In Vitro Conservation and Regeneration of Potato (Solanum tuberosum L.): Role of Paclobutrazol and Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Effect of AgNPs and PAC on In Vitro Growth
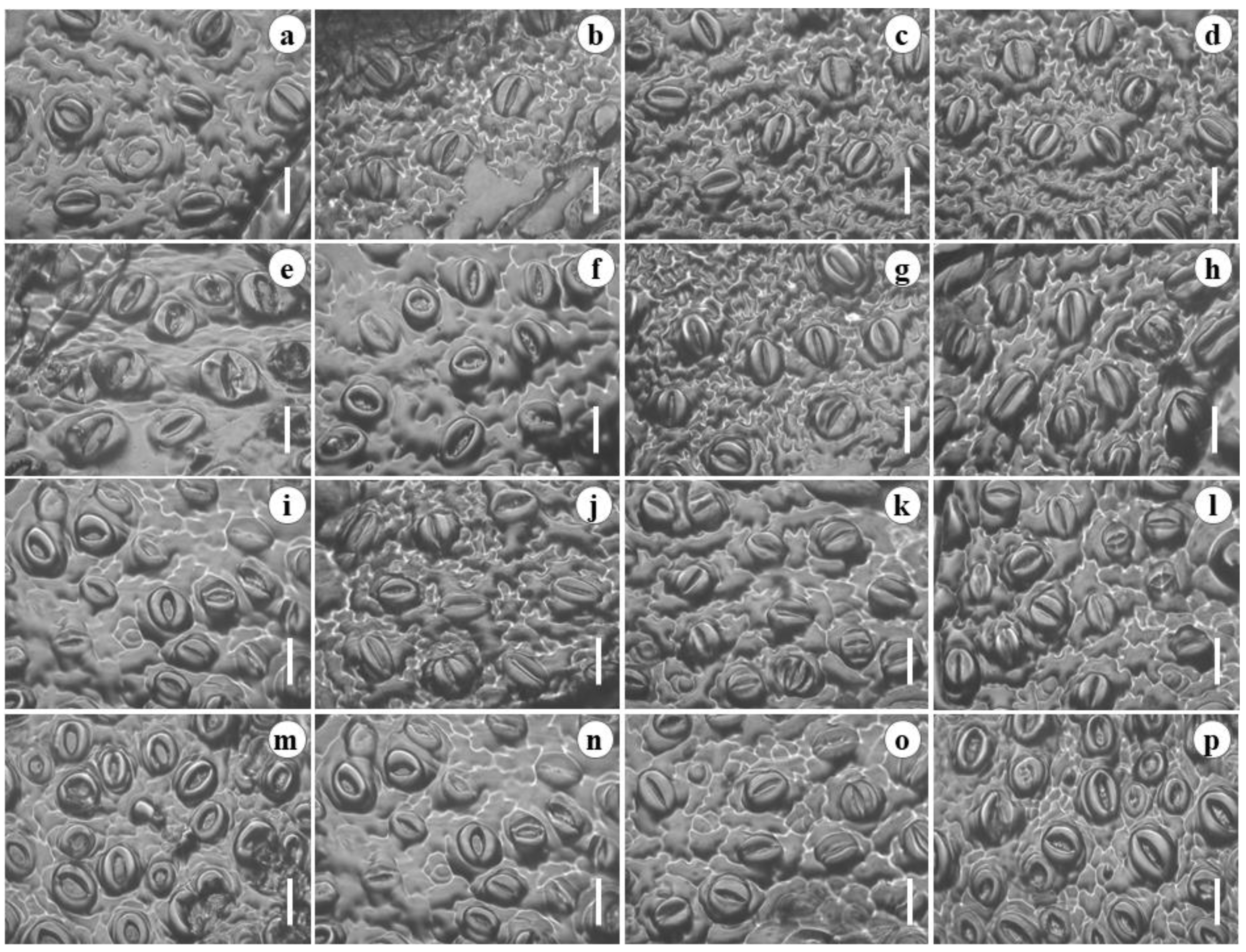
2.3. Stomatal Density
2.4. Total Chlorophyll
2.5. In Vitro Regeneration
2.6. Data Analysis and Experimental Design
3. Results
3.1. Effect of AgNPs and PAC on Physiological and Biochemical Parameters
3.2. In Vitro Regeneration after PAC and AgNP Treatments
4. Discussion
4.1. Effect of PAC and AgNPs on Physiological and Biochemical Parameters
4.2. In Vitro Regeneration after PAC and AgNP Treatments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2022. Available online: http://www.fao.org/faostat/es/#data (accessed on 25 April 2023).
- Zsögön, A.; Peres, L.E.P.; Xiao, Y.; Yan, J.; Fernie, A.R. Enhancing crop diversity for food security in the face of climate uncertainty. Plant J. 2022, 109, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Kulak, V.; Longboat, S.; Brunet, N.D.; Shukla, M.; Saxena, P. In Vitro Technology in Plant Conservation: Relevance to Biocultural Diversity. Plants 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Panis, B.; Nagel, M.; Van den Houwe, I. Challenges and prospects for the conservation of crop genetic resources in field genebanks, in In vitro collections and/or in liquid nitrogen. Plants 2020, 12, 1634. [Google Scholar] [CrossRef] [PubMed]
- Engels, J.M.M.; Andreas, W.E. A critical review of the current global ex situ conservation system for plant agrobiodiversity. I. History of the development of the global system in the context of the political/legal framework and its major conservation components. Plants 2021, 10, 1557. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Pérez-Sato, J.A.; Schettino-Salomón, S.S.; Bello-Bello, J.J. An alternative method for medium-term in vitro conservation of different plant species through gibberellin inhibitors. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 606–614. [Google Scholar] [CrossRef]
- Ruta, C.; Lambardi, M.; Ozudogru, E.A. Biobanking of vegetable genetic resources by in vitro conservation and cryopreservation. Biodivers. Conserv. 2020, 29, 3495–3532. [Google Scholar] [CrossRef]
- Bello-Bello, J.; Canto-Flick, A.; Balam-Uc, E.; Robert, L. Improvement of in vitro proliferation and elongation of Habanero pepper shoots (Capsicum chinense Jacq.) by temporary immersion. HortScience 2010, 45, 1093–1098. [Google Scholar] [CrossRef]
- Ding, Y.; Sheng, J.; Li, S.; Nie, Y.; Zhao, J.; Zhu, Z.; Wang, Z.; Tang, X. The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2015, 101, 88–95. [Google Scholar] [CrossRef]
- Zeid, I.M.A.; Soliman, H.I.; Metwali, E.M. In Vitro Evaluation of Some High Yield Potato (Solanum tuberosum L.) Cultivars under Imposition of Salinity at the Cellular and Organ Levels. Saudi J. Biol. Sci. 2022, 29, 2541–2551. [Google Scholar] [CrossRef]
- Benelli, C.; Tarraf, W.; Izgu, T.; De Carlo, A. In Vitro Conservation through Slow Growth Storage Technique of Fruit Species: An Overview of the Last 10 Years. Plants 2022, 11, 3188. [Google Scholar] [CrossRef] [PubMed]
- Adly, W.M.R.M.; Mazrou, Y.S.A.; EL-Denary, M.E.; Mohamed, M.A.; Abd El-Salam, E.-S.T.; Fouad, A.S. Boosting Polyamines to Enhance Shoot Regeneration in Potato (Solanum tuberosum L.) Using AgNO3. Horticulturae 2022, 8, 113. [Google Scholar] [CrossRef]
- Santana-Buzzy, N.; Canto-Flick, A.; Iglesias-Andreu, L.G.; Montalvo-Peniche, M.d.C.; López-Puc, G.; Barahona-Pérez, F. Improvement of In Vitro Culturing of Habanero Pepper by Inhibition of Ethylene Effects. HortSci 2006, 41, 405–409. [Google Scholar] [CrossRef]
- Sarmast, M.K.; Salehi, H. Sub-lethal concentrations of silver nanoparticles mediate a phytostimulatory response in tobacco via the suppression of ethylene biosynthetic genes and the ethylene signaling pathway. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 1–14. [Google Scholar] [CrossRef]
- Mahajan, S.; Kadam, J.; Dhawal, P. Application of silver nanoparticles in in-vitro plant growth and metabolite production: Revisiting its scope and feasibility. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 150, 15–39. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wilkinson, H.P. The plant surface (mainly leaf). In Anatomy of the Dicotyledons; Metcalfe, C.R., Chalk, L.I., Eds.; Clarendon Press: Oxford, UK, 1980; pp. 97–165. [Google Scholar]
- Harborne, J.B. Phenolic compounds. In Phytochemical Methods; Springer: Dordrecht, The Netherlands, 1973; pp. 33–88. [Google Scholar]
- Bello-Bello, J.; Poot-Poot, W.; Iglesias-Andreu, L.; Caamal-Velázquez, H.; de la Cruz Diaz-Sánchez, M. Comparison of effect of osmoregulators and growth inhibitors on in vitro conservation of sugarcane. Agrociencia 2014, 48, 439–446. [Google Scholar]
- Cruz-Cruz, C.A.; González-Arnao, M.T.; Bautista-Aguilar, J.R.; Ramírez-Mosqueda, M.A. In vitro short-term storage of Stanhopea tigrina Bateman ex Lind. S. Afr. J. Bot. 2022, 151, 334–338. [Google Scholar] [CrossRef]
- Castro-González, C.G.; Sánchez-Segura, L.; Gómez-Merino, F.C.; Bello-Bello, J.J. Exposure of stevia (Stevia rebaudiana B.) to silver nanoparticles in vitro: Transport and accumulation. Sci. Rep. 2019, 9, 10372. [Google Scholar] [CrossRef]
- Kaur, A.; Kumar, A. The effect of gelling agent, medium pH and silver nitrate on adventitious shoot regeneration in Solanum tuberosum. bioRxiv 2020. [Google Scholar] [CrossRef]
- Elatafi, E.; Fang, J. Effect of Silver Nitrate (AgNO3) and Nano-Silver (Ag-NPs) on Physiological Characteristics of Grapes and Quality during Storage Period. Horticulturae 2022, 8, 419. [Google Scholar] [CrossRef]
- Syu, Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kazama, H.; Dan, H.; Imaseki, H.; Wasteneys, G.O. Transient Exposure to Ethylene Stimulates Cell Division and Alters the Fate and Polarity of Hypocotyl Epidermal Cells. Plant Physiol. 2004, 134, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Manokari, M.; Raj, M.C.; Dey, A.; Faisal, M.; Alatar, A.A.; Joshee, N.; Shekhawat, M.S. Silver nanoparticles improved morphogenesis, biochemical profile and micro-morphology of Gaillardia pulchella Foug cv. ‘Torch Yellow’. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 153, 1–13. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Tung, H.T.; Thuong, T.T.; Cuong, D.M.; Luan, V.Q.; Hien, V.T.; Hieu, T.; Nam, N.B.; Phuong, H.T.N.; Vinh, B.V.T.; Khai, H.D.; et al. Silver nanoparticles improved explant disinfection, in vitro growth, runner formation and limited ethylene accumulation during micropropagation of strawberry (Fragaria× ananassa). Plant Cell Tissue Organ Cult. (PCTOC) 2021, 145, 393–403. [Google Scholar] [CrossRef]
- Kumar, A.; Ram, S.; Singh, C.P. Application methods of paclobutrazol in mango Cvs & Its residual effect in leaf, fruit and orchard soil. Int. J. Res. Agric. For. 2020, 2, 24–39. [Google Scholar]


| PAC (mg L−1) | AgNPs (mg L−1) | Shoot Length (cm) | No. of Lateral Branches | No. of Leaves Per Explant | No. of Roots Per Explant | Root Length (cm) | Stomatal Density | Total Chl (mg g−1 FW) |
|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 15.80 ± 0.19 a | 19.95 ± 0.40 a | 26.85 ± 0.89 a | 10.20 ± 0.28 a | 11.85 ± 0.24 a | 194.25 ± 7.02 d | 0.13 ± 0.00 c |
| 0.5 | 10.90 ± 0.48 bc | 19.90 ± 0.44 a | 26.80 ± 1.00 a | 10.30 ± 0.29 a | 11.35 ± 0.22 a | 195.50 ± 7.35 d | 0.14 ± 0.00 c | |
| 1 | 9.45 ± 0.40 c | 19.65 ± 0.56 a | 28.60 ± 0.93 a | 10.65 ± 0.27 a | 7.00 ± 0.16 b | 194.50 ± 4.57 d | 0.14 ± 0.00 c | |
| 2 | 7.69 ± 0.49 cd | 19.44 ± 0.40 a | 27.55 ± 1.05 a | 10.11 ± 0.29 a | 6.83 ± 0.20 b | 195.75 ± 5.35 cd | 0.13 ± 0.00 c | |
| 0 | 50 | 15.66 ± 0.22 a | 19.00 ± 0.41 a | 26.00 ± 0.90 ab | 10.55 ± 0.29 a | 11.70 ± 0.24 a | 191.75 ± 6.56 d | 0.12 ± 0.00 c |
| 0.5 | 15.23 ± 0.30 ab | 9.10 ± 0.42 b | 24.26 ± 1.14 ab | 9.78 ± 0.32 ab | 7.00 ± 0.20 b | 235.00 ± 7.35 b | 0.58 ± 0.02 a | |
| 1 | 7.22 ± 0.21 cd | 6.55 ± 0.37 c | 17.70 ± 0.85 d | 8.00 ± 0.34 cd | 7.15 ± 0.18 b | 246.75 ± 4.49 b | 0.59 ± 0.03 a | |
| 2 | 5.58 ± 0.19 e | 4.83 ± 0.32 c | 11.77 ± 0.67 e | 6.72 ± 0.26 d | 5.50 ± 0.23 c | 289.25 ± 1.88 a | 0.64 ± 0.02 a | |
| 0 | 100 | 15.8 ± 0.23 a | 9.47 ± 0.38 b | 22.21 ± 1.36 bc | 10.00 ± 0.35 a | 11.26 ± 0.26 a | 230.25 ± 8.29 bc | 0.54 ± 0.03 ab |
| 0.5 | 7.14 ± 0.23 d | 9.41 ± 0.45 b | 17.23 ± 0.87 d | 9.64 ± 0.35 ab | 11.11 ± 0.26 a | 241.50 ± 6.84 b | 0.44 ± 0.02 b | |
| 1 | 6.71 ± 0.15 de | 6.31 ± 0.38 c | 17.47 ± 0.69 d | 8.10 ± 0.34 cd | 7.31 ± 0.18 b | 240.75 ± 6.15 b | 0.53 ± 0.03 ab | |
| 2 | 5.40 ± 0.23 e | 4.62 ± 0.35 c | 11.68 ± 0.64 e | 6.75 ± 0.28 d | 5.56 ± 0.30 c | 287.25 ± 4.06 a | 0.61 ± 0.02 a | |
| 0 | 200 | 14.95 ± 0.29 ab | 8.90 ± 0.49 b | 18.80 ± 0.77 cd | 9.95 ± 0.32 a | 6.95 ± 0.19 b | 231.00 ± 8.22 bc | 0.54 ± 0.02 ab |
| 0.5 | 8.70 ± 0.38 c | 9.45 ± 0.38 b | 18.10 ± 0.70 cd | 8.30 ± 0.31 bc | 6.90 ± 0.19 b | 237.50 ± 9.81 b | 0.54 ± 0.02 ab | |
| 1 | 6.66 ± 0.25 de | 9.42 ± 0.42 b | 18.10 ± 0.69 cd | 8.00 ± 0.35 cd | 6.84 ± 0.19 b | 245.75 ± 11.34 b | 0.52 ± 0.03 ab | |
| 2 | 5.57 ± 0.17 e | 5.47 ± 0.24 c | 11.36 ± 0.39 e | 6.68 ± 0.27 d | 5.15 ± 0.25 c | 289.25 ± 4.21 a | 0.62 ± 0.01 a | |
| p-value | ||||||||
| p (PAC) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| p (AgNPs) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| p (PAC × AgNPs) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| PAC (mg L−1) | AgNPs (mg L−1) | Response (%) | No. of Shoots Per Explant | Shoot Length (cm) |
|---|---|---|---|---|
| 0 | 0 | 43.75 ±12.50 c | 2.30 ± 0.26 b | 3.78 ± 0.24 a |
| 0.5 | 43.75 ± 12.50 c | 2.16 ± 0.40 b | 3.97 ± 0.30 a | |
| 1 | 37.50 ± 14.43 c | 2.00 ± 0.36 b | 3.55 ± 0.28 a | |
| 2 | 37.50 ± 14.43 c | 2.16 ± 0.24 b | 3.64 ± 0.27 a | |
| 0 | 50 | 62.50 ± 14.43 c | 2.07 ± 0.22 b | 3.34 ± 0.31 a |
| 0.5 | 93.75 ± 12.50 ab | 2.93 ± 0.23 b | 3.08 ± 0.17 a | |
| 1 | 100.00 ± 0.00 a | 4.66 ± 0.18 a | 3.42 ± 0.10 a | |
| 2 | 100.00 ± 0.00 a | 4.66 ± 0.18 a | 3.34 ± 0.10 a | |
| 0 | 100 | 62.50 ± 14.43 bc | 2.00 ± 0.44 b | 3.95 ± 0.28 a |
| 0.5 | 87.50 ± 14.43 ab | 3.00 ± 0.30 b | 3.07 ± 0.16 a | |
| 1 | 87.50 ± 14.43 ab | 3.36 ± 0.33 ab | 3.56 ± 0.13 a | |
| 2 | 93.75 ± 12.50 ab | 3.21 ± 0.31 ab | 3.45 ± 0.24 a | |
| 0 | 200 | 87.50 ± 14.43 ab | 2.00 ± 0.57 b | 3.00 ± 0.35 a |
| 0.5 | 68.75 ± 12.50 bc | 2.37 ± 0.18 b | 2.85 ± 0.21 a | |
| 1 | 87.50 ± 14.43 ab | 2.87 ± 0.22 b | 3.30 ± 0.18 a | |
| 2 | 81.25 ± 12.50 abc | 2.76 ± 0.23 b | 2.94 ± 0.16 a | |
| p-value | ||||
| p (PAC) | 0.000 | 0.000 | 0.386 | |
| p (AgNPs) | 0.005 | 0.000 | 0.001 | |
| p (PAC × AgNPs) | 0.002 | 0.000 | 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltazar Bernal, O.; Spinoso-Castillo, J.L.; Mancilla-Álvarez, E.; Muñoz-Márquez Trujillo, R.A.; Bello-Bello, J.J. In Vitro Conservation and Regeneration of Potato (Solanum tuberosum L.): Role of Paclobutrazol and Silver Nanoparticles. Horticulturae 2023, 9, 676. https://doi.org/10.3390/horticulturae9060676
Baltazar Bernal O, Spinoso-Castillo JL, Mancilla-Álvarez E, Muñoz-Márquez Trujillo RA, Bello-Bello JJ. In Vitro Conservation and Regeneration of Potato (Solanum tuberosum L.): Role of Paclobutrazol and Silver Nanoparticles. Horticulturae. 2023; 9(6):676. https://doi.org/10.3390/horticulturae9060676
Chicago/Turabian StyleBaltazar Bernal, Obdulia, José Luis Spinoso-Castillo, Eucario Mancilla-Álvarez, Rafael Arturo Muñoz-Márquez Trujillo, and Jericó Jabín Bello-Bello. 2023. "In Vitro Conservation and Regeneration of Potato (Solanum tuberosum L.): Role of Paclobutrazol and Silver Nanoparticles" Horticulturae 9, no. 6: 676. https://doi.org/10.3390/horticulturae9060676
APA StyleBaltazar Bernal, O., Spinoso-Castillo, J. L., Mancilla-Álvarez, E., Muñoz-Márquez Trujillo, R. A., & Bello-Bello, J. J. (2023). In Vitro Conservation and Regeneration of Potato (Solanum tuberosum L.): Role of Paclobutrazol and Silver Nanoparticles. Horticulturae, 9(6), 676. https://doi.org/10.3390/horticulturae9060676







