8.00 ± 0.82 a | 12.65 ± 0.58 a | 10.33 ± 0.87 a | 1.07 ± 0.12 a | 0.74 ± 0.51 a |
| - | 1.0 | 12.50 ± 0.58 ab | 5.00 ± 0.58 b | 10.65 ± 0.50 b | 8.67 ± 0.50 b | 0.97 ± 0.36 ab | 0.71 ± 0.16 ab |
| - | 1.5 | 13.25 ± 0.50 ab | 6.50 ± 1.41 b | 11.00 ± 0.43 b | 6.55 ± 0.72 c | 1.01 ± 0.21 a | 0.56 ± 0.20 b |
| - | 2.0 | 11.25 ± 0.96 b | 2.00 ± 0.82 c | 8.50 ± 0.58 c | 6.00 ± 0.23 c | 0.74 ± 0.23 ab | 0.43 ± 0.11 b |
| Plant Growth Regulators (mg/L) | Percentage Number of Protocorm with Leaf (% ± SD) | Leaf Number (±SD) | Percentage Number of Protocorm with Root (% ± SD) | Root Number (±SD) | Length (mm ± SD) | ||
|---|---|---|---|---|---|---|---|
| BAP | NAA | Leaf | Root | ||||
| 0.5 | 0.5 | 11.00 ± 0.63 b | 4.75 ± 0.21 b | 11.00 ± 0.48 b | 5.00 ± 0.18 b | 1.34 ± 0.72 c | 0.59 ± 0.12 c |
| 0.5 | 1.0 | 34.75 ± 0.56 a | 5.75 ± 0.63 a | 18.50 ± 0.57 ab | 8.65 ± 0.86 a | 2.94 ± 0.72 c | 1.84 ± 0.59 c |
| 0.5 | 1.5 | 10.33 ± 0.31 b | 4.25 ± 0.25 b | 6.67 ± 0.34 b | 2.00 ± 0.23 c | 1.25 ± 0.34 b | 1.33 ± 0.64 b |
| 1.0 | 0.5 | 25.76 ± 0.54 b | 2.75 ± 0.50 c | 16.00 ± 0.50 b | 3.33 ± 0.21 c | 2.62 ± 0.54 b | 0.50 ± 0.37 c |
| 1.0 | 1.0 | 30.00 ± 0.87 b | 5.25 ± 0.96 b | 16.33 ± 0.33 a | 6.00 ± 0.54 b | 2.78 ± 0.19 b | 1.00 ± 0.63 b |
| 1.0 | 1.5 | 10.00 ± 0.25 bc | 4.00 ± 0.63 b | 5.00 ± 0.40 c | 2.33 ± 0.97 c | 0.79 ± 0.14 c | 1.56 ± 0.76 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, H.V.; Gansau, J.A.; Mus, A.A.; Mohammad, N.N.; Shamsudin, N.A.; Amin, J.; Rusdi, N.A. Developing Paraphalaenopsis labukensis (Shim, A. Lamb & C.L. Chan), an Orchid Endemic to Sabah, Borneo, Asymbiotic Seed Germination and In Vitro Seedling Development. Horticulturae 2023, 9, 681. https://doi.org/10.3390/horticulturae9060681
Nelson HV, Gansau JA, Mus AA, Mohammad NN, Shamsudin NA, Amin J, Rusdi NA. Developing Paraphalaenopsis labukensis (Shim, A. Lamb & C.L. Chan), an Orchid Endemic to Sabah, Borneo, Asymbiotic Seed Germination and In Vitro Seedling Development. Horticulturae. 2023; 9(6):681. https://doi.org/10.3390/horticulturae9060681
Chicago/Turabian StyleNelson, Heira Vanessa, Jualang Azlan Gansau, Ahmad Asnawi Mus, Nurul Najwa Mohammad, Nor Amirah Shamsudin, Jumatiah Amin, and Nor Azizun Rusdi. 2023. "Developing Paraphalaenopsis labukensis (Shim, A. Lamb & C.L. Chan), an Orchid Endemic to Sabah, Borneo, Asymbiotic Seed Germination and In Vitro Seedling Development" Horticulturae 9, no. 6: 681. https://doi.org/10.3390/horticulturae9060681
APA StyleNelson, H. V., Gansau, J. A., Mus, A. A., Mohammad, N. N., Shamsudin, N. A., Amin, J., & Rusdi, N. A. (2023). Developing Paraphalaenopsis labukensis (Shim, A. Lamb & C.L. Chan), an Orchid Endemic to Sabah, Borneo, Asymbiotic Seed Germination and In Vitro Seedling Development. Horticulturae, 9(6), 681. https://doi.org/10.3390/horticulturae9060681







