Establishment of an Efficient Somatic Embryogenesis Protocol for Giant Reed (Arundo donax L.) and Multiplication of Obtained Shoots via Semi-Solid or Liquid Culture
Abstract
:1. Introduction
2. Materials and Methods
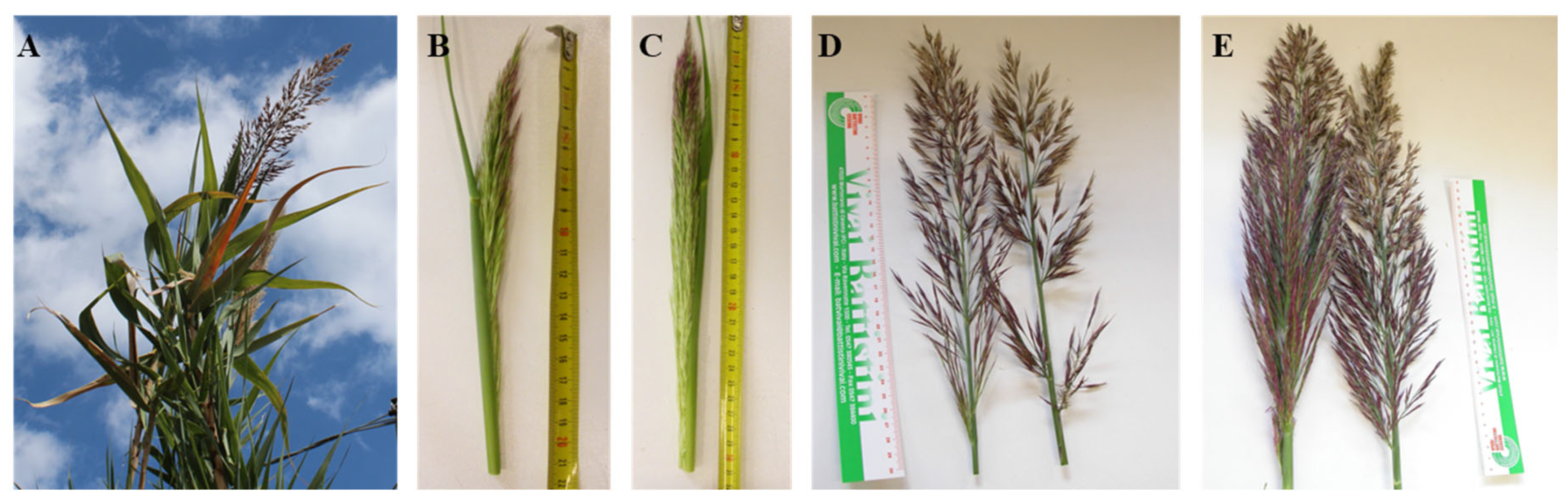
2.1. Plant Material
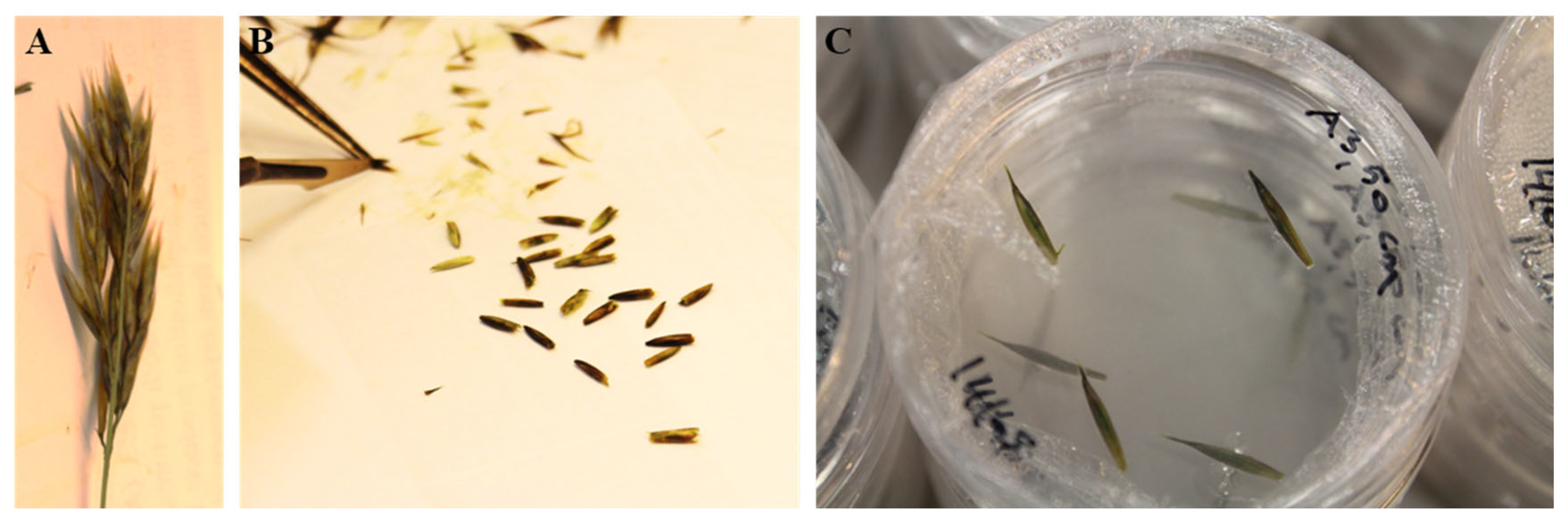
2.2. Decontamination of the Plant Material and Explant Preparation
2.3. Induction of Somatic Embryogenesis
2.4. Somatic Embryo Maturation and Plantlet Formation
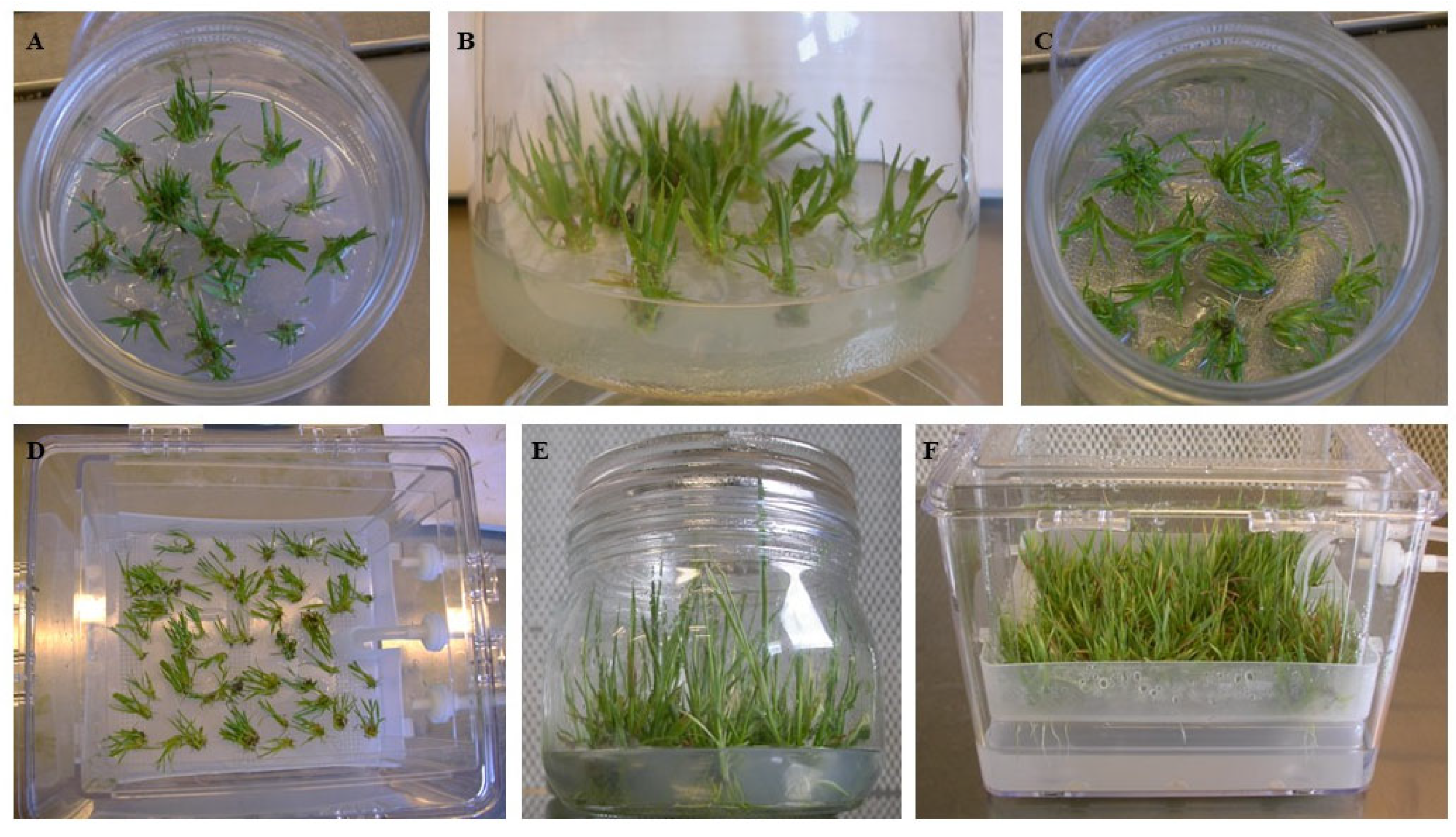
2.5. Multiplication of Shoots Derived from Somatic Embryogenesis
2.6. Experimental Design, Data Collection, and Statistical Analysis
3. Results
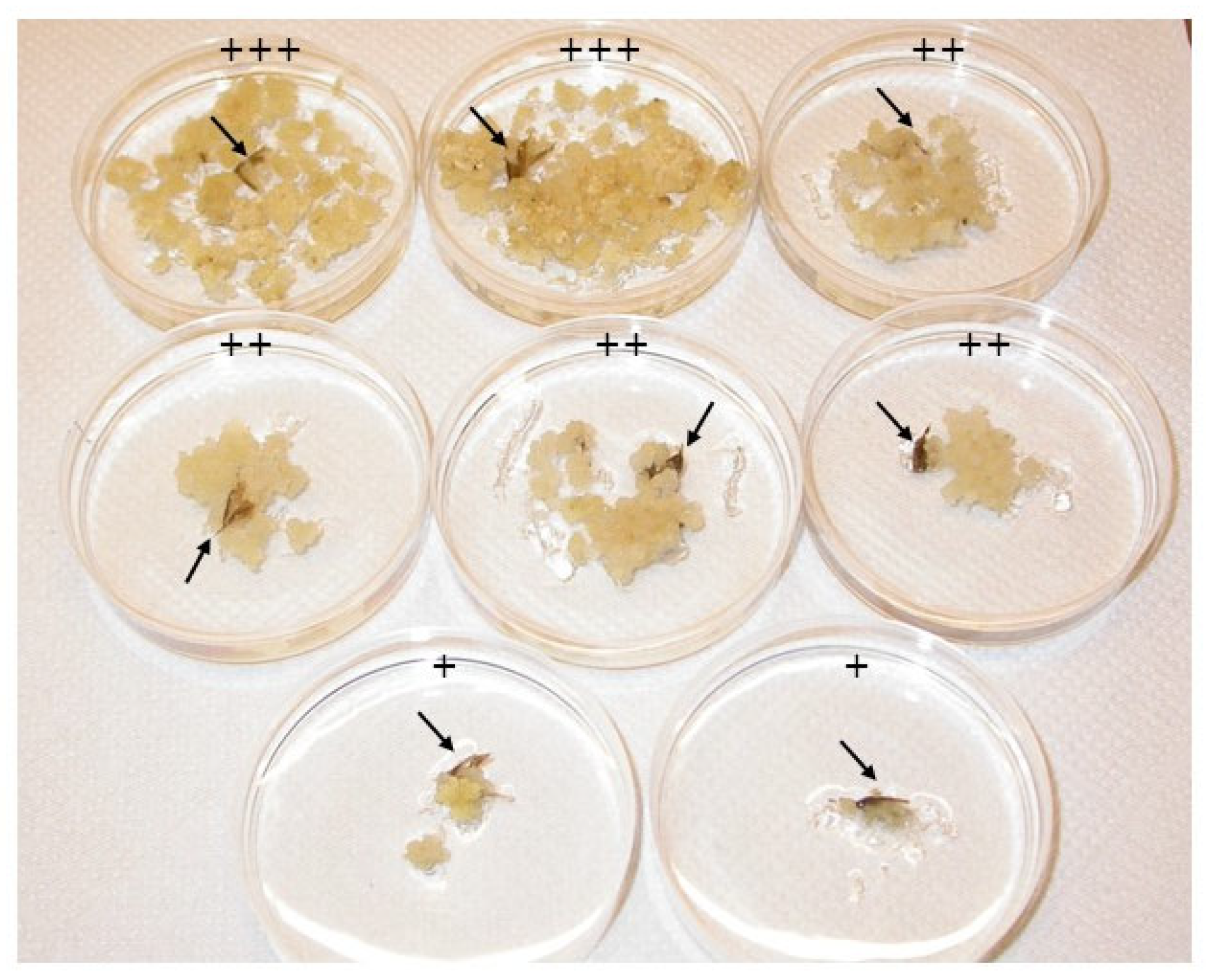
3.1. Callus Induction on Gelled Medium
3.2. Maturation of the Embryogenic Callus and Emergence of the Plantlets
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiménez-Ruiz, J.; Hardion, L.; Pablo, J.; Vila, B.; Santín-Montanyá, M.I. Monographs on Invasive Plants in Europe N° 4: Arundo donax L. Bot. Lett. 2021, 168, 131–151. [Google Scholar]
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The Development and Current Status of Perennial Rhizomatous Grasses as Energy Crops in the US and Europe. Biomass Bioenerg. 2003, 25, 335–361. [Google Scholar]
- Häfliger, E.; Scholz, H. Grass Weeds (2 Bde): Weeds of the Subfamilies Chloridoideae, Pooideae, Oryzoideae; Giba-Geigy: Basel, Switzerland, 1981; p. 223. [Google Scholar]
- Bell, C.E.; DiTomaso, J.M.; Brooks, M.L. Wildfires and invasive plants in southern California. Proc. Calif. Weed Sci. Soc. 2006, 58, 40–42. [Google Scholar]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar]
- Pyšek, P.; Richardson, D.M. The biogeography of naturalization in alien plants. J. Biogeogr. 2006, 33, 2040–2050. [Google Scholar]
- Ceotto, E.; Di Candilo, M. Shoot Cuttings Propagation of Giant Reed (Arundo donax L.) in Water and Moist Soil: The Path Forward? Biomass Bioenerg. 2010, 34, 1614–1623. [Google Scholar]
- Mariani, C.; Cabrini, R.; Danin, A.; Piffanelli, P.; Fricano, A.; Gomarasca, S.; Dicandilo, M.; Grassi, F.; Soave, C. Origin, Diffusion and Reproduction of the Giant Reed (Arundo donax L.): A Promising Weedy Energy Crop. Ann. Appl. Biol. 2010, 157, 191–202. [Google Scholar]
- Jahirul, M.; Rasul, M.; Chowdhury, A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar]
- Muench, S.; Guenther, E. A Systematic Review of Bioenergy Life Cycle Assessments. Appl. Energy 2013, 112, 257–273. [Google Scholar]
- Roos, A.; Ahlgren, S. Consequential Life Cycle Assessment of Bioenergy Systems—A Literature Review. J. Clean. Prod. 2018, 189, 358–373. [Google Scholar]
- Angelini, L.G.; Ceccarini, L.; Nassi o Di Nasso, N.; Bonari, E. Comparison of Arundo donax L. and Miscanthus giganteus in a Long-Term Field Experiment in Central Italy: Analysis of Productive Characteristics and Energy Balance. Biomass Bioenerg. 2009, 33, 635–643. [Google Scholar]
- Pigna, G.; Dhillon, T.; Dlugosz, E.M.; Yuan, J.S.; Gorman, C.; Morandini, P.; Lenaghan, S.C.; Stewart, C.N. Methods for Suspension Culture, Protoplast Extraction, and Transformation of High-Biomass Yielding Perennial Grass Arundo donax. Biotechnol. J. 2016, 11, 1657–1666. [Google Scholar]
- Basso, M.C.; Cerrella, E.G.; Buonomo, E.L.; Bonelli, P.R.; Cukierman, A.L. Thermochemical Conversion of Arundo donax into Useful Solid Products. Energy Sources 2005, 27, 1429–1438. [Google Scholar] [CrossRef]
- Peng, F.; Bian, J.; Peng, P.; Xiao, H.; Ren, J.; Xu, F.; Sun, R.-C. Separation and Characterization of Acetyl and Non-Acetyl Hemicelluloses of Arundo donax by Ammonium Sulfate Precipitation. J. Agric. Food Chem. 2012, 60, 4039–4047. [Google Scholar]
- Bajguz, A.; Hayat, S. Effects of Brassinosteroids on the Plant Responses to Environmental Stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar]
- Zhang, J.; Lu, Y.; Zhang, S.; Lu, F.; Yang, H. Identification of Transcriptome Involved in Atrazine Detoxification and Degradation in Alfalfa (Medicago Sativa) Exposed to Realistic Environmental Contamination. Ecotoxicol. Environ. Saf. 2016, 130, 103–112. [Google Scholar]
- Etienne, H. Somatic embryogenesis protocol: Coffee (Coffea arabica L. and C. canephora P.). In Protocols for Somatic Embryogenesis in Woody Plants; Jain, S.M., Gupta, P.K., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 167–179. [Google Scholar]
- Ramirez-Mosqueda, M.A.; Iglesias-Andreu, L.G.; Ramirez-Madero, G.; Hernandez-Rincon, E.U. Micropropagation of Steviare baudiana Bert. in temporary immersion systems and evaluation of genetic fidelity. S. Afr. J. Bot. 2016, 106, 238–243. [Google Scholar]
- Othmani, A.; Bayoudh, C.; Sellemi, A.; Drira, N. Temporary immersion system for date palm micropropagation. In Date Palm Biotechnology Protocols Volume 1: Tissue Culture Applications, Methods in Molecular Biology; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer Science + Business Media LLC: Berlin/Heidelberg, Germany, 2018; Volume 1637, pp. 239–250. [Google Scholar] [CrossRef]
- Rico, S.; Garrido, J.; Sánchez, C.; Ferreiro-Vera, C.; Codesido, V.; Vidal, N. A Temporary Immersion System to Improve Cannabis sativa Micropropagation. Front. Plant Sci. 2022, 13, 895971. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A novel temporary immersion bioreactor system for large scale multiplication of banana (Rasthali AAB-Silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 381–385. [Google Scholar]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut Juglans hindsii x Juglans regia rootstocks. HortScience 1984, 19, 507–509. [Google Scholar]
- Roncasaglia, R.; Benelli, C.; De Carlo, A.; Dradi, G.; Lambardi, M.; Ozudogru, E.A. Fattori che influiscono sulla conservazione in crescita rallentata di specie da frutto. Italus Hortus 2009, 16, 234–238. [Google Scholar]
- Marascuilo, L.A.; McSweeney, M. Post hoc multiple comparisons in sample preparations for test of homogenesity. In Non-Parametric and Distribution FreeMethods of the Social Sciences; McSweeney, M., Marasculio, L.A., Eds.; Books/Cole Publ: Belmont, CA, USA, 1977; pp. 141–147. [Google Scholar]
- Herrera-Alamillo, M.Á.; Robert, M.L. Liquid In Vitro Culture for the Propagation of Arundo donax. In Plant Cell Culture Protocols. Methods in Molecular Biology, 1st ed.; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 877, pp. 153–160. [Google Scholar]
- Corno, L.; Pilu, R.; Adani, F. Arundo donax L.: A Non-Food Crop for Bioenergy and Bio-Compound Production. Biotechnol. Adv. 2014, 32, 1535–1549. [Google Scholar] [PubMed]
- Jeguirim, M.; Trouvé, G. Pyrolysis Characteristics and Kinetics of Arundo donax Using Thermogravimetric Analysis. Bioresour. Technol. 2009, 100, 4026–4031. [Google Scholar]
- Márton, L.; Czakó, M. Method for Micropropagation of Monocots Based on Sustained Totipotent Cell Cultures. EP2150100B1, 11 July 2007. [Google Scholar]
- Márton, L.; Czakó, M. Propagating Arundo Species in Nutrient Broth and on Teriary Medium Containing Auxin and Cytokinin. U.S. Patent US 7863046 B2, 7 May 2007. [Google Scholar]
- Takahashi, W.; Takamizo, T.; Kobayashi, M.; Ebina, M. Plant Regeneration from Calli in Giant Reed (Arundo donax L.). Grassl. Sci. 2010, 56, 224–229. [Google Scholar]
- Cavallaro, V.; Patanè, C.; Cosentino, S.L.; di Silvestro, I.; Copani, V. Optimizing in Vitro Large Scale Production of Giant Reed (Arundo donax L.) by Liquid Medium Culture. Biomass Bioenerg. 2014, 69, 21–27. [Google Scholar]
- Antal, G.; Kurucz, E.; Miklós, F.; Popp, J. Tissue Culture and Agamic Propagation of Winter-Frost Tolerant “Longicaulis”. Arundo donax L. Environ. Eng. Manag. J. 2014, 13, 2709–2715. [Google Scholar] [CrossRef]
- Ozudogru, E.A.; Karlik, E.; Elazab, D.; Lambardi, M. Establishment of direct organogenesis protocol for Arachis hypogaea cv. Virginia in liquid medium by temporary immersion system (TIS). Horticulturae 2022, 8, 1129–1143. [Google Scholar]


| Callus Induction Medium | % of Regenerating Explant | Density of Callus after 4 Subcultures ** | ||
|---|---|---|---|---|
| After 1st Subculture | After 2nd Subculture | After 3rd Subculture | ||
| Leaf fragments | ||||
| A1—MS + 1.1 mg/L 2,4-D | 0.0 d * | 0.0 d | 0.0 d | - |
| A2—MS + 2.2 mg/L 2,4-D | 0.0 d | 0.0 d | 0.0 d | - |
| A3—MS + 4.4 mg/L 2,4-D | 0.0 d | 0.0 d | 0.0 d | - |
| A4—MS + 1.9 mg/L NAA | 0.0 d | 0.0 d | 0.0 d | - |
| A5—MS + 1.1 mg/L 2,4-D + 0.9 mg/L NAA | 0.0 d | 0.0 d | 0.0 d | - |
| 30 cm long inflorescences, collected in May, from Sesto Fiorentino (SF, 30 cm, May) | ||||
| A1—MS + 1.1 mg/L 2,4-D | 20.0 a | 46.7 a | 53.3 a | 13.3%, +++; 20%, ++; 20%, + |
| A2—MS + 2.2 mg/L 2,4-D | 15.0 a | 14.3 b | 14.3 b | 6.7%, +++; 7.6%, + |
| A3—MS + 4.4 mg/L 2,4-D | 0.0 d | 13.3 b | 15.4 b | 7.7%, ++; 7.7%, + |
| A4—MS + 1.9 mg/L NAA | 6.7 b | 6.7 c | 6.7 c | 6.7%, +++ |
| A5—MS + 1.1 mg/L 2,4-D + 0.9 mg/L NAA | 6.7 b | 33.3 a | 33.3 a | 6.7%, +++; 6.7%, ++ 19.9%, + |
| 40 cm long inflorescences, collected in May, from Sesto Fiorentino (SF, 40 cm, May) | ||||
| A1—MS + 1.1 mg/L 2,4-D | 17.4 a | 30.8 a | 33.3 a | 8.3%, +++; 25%, + |
| A2—MS + 2.2 mg/L 2,4-D | 0.0 d | 0.0 d | 11.1 bc | 11.1%, + |
| A3—MS + 4.4 mg/L 2,4-D | 0.0 d | 8.3 b | 8.3 c | 8.3%, + |
| A4—MS + 1.9 mg/L NAA | 0.0 d | 0.0 d | 0.0 d | - |
| A5—MS + 1.1 mg/L 2,4-D + 0.9 mg/L NAA | 0.0 d | 0.0 d | 0.0 d | - |
| 50 cm long inflorescences, collected in May, from Sesto Fiorentino (SF, 50 cm, May) | ||||
| A1—MS + 1.1 mg/L 2,4-D | 0.0 d | 0.0 d | 25.0 ab | 25%, + |
| A2—MS + 2.2 mg/L 2,4-D | 0.0 d | 8.3 c | 8.3 c | 8.3%, +++ |
| A3—MS + 4.4 mg/L 2,4-D | 0.0 d | 0.0 d | 0.0 d | - |
| A4—MS + 1.9 mg/L NAA | 0.0 d | 0.0 d | 0.0 d | - |
| A5—MS + 1.1 mg/L 2,4-D + 0.9 mg/L NAA | 0.0 d | 0.0 d | 6.7 c | 6.7%, + |
| 30 cm long inflorescences, collected in October, from Sesto Fiorentino (SF, 30 cm, October) | ||||
| A1—MS + 1.1 mg/L 2,4-D | 5.5 b | 13.7 b | 15.3 b | 10.3%, +++; 5%, ++ |
| A2—MS + 2.2 mg/L 2,4-D | 2.7 c | 3.5 c | 5.0 c | 5%, + |
| A3—MS + 4.4 mg/L 2,4-D | 3.2 c | 5.0 c | 5.0 c | 5%, + |
| A4—MS + 1.9 mg/L NAA | 0.0 d | 0.0 d | 0.0 d | - |
| A5—MS + 1.1 mg/L 2,4-D + 0.9 mg/L NAA | 3.5 c | 5.0 c | 6.7 c | 6.7%, ++ |
| 20 cm long inflorescences, collected in September, from Piombino (P, 20 cm, September) | ||||
| A1—MS + 1.1 mg/L 2,4-D | 0.0 d | 10.0 b | 10.0 bc | 5%, +++; 5%, ++ |
| A2—MS + 2.2 mg/L 2,4-D | 5.0 b | 5.0 c | 5.0 c | 5%, +++ |
| A3—MS + 4.4 mg/L 2,4-D | 0.0 d | 0.0 d | 0.0 d | - |
| A4—MS + 1.9 mg/L NAA | 5.3 b | 0.0 d | 0.0 d | - |
| A5—MS + 1.1 mg/L 2,4-D + 0.9 mg/L NAA | 5.0 b | 5.0 c | 5.0 c | 5%, +++ |
| 30 cm long inflorescences, collected in September, from Piombino (P, 30 cm, September) | ||||
| A1—MS + 1.1 mg/L 2,4-D | 4.5 bc | 9.1 b | 9.7 bc | 5% +++; 4.7%, + |
| A2—MS + 2.2 mg/L 2,4-D | 0.0 d | 0.0 d | 0.0 d | - |
| A3—MS + 4.4 mg/L 2,4-D | 0.0 d | 0.0 d | 0.0 d | - |
| A4—MS + 1.9 mg/L NAA | 0.0 d | 0.0 d | 0.0 d | - |
| A5—MS + 1.1 mg/L 2,4-D + 0.9 mg/L NAA | 2.3 c | 4.5 c | 4.5 c | 2.3%, +++; 2.2%, ++ |
| Selected Callus Lines | Province of Origin/Callus Induction Medium | % of Plantlet Induction on * | |
|---|---|---|---|
| MS.0 | MS + AC | ||
| IBE/SE-1 | SF, 30 cm, May/A5 | 0.0 d | 0.0 d |
| IBE/SE-2 | SF, 40 cm, May/A1 | 0.0 d | 0.0 d |
| IBE/SE-3 | SF, 30 cm, October/A1 | 10.5 c | 16.7 c |
| IBE/SE-4 | P, 30 cm, September/A1 | 0.0 d | 0.0 d |
| IBE/SE-5 | SF, 30 cm, May/A1 | 16.7 c | 25.7 c |
| IBE/SE-6 | SF, 30 cm, May/A1 | 75.5 a | 40.7 b |
| IBE/SE-7 | SF, 30 cm, October/A1 | 25.0 c | 20.0 c |
| IBE/SE-8 | P, 30 cm, September/A5 | 52.7 b | 50.0 b |
| IBE/SE-9 | SF, 30 cm, May/A2 | 20.0 c | 0.0 d |
| IBE/SE-10 | SF, 30 cm, May/A5 | 97.1 a | 46.0 b |
| IBE/SE-11 | SF, 30 cm, May/A1 | 93.1 a | 86.1 a |
| IBE/SE-12 | SF, 50 cm, May/A2 | 81.2 a | 75.0 a |
| Selected Callus Lines | Initial Plant Weight (gr) | Final Plant Weight (gr) | RGR * |
|---|---|---|---|
| Gelled DKW multiplication medium | |||
| IBE/SE-10 | 2.74 | 22.21 | 6.77 |
| IBE/SE-11 | 2.03 | 17.86 | 7.00 |
| IBE/SE-12 | 2.42 | 19.83 | 6.80 |
| Stationary liquid DKW multiplication medium | |||
| IBE/SE-10 | 2.78 | 17.52 | 5.93 |
| IBE/SE-11 | 2.28 | 13.52 | 5.74 |
| IBE/SE-12 | 3.43 | 16.15 | 5.00 |
| Liquid DKW multiplication medium in TIS (12 min/8 h) | |||
| IBE/SE-10 | 7.21 | 86.90 | 8.03 |
| IBE/SE-11 | 7.16 | 86.92 | 8.06 |
| IBE/SE-12 | 7.05 | 87.75 | 8.12 |
| Liquid DKW multiplication medium in TIS (8 min/16 h) | |||
| IBE/SE-10 | 8.51 | 45.35 | 5.38 |
| IBE/SE-11 | 7.24 | 43.16 | 5.74 |
| IBE/SE-12 | 8.06 | 44.08 | 5.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozudogru, E.A.; Karlik, E.; Elazab, D.; Lambardi, M. Establishment of an Efficient Somatic Embryogenesis Protocol for Giant Reed (Arundo donax L.) and Multiplication of Obtained Shoots via Semi-Solid or Liquid Culture. Horticulturae 2023, 9, 735. https://doi.org/10.3390/horticulturae9070735
Ozudogru EA, Karlik E, Elazab D, Lambardi M. Establishment of an Efficient Somatic Embryogenesis Protocol for Giant Reed (Arundo donax L.) and Multiplication of Obtained Shoots via Semi-Solid or Liquid Culture. Horticulturae. 2023; 9(7):735. https://doi.org/10.3390/horticulturae9070735
Chicago/Turabian StyleOzudogru, Elif Aylin, Elif Karlik, Doaa Elazab, and Maurizio Lambardi. 2023. "Establishment of an Efficient Somatic Embryogenesis Protocol for Giant Reed (Arundo donax L.) and Multiplication of Obtained Shoots via Semi-Solid or Liquid Culture" Horticulturae 9, no. 7: 735. https://doi.org/10.3390/horticulturae9070735
APA StyleOzudogru, E. A., Karlik, E., Elazab, D., & Lambardi, M. (2023). Establishment of an Efficient Somatic Embryogenesis Protocol for Giant Reed (Arundo donax L.) and Multiplication of Obtained Shoots via Semi-Solid or Liquid Culture. Horticulturae, 9(7), 735. https://doi.org/10.3390/horticulturae9070735








