Changes in Agronomic, Antioxidant Compounds, and Morphology Parameters of Green and Red Lettuces (Lactuca sativa L.) by Successive Harvests and UV-B Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Experimental Design
2.1.1. Experiment 1: Effect of the Cutting by Harvest on Green and Red Lettuces
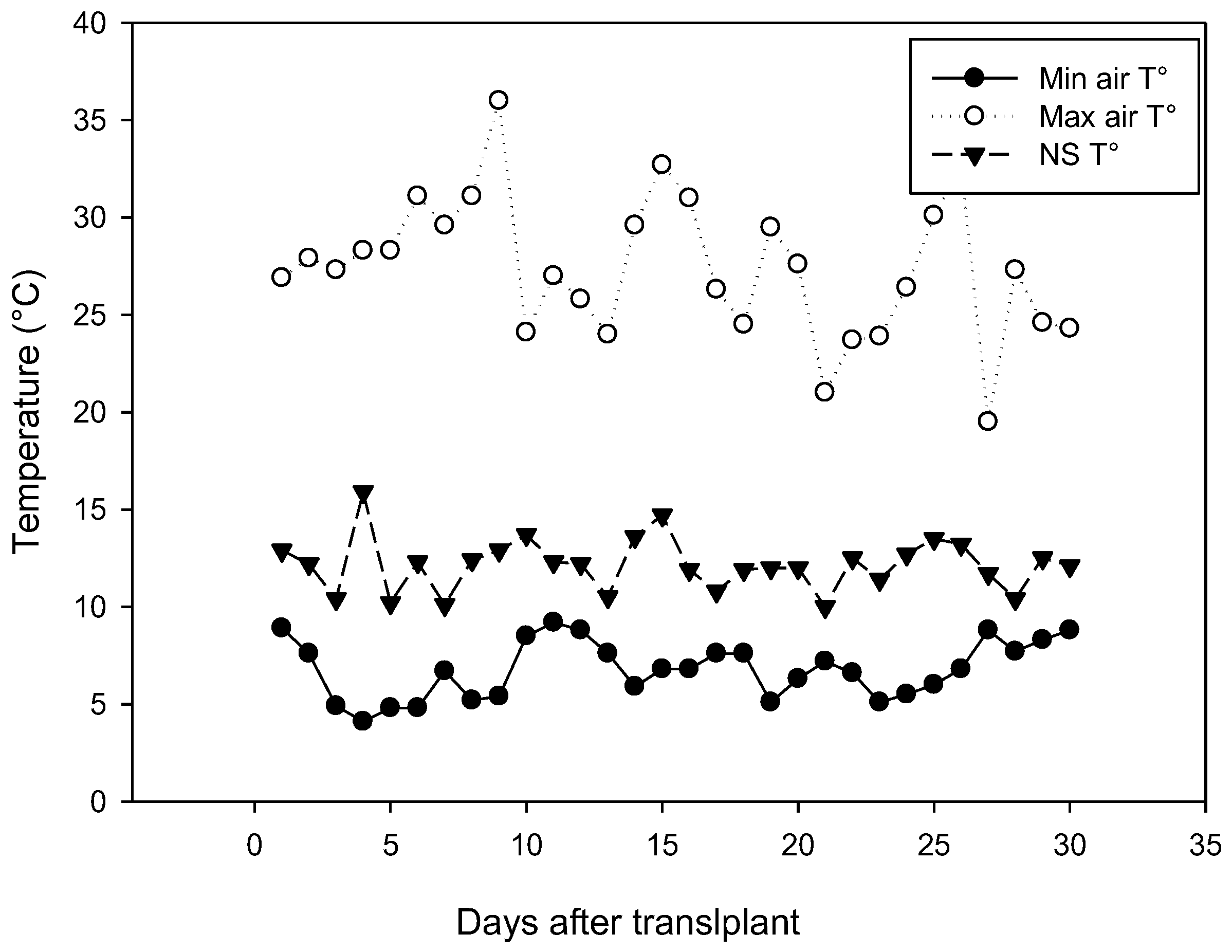
2.1.2. Experiment 2: Effect of UV-B Radiation and Successive Harvests on Green and Red Lettuces
2.2. Plant Growth Analytical Parameters
2.3. Color Parameters
2.4. Antioxidant Extraction
2.5. Total Phenolic Content (TPC)
2.6. Total Flavonoid Content (TFC)
2.7. Total Anthocyanin Content (TAC)
2.8. Antioxidant Capacity (AC)
2.9. Total Proline Content (TPrC)
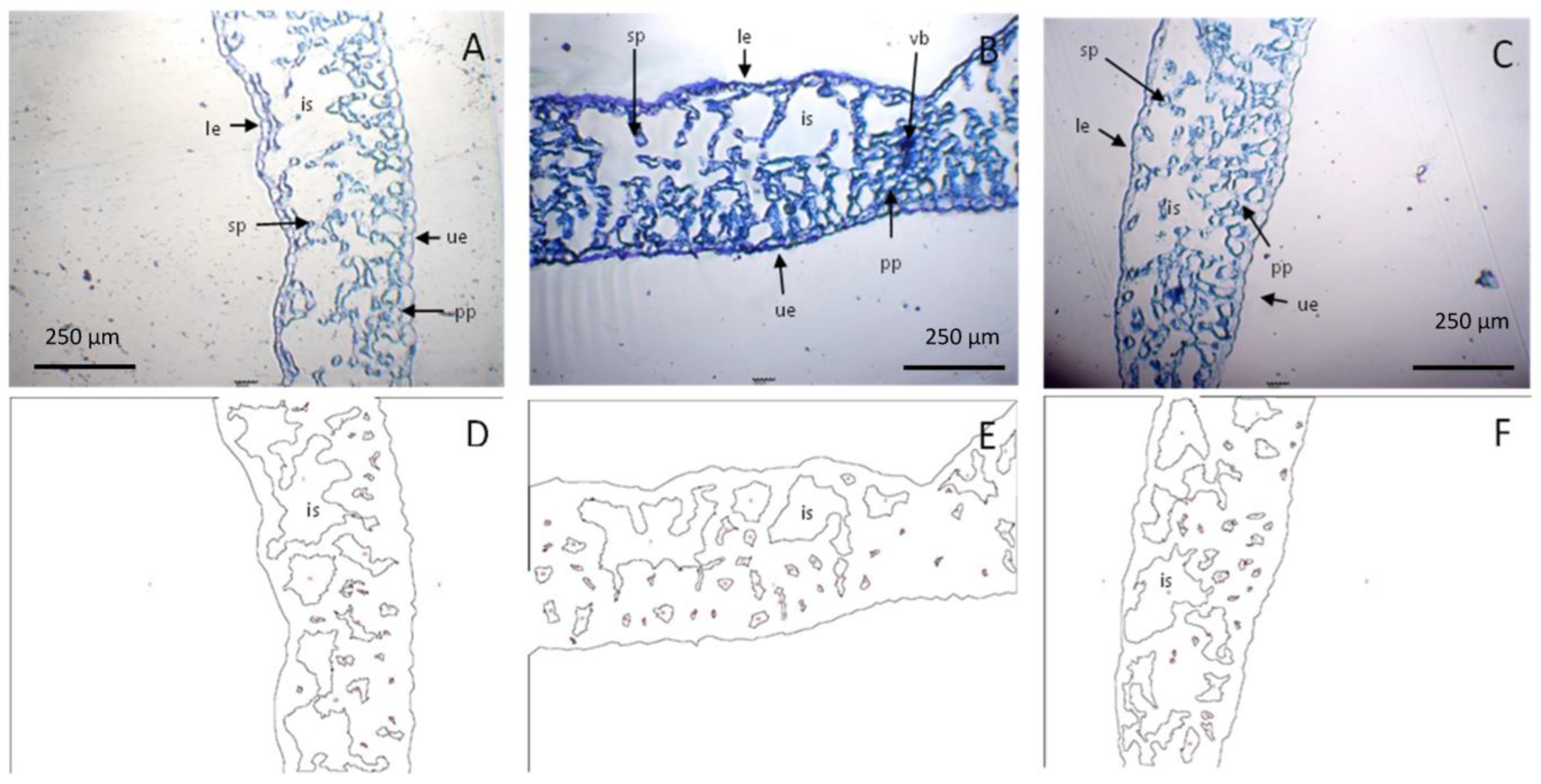
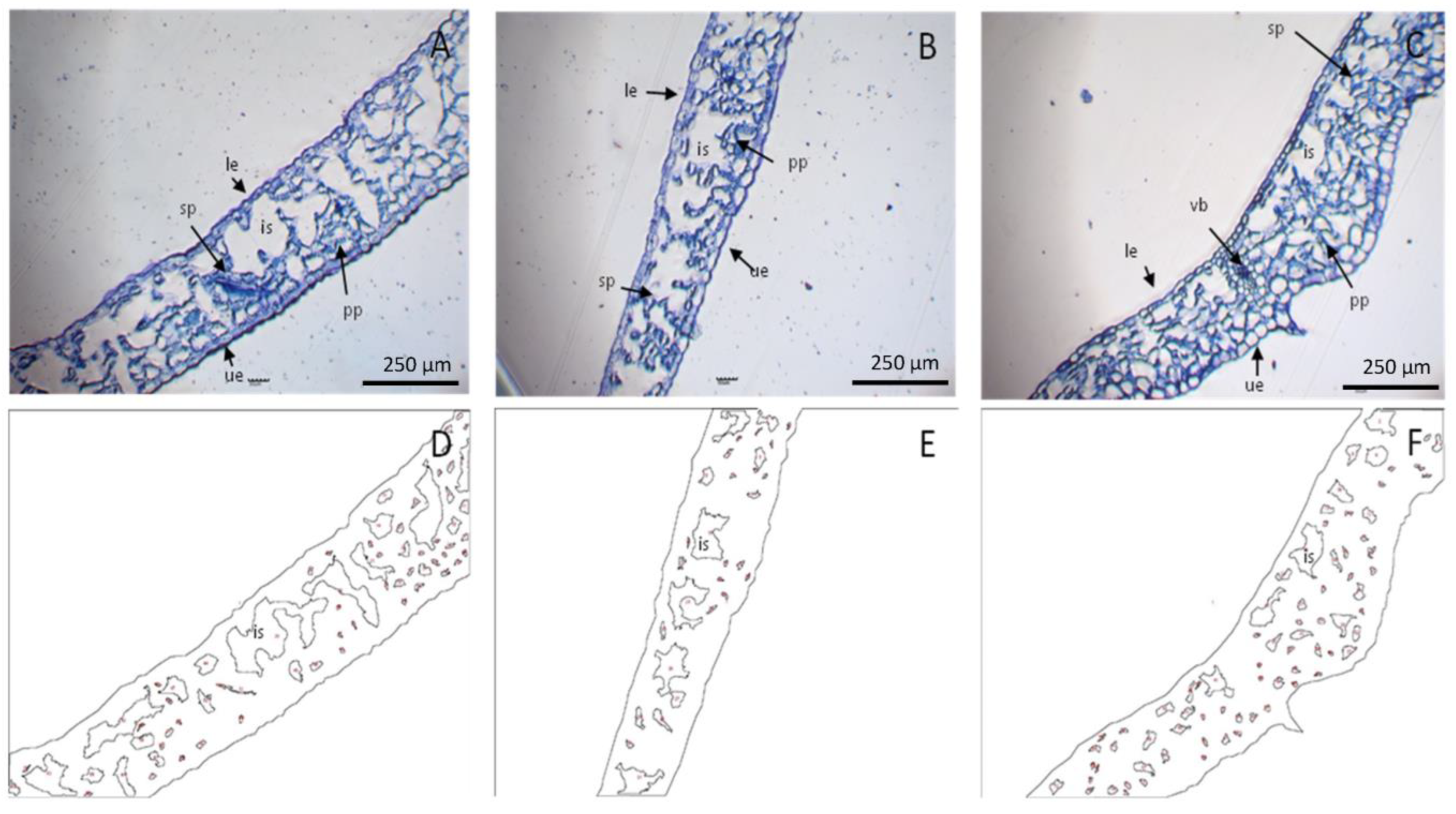
2.10. Microscopic Cell Analysis
2.10.1. Stomatal and Cellular Densities
2.10.2. Intercellular Space
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Successive Harvests on Green and Red Lettuces (Experiment 1)
3.1.1. Plant Growth Analytical Parameters
3.1.2. Color Parameters
3.1.3. Total Phenolic, Flavonoid, Anthocyanin Content, and Antioxidant Capacity
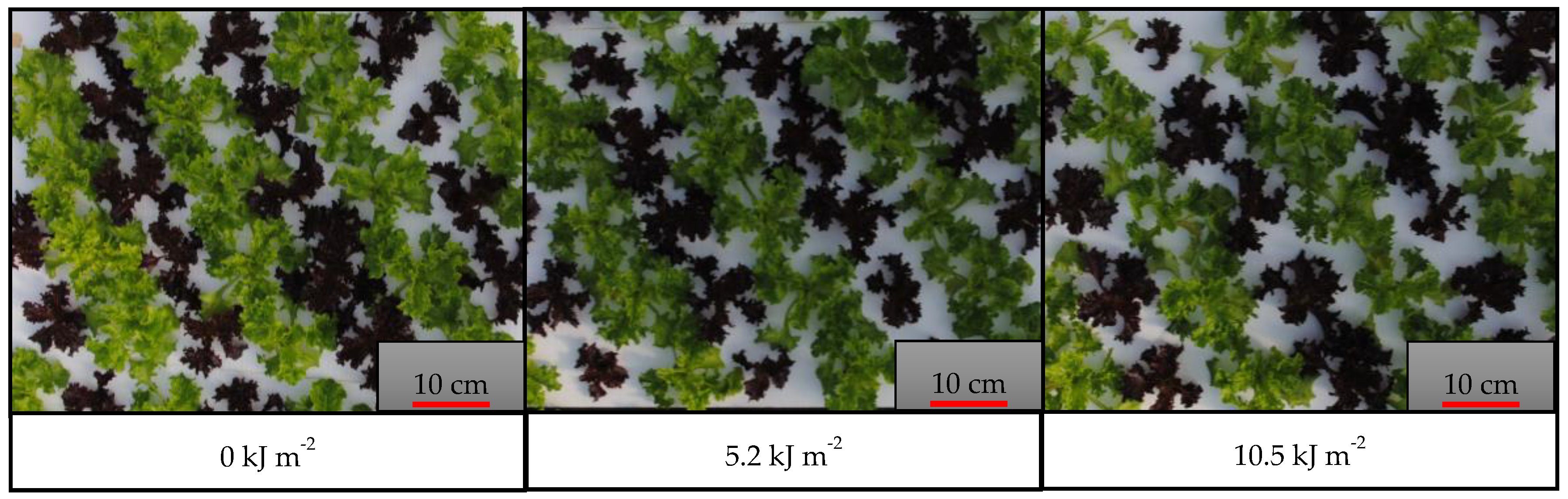
3.2. Effect of UV-B Radiation and Harvest Times on Green and Red Lettuces (Experiment 2)
3.2.1. Plant Growth Analytical Parameters
3.2.2. Color Parameters
3.2.3. Total Phenolic, Flavonoid, and Anthocyanin Contents, and Antioxidant Capacity
3.2.4. Total Proline Content
3.2.5. Stomatal, Cellular Density, and Intercellular Space
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolle, C.; Cardinault, N.; Gueux, E.; Jaffrelo, L.; Rock, E.; Mazur, A.; Amouroux, P.; Rémésy, C. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004, 23, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Amodio, M.L.; Derossi, A.; Colelli, G. Modeling phenolic content during storage of cut fruit and vegetables: A consecutive reaction mechanism. J. Food Eng. 2014, 140, 1–8. [Google Scholar] [CrossRef]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñóz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, X.; Cai, L.; Lu, X.; Ying, T.; Jiang, Z. Postharvest UV-B irradiation maintains sensory qualities and enhances antioxidant capacity in tomato fruit during storage. Postharvest Biol. Technol. 2011, 59, 232–237. [Google Scholar] [CrossRef]
- Sloan, E. Top 10 Food Trends. Food Technology. 1 April 2019. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2019/april/features/2019-top-10-food-trends (accessed on 22 January 2023).
- Mulabagal, V.; Ngouajio, M.; Nair, A.; Zhang, Y.; Gottumukkala, A. In vitro evaluation of red and green lettuce (Lactuca sativa) for functional food properties. Food Chem. 2010, 118, 300–306. [Google Scholar] [CrossRef]
- Kim, M.; Moon, Y.; Tou, J.; Mou, B.; Waterland, N. Nutritional value, bioactive compounds, and health benefits of lettuce (Lactuca sativa L.). J. Food Comp. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, C.Y. Comprehensive Study on Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Various Polyphenolics in Scavenging a Free Radical and its Structural Relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and DPPH* radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT Food Sci. Technol. 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Terfa, M.T.; Roro, A.G.; Olsen, J.E.; Torré, S. Effects of UV radiation on growth and postharvest characteristics of three pot rose cultivars grown at different altitudes. Sci. Hortic. 2014, 178, 184–191. [Google Scholar] [CrossRef]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds, and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hort. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X.L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hort. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations-perspectives for applications in horticulture. Environ. Exper. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Nocchi, N.; Monteiro, H.; Crespo, R.; Ungaretti, T.; Ribeiro, A. Effects of UV-B radiation on secondary metabolite production, antioxidant activity, photosynthesis, and herbivory interactions in Nymphoides humboldtiana (Menyanthaceae). J. Photochem. Photobiol. B Biol. 2020, 212, 112021. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Changing scenario in plant UV-B research:UV-B from a generic stressor to a specific regulator. J. Photochem. Photobiol. B Biol. 2015, 153, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Hectors, K.; O’Brien, N.; Guisez, Y.; Potters, G. Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Sci. 2008, 175, 449–458. [Google Scholar] [CrossRef]
- Ebisawa, M.; Shoiji, K.; Kato, M.; Shimomura, K.; Goto, F.; Yoshihara, T. Supplementary ultraviolet radiation B together with blue light at night increased quercetin content and flavonol synthase gene expression in leaf lettuce (Lactuca sativa L.). Environ. Control Biol. 2008, 46, 1–11. [Google Scholar] [CrossRef]
- Xu, C.; Natarajan, S.; Sullian, J.H. Impact of solar ultraviolet-B radiation on the antioxidant defense system in soybean lines differing in flavonoid contents. Environ. Exp. Bot. 2008, 63, 39–48. [Google Scholar] [CrossRef]
- Brown, B.A.; Cloix, C.; Jiang, G.H.; Kaiserli, E.; Herzyk, P.; Kliebenstein, D.J.; Jenkins, G.I. A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA 2005, 102, 18225–18230. [Google Scholar] [CrossRef]
- Larson, R. The antioxidants of higher plants. Phytochemistry 1988, 27, 969–978. [Google Scholar] [CrossRef]
- Wargent, J.; Elfadly, E.; Moore, J.; Paul, N.D. Increased exposure to UV-B radiation during early development leads to enhanced photoprotection and improved long-term performance in Lactuca sativa. Plant Cell Environ. 2011, 34, 1401–1413. [Google Scholar] [CrossRef]
- Coffey, A.; Prinsen, E.; Jansen, M.A.K.; Conway, J. The UVB photoreceptor UVR8 mediates accumulation of UV absorbing pigments, but not changes in plant morphology, under outdoor conditions. Plant Cell Environ. 2017, 40, 2250–2260. [Google Scholar] [CrossRef]
- Ioannidis, D.; Bonner, L.; Johnson, C. UV-B is Required for Normal Development of Oil Glands in Ocimum basilicum L. (Sweet Basil). Ann. Bot. 2002, 90, 453–460. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef]
- Eichholz, I.; Rohn, S.; Gamm, A.; Beesk, N.; Herppich, W.B.; Kroh, L.W. UV-B-mediated flavonoid synthesis in white asparagus (Asparagus officinalis L.). Food Res. Int. 2012, 48, 196–201. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Henbest, R.G.C.; Davis, N.H.; Hadley, P.; Wagstaffe, A. UV irradiance as a major influence on growth, development, and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Sakalauskaité, J.; Viskelis, P.; Dambrauskiene, E.; Sakalauskiené, S.; Samuoliené, G.; Brazaityte, A.; Duchovskis, P.; Urbonavičienė, D. The effects of different UVB radiation intensities on morphological and biochemical characteristics in Ocimum basilicum L. J. Sci. Food. Agric. 2012, 93, 1266–1271. [Google Scholar] [CrossRef]
- Staxén, I.; Bornman, J. A morphological and cytological study of Petunia hybrida exposed to UV-B radiation. Physiol. Plant. 1994, 91, 735–740. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet-B radiation: A review. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Hollosy, F. Effects of ultraviolet radiation on plant cells. Micron 2002, 33, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, O.; Tabay, D.; Esringu, A.; Icoglu Aksakal, F.; Esim, N. Effect of Proline on Biochemical and Molecular Mechanism in Lettuce (Lactuca sativa L.) Exposed to UV-B radiation. Photochem. Photobiol. Sci. 2016, 16, 246–254. [Google Scholar] [CrossRef]
- Bi, J.L.; Felton, G.W. Foliar oxidative stress and insect herbivory: Primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J. Chem. Ecol. 1995, 21, 1511–1530. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Usha Rami, P.; Jyothsna, Y. Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol. Plant. 2010, 32, 695–704. [Google Scholar] [CrossRef]
- Flores, M.; Amóros, A.; Escalona, V.H. Effect of NaCl and harvest time on antioxidant compounds and morphological cell changes in Lollo Bionda and Lollo Rosso lettuces. Chilean JAR 2022, 82, 537–551. [Google Scholar] [CrossRef]
- Sonneveld, C.; Straver, N. Nutrient Solutions for Vegetables and Flowers Grown in Water or Substrates, 10th ed.; PBG: Aalsmeer, The Netherlands; Naaldwijk, The Netherlands, 1994. [Google Scholar]
- McGuire, R. Reporting of Objective Color Measurements. Hort. Sci. 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Tharasena, B.; Lawan, S. Phenolics, flavonoids and antioxidant activity of vegetables as Thai side dish. APCBEE Procedia 2014, 8, 99–104. [Google Scholar] [CrossRef]
- Du, W.X.; Avena-Bustillos, R.; Breksa, A.; McHugh, T. UV-B light as a factor affecting total soluble phenolic contents of various whole and fresh-cut specialty crops. Postharvest Biol. Technol. 2014, 93, 72–82. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterization of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Gupta, S.; Prakash, J. Studies on Indian Green Leafy Vegetables for Their Antioxidant Activity. Plant Foods Hum. Nutr. 2009, 64, 39–45. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, R.; Qu, Y.; Miao, Z.; Zhang, Y.; Shen, X.; Wang, T.; Dong, J. Overexpression of MtCAS31 enhances drought tolerance in transgenic Arabidopsis by reducing stomatal density. New Phytol. 2012, 195, 124–135. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Sela, N.; Feygenberg, O.; Zemach, H.; Maurer, D.; Alkan, N. Transcriptome dynamics in mango fruit peel reveals mechanisms of chilling stress. Front. Plant Sci. 2016, 7, 1579. [Google Scholar] [CrossRef]
- Sumner, M.J. Epoxy resins for light and transmission electron microscopy. In Plant Micro Techniques and Protocols; Yeung, E.C.T., Stasolla, C., Summer, M., Huang, B., Eds.; Springer: Cham, Switzerland, 2015; pp. 83–101. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión; Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Córdoba, Argentina. 2017. Available online: http://www.infostat.com.ar (accessed on 22 January 2023).
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoran (Origanum majorana L.). Ind. Crops Prod. 2009, 30, 95–402. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.; Aguilar, A.; Ferreira, I. Changes in macro minerals, trace elements and pigments content during lettuce (Lactuca sativa L.) growth: Influence of soil composition. Food Chem. 2014, 152, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, U.; Pinzino, C.; Quartacci, F.; Ranieri, A.; Sgherri, C. Phenolic Composition and Related Antioxidant Properties in Differently Colored Lettuces: A Study by Electron Paramagnetic Resonance (EPR) Kinetics. J. Agric. Food Chem. 2014, 62, 12001–12007. [Google Scholar] [CrossRef]
- Sgherri, C.; Pérez-López, U.; Micaelli, F.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A.; Quartacci, M.F. Elevated CO2 and salinity are responsible for phenolics-enrichment in two differently pigmented lettuces. Plant Physiol. Biochem. 2017, 115, 269–278. [Google Scholar] [CrossRef]
- Zlotek, U.; Swieca, M.; Jakubczyk, A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.). Food Chem. 2014, 148, 253–260. [Google Scholar] [CrossRef]
- Gazula, A.; Kleinhenz, M.; Scheerens, J.; Ling, P. Anthocyanin Levels in Nine Lettuce (Lactuca sativa) Cultivars: Influence of Planting Date and Relations among Analytic, Instrumented, and Visual Assessments of Color. HortScience 2007, 42, 232–238. [Google Scholar] [CrossRef]
- Zhao, X.; Carey, E.; Young, J.; Wang, W.; Iwamoto, T. Influences of organic fertilization, high tunnel environment, and postharvest storage on phenolic compounds in lettuce. HortScience 2007, 42, 71–76. Available online: http://hortsci.ashspublications.org/content/42/1/71.full (accessed on 22 January 2023). [CrossRef]
- Krizek, D. Influence of PAR and UV-A in Determining Plant Sensitivity and Photomorphogenic Responses to UV-B Radiation. Photochem. Photobiol. 2004, 79, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.; Brosché, M.; Vainonen, J.; Jenkins, G.; Wargent, J.; Sipari, N.; Strid, A.; Lindfors, A.; Tegelberg, R.; Aphalo, P. Multiple Roles for UV RESISTANCE LOCUS8 in Regulating Gene Expression and Metabolite Accumulation in Arabidopsis under Solar Ultraviolet Radiation. Plant Physiol. 2013, 161, 744–759. [Google Scholar] [CrossRef]
- Caldwell, C. Alkylperoxyl radical scavenging activity of red leaf lettuce (Lactuca sativa L.) phenolics. J. Agric. Food Chem. 2003, 51, 4589–4595. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Chadzinikolau, T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci. 2013, 213, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Bernardo, L. Comparison of proteome response to saline and zinc stress in lettuce. Front. Plant Sci. 2015, 6, 240. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Zdunek-Zastocka, E.; Grabowska, A.; Michniewska, B.; Orzechowski, S. Proline concentration and its metabolism are regulated in a leaf age dependent manner but not by abscisic acid in pea plants exposed to cadmium stress. Cells 2021, 10, 946. [Google Scholar] [CrossRef]
| Factor | Level | FW (g) | DW (%) | ||
|---|---|---|---|---|---|
| ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ||
| Harvest time (1) | 1st | (7.82) | (6.72) | (9.04) | (8.54) |
| 2nd | 18.93 | 14.6 | 6.80 b | 7.11 | |
| 3rd | 20.31 | 18.98 | 8.64 a | 7.12 | |
| Cutting (2) | With (w) | 16.58 b | 12.15 | 7.93 | 7.53 |
| Without (wo) | 22.66 a | 21.43 | 7.50 | 6.70 | |
| 1·2 | 2nd·w | 16.55 | 11.87 c | 7.06 | 7.23 b |
| 3rd·w | 16.61 | 12.42 c | 8.80 | 7.84 a | |
| 2nd·wo | 21.31 | 17.32 b | 6.54 | 6.99 b | |
| 3rd·wo | 24.00 | 25.53 a | 8.47 | 6.41 c | |
| p-value 1 | ns | ** | *** | ns | |
| p-value 2 | *** | *** | ns | *** | |
| p-value 1·2 | ns | ** | ns | ** | |
| Factor | Level | Luminosity | Chroma | Hue | |||
|---|---|---|---|---|---|---|---|
| ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ||
| Harvest time (1) | 1st | (46.47) | (4.80) | (51.11) | (17.49) | (106.23) | (22.54) |
| 2nd | 48.27 b | 6.32 | 49.07 a | 18.90 | 105.28 b | 25.14 | |
| 3rd | 52.54 a | 11.21 | 46.73 b | 19.92 | 108.24 a | 42.03 | |
| Cutting (2) | With (w) | 48.54 | 12.25 | 49.05 | 16.73 b | 106.76 | 33.61 |
| Without (wo) | 49.65 | 5.29 | 48.90 | 22.09 a | 106.40 | 26.19 | |
| 1·2 | 2nd·w | 47.62 | 6.05 b | 47.85 | 16.42 | 105.06 | 22.79 |
| 3rd·w | 51.53 | 4.52 b | 47.73 | 17.03 | 107.92 | 33.24 | |
| 2nd·wo | 48.93 | 6.60 b | 50.29 | 21.38 | 105.51 | 27.49 | |
| 3rd·wo | 53.55 | 17.09 a | 45.74 | 22.81 | 108.55 | 50.82 | |
| p-value 1 | *** | *** | *** | ns | *** | ns | |
| p-value 2 | ns | *** | ns | *** | ns | ns | |
| p-value 1·2 | ns | *** | ns | ns | ns | ns | |
| Factor | Level | Phenolics (TPC, mg GAE 100g−1 FW) | Flavonoids (TFC, mg Rut eq 100g−1 FW) | Anthocyanins (TAC, mg Cyn3gluc eq 100g−1 FW) | FRAP (mg Trolox eq 100 g−1 FW) | DPPH (mg Trolox eq 100 g−1 FW) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ||
| Harvest time (1) | 1st | (1811.16) | (2944.71) | (953.04) | (1464.47) | (5.81) | (469.64) | (787.49) | (163.05) | (321.33) |
| 2nd | 1280.44 b | 2706.75 | 737.69 b | 1398.22 | 5.41 | 334.97 | 752.39 | 131.21 b | 269.36 | |
| 3rd | 1654.15 a | 2774.52 | 1029.72 a | 1336.71 | 6.06 | 414.58 | 801.63 | 159.95 a | 271.91 | |
| Cutting (2) | With (w) | 1532.54 | 3057.98 | 928.39 | 1561.53 | 6.44 a | 389.40 | 797.52 | 146.53 | 278.66 |
| Without (wo) | 1402.06 | 2423.30 | 839.02 | 1173.39 | 5.04 b | 360.14 | 756.50 | 144.62 | 262.61 | |
| 1·2 | 2nd·w | 1362.19 | 2821.10 b | 797.06 | 1471.56 b | 5.89 | 326.93 b | 732.91 b | 132.51 | 266.74 c |
| 3rd·w | 1702.89 | 3294.86 a | 1059.71 | 1651.51 a | 7.00 | 451.88 a | 862.13 a | 160.55 | 290.59 a | |
| 2nd·wo | 1198.70 | 2592.41 b | 678.32 | 1324.87 c | 4.94 | 343.01 b | 771.87 ab | 129.90 | 271.99 b | |
| 3rd·wo | 1605.42 | 2254.19 c | 999.72 | 1021.90 d | 5.13 | 377.28 b | 741.13 b | 159.34 | 253.23 d | |
| p-value 1 | ** | ns | *** | ns | ns | *** | ns | *** | *** | |
| p-value 2 | ns | *** | ns | *** | * | ns | ns | ns | ns | |
| p-value 1·2 | ns | *** | ns | *** | ns | * | * | ns | *** | |
| Factor | Level | FW (g) | DW (%) | ||
|---|---|---|---|---|---|
| ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ||
| Harvest time (1) | 1st | 4.64 c | 3.34 b | 6.55 a | 5.80 b |
| 2nd | 9.55 b | 4.84 a | 6.01 b | 5.83 b | |
| 3rd | 15.52 a | 4.66 a | 6.72 a | 7.46 a | |
| UV-B Doses (2) | 0 kJ m−2 | 10.93 a | 4.09 | 6.30 b | 6.30 |
| 5.2 kJ m−2 | 10.12 b | 4.33 | 6.36 b | 6.38 | |
| 10.5 kJ m−2 | 8.66 c | 4.42 | 6.62 a | 6.41 | |
| p-value 1 | *** | *** | *** | *** | |
| p-value 2 | *** | ns | ** | ns | |
| p-value 1·2 | ns | ns | ns | ns | |
| Factor | Level | Luminosity | Chroma | Hue | |||
|---|---|---|---|---|---|---|---|
| ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ||
| Harvest time (1) | 1st | 40.74 | 1.78 a | 50.03 c | 9.51 a | 105.74 a | 16.27 |
| 2nd | 42.15 | 0.94 b | 55.82 a | 5.06 b | 104.54 b | 15.59 | |
| 3rd | 40.43 | 1.89 b | 52.87 b | 8.78 a | 104.75 b | 15.22 | |
| UV-B Doses (2) | 0 kJ m−2 | 42.75 a | 1.61 | 52.69 | 8.63 | 105.10 | 15.96 |
| 5.2 kJ m−2 | 40.55 b | 1.74 | 53.82 | 7.83 | 105.08 | 15.87 | |
| 10.5 kJ m−2 | 40.02 b | 1.28 | 52.20 | 6.89 | 104.87 | 15.26 | |
| p-value 1 | ns | ** | *** | *** | *** | ns | |
| p-value 2 | ** | ns | ns | ns | ns | ns | |
| p-value 1·2 | ns | ns | ns | ns | ns | ns | |
| Factor | Level | Phenolics (TPC, mg GAE 100 g−1 FW) | Flavonoids (TFC, mg Rut eq 100 g−1 FW) | Anthocyanins (TAC, mg Cyn3gluc eq 100 g−1 FW) | FRAP (mg Trolox eq 100 g−1 FW) | DPPH (mg Trolox eq 100 g−1 FW) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ||
| Harvest time (1) | 1st | 207.04 b | 430.85 c | 692.18 | 1130.18 c | 5.22 c | 273.63 b | 891.91 b | 239.05 b | 289.25 c |
| 2nd | 222.70 b | 505.72 b | 813.02 | 1595.79 b | 7.43 b | 305.09 b | 782.91 b | 267.59 b | 334.77 b | |
| 3rd | 271.91 a | 650.73 a | 1069.67 | 2214.71 a | 8.61 a | 398.84 a | 1075.08 a | 333.70 a | 419.71 a | |
| UV-B Doses (2) | 0 kJ m−2 | 217.99 | 500.69 | 776.92 | 1522.25 b | 6.57 b | 310.88 | 893.34 | 256.35 | 318.34 b |
| 5.2 kJ m−2 | 247.40 | 537.35 | 906.07 | 1681.16 a | 7.63 a | 342.36 | 929.15 | 300.07 | 351.81 a | |
| 10.5 kJ m−2 | 236.26 | 549.26 | 891.89 | 1737.27 a | 7.06 ab | 324.31 | 927.41 | 283.91 | 373.58 a | |
| 1·2 | 1st·0 | 198.16 | 407.10 | 657.92 de | 993.95 | 5.02 | 261.97 | 873.00 | 223.98 | 257.33 |
| 2nd·0 | 219.44 | 484.58 | 792.31 cde | 1560.72 | 6.85 | 316.16 | 757.57 | 267.26 | 314.37 | |
| 3rd·0 | 236.37 | 610.38 | 880.54 bc | 2012.09 | 7.84 | 354.52 | 1049.44 | 277.82 | 383.31 | |
| 1st·5.2 | 232.81 | 430.78 | 805.61 cde | 1142.24 | 5.37 | 314.62 | 904.55 | 270.52 | 284.99 | |
| 2nd·5.2 | 228.13 | 515.23 | 841.10 cd | 1615.40 | 8.01 | 315.40 | 805.32 | 279.16 | 339.48 | |
| 3rd·5.2 | 281.25 | 666.04 | 1071.48 ab | 2285.83 | 9.51 | 397.06 | 1077.59 | 350.54 | 430.97 | |
| 1st·10.5 | 190.15 | 454.68 | 613.02 e | 1254.33 | 5.29 | 244.30 | 898.20 | 222.64 | 325.43 | |
| 2nd·10.5 | 220.52 | 517.33 | 805.65 cde | 1611.25 | 7.43 | 283.71 | 785.83 | 256.35 | 350.47 | |
| 3rd·10.5 | 298.10 | 675.77 | 1257.0 a | 2346.21 | 8.46 | 444.93 | 1098.20 | 372.74 | 444.85 | |
| p-value 1 | *** | *** | *** | *** | *** | *** | ** | *** | *** | |
| p-value 2 | ns | ns | ns | * | * | ns | ns | ns | ** | |
| p-value 1·2 | ns | ns | * | ns | ns | ns | ns | ns | ns | |
| Factor | Level | Proline (µg·100 g−1 FW) | |
|---|---|---|---|
| ‘Levistro’ | ‘Carmoli’ | ||
| Harvest time (1) | 1st | 52.65 a | 14.82 a |
| 2nd | 13.46 b | 7.56 b | |
| 3rd | 16.02 b | 7.04 b | |
| UV-B Doses (2) | 0 kJ·m−2 | 26.24 | 9.95 |
| 5.2 kJ·m−2 | 28.86 | 10.48 | |
| 10.5 kJ·m−2 | 27.02 | 9.00 | |
| p-value 1 | *** | *** | |
| p-value 2 | ns | ns | |
| p-value 1·2 | ns | ns | |
| Factor | Level | Stomatal Density Stomata mm−2 | Cellular Density Cell mm−2 | Stomatal Index | |||
|---|---|---|---|---|---|---|---|
| ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ‘Levistro’ | ‘Carmoli’ | ||
| Harvest time (1) | 1st | 44.8 | 58.0 | 432.7 c | 715.4 b | 9.4 a | 8.0 a |
| 2nd | 45.6 | 51.4 | 546.3 b | 923.4 a | 7.8 b | 5.3 b | |
| 3rd | 59.7 | 54.7 | 682.2 a | 719.5 b | 8.1 b | 7.2 a | |
| UV-B Doses (2) | 0 kJ m−2 | 43.9 | 51.4 b | 527.2 b | 695.5 b | 7.8 | 7.1 a |
| 5.2 kJ m−2 | 50.6 | 63.0 a | 497.4 b | 746.0 b | 9.2 | 8.0 a | |
| 10.5 kJ m−2 | 55.5 | 49.7 b | 636.6 a | 916.8 a | 8.2 | 5.4 b | |
| 1·2 | 1st·0 | 44.8 bc | 59.7 | 455.1 | 567.0 | 8.9 | 9.5 |
| 2nd·0 | 42.3 bc | 44.8 | 489.9 | 850.5 | 7.9 | 4.9 | |
| 3rd·0 | 44.8 bc | 49.7 | 636.6 | 669.0 | 6.6 | 6.9 | |
| 1st·5.2 | 47.2 bc | 59.7 | 397.9 | 666.5 | 10.6 | 8.4 | |
| 2nd·5.2 | 37.3 c | 64.7 | 457.6 | 937.5 | 7.6 | 6.6 | |
| 3rd·5.2 | 67.1 a | 64.7 | 636.6 | 634.1 | 9.3 | 9.0 | |
| 1st·10.5 | 42.3 bc | 54.7 | 445.1 | 912.7 | 8.7 | 6.2 | |
| 2nd·10.5 | 57.2 ab | 44.8 | 691.3 | 982.3 | 7.9 | 4.5 | |
| 3rd·10.5 | 67.1 a | 49.8 | 773.4 | 855.4 | 8.2 | 5.6 | |
| p-value 1 | ** | ns | *** | *** | * | *** | |
| p-value 2 | ns | * | *** | *** | ns | *** | |
| p-value 1·2 | * | ns | ns | ns | ns | ns | |
| Factor | Level | Intercellular Space (% Total Area) | |
|---|---|---|---|
| ‘Levistro’ | ‘Carmoli’ | ||
| UV-B Doses | 0 kJ m−2 | 24.02 b | 17.17 a |
| 5.2 kJ m−2 | 19.57 b | 16.17 a | |
| 10.5 kJ m−2 | 37.07 a | 8.25 b | |
| p-value | *** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, M.; Amorós, A.; Escalona, V.H. Changes in Agronomic, Antioxidant Compounds, and Morphology Parameters of Green and Red Lettuces (Lactuca sativa L.) by Successive Harvests and UV-B Supplementation. Horticulturae 2023, 9, 677. https://doi.org/10.3390/horticulturae9060677
Flores M, Amorós A, Escalona VH. Changes in Agronomic, Antioxidant Compounds, and Morphology Parameters of Green and Red Lettuces (Lactuca sativa L.) by Successive Harvests and UV-B Supplementation. Horticulturae. 2023; 9(6):677. https://doi.org/10.3390/horticulturae9060677
Chicago/Turabian StyleFlores, Mónica, Asunción Amorós, and Víctor Hugo Escalona. 2023. "Changes in Agronomic, Antioxidant Compounds, and Morphology Parameters of Green and Red Lettuces (Lactuca sativa L.) by Successive Harvests and UV-B Supplementation" Horticulturae 9, no. 6: 677. https://doi.org/10.3390/horticulturae9060677
APA StyleFlores, M., Amorós, A., & Escalona, V. H. (2023). Changes in Agronomic, Antioxidant Compounds, and Morphology Parameters of Green and Red Lettuces (Lactuca sativa L.) by Successive Harvests and UV-B Supplementation. Horticulturae, 9(6), 677. https://doi.org/10.3390/horticulturae9060677






