Abstract
This paper presents the results of breeding and genetic studies of Raphanus sativus L. under the controlled conditions of the biopoligon of the Agrophysical Research Institute (St. Petersburg, Russia). The aim of this study was to create new R. sativus forms for controlled environments with artificial lightning (CEAL). An original technique for accelerated transgressive plant breeding was used. It is based on the methodology for predicting transgressions by economically valuable plant traits when evaluating breeding traits under controlled conditions. Using it in a short period of time (4–5 years) greatly increases productive accessions of small radish and radish adapted to light culture, and a set of valuable characteristics (resistance to bolting, glabrous leaves, compact leaf rosette) were obtained. The yield of roots of new forms was 4.30–4.98 kg/m2 (small radish) and 5.46–7.66 kg/m2 (radish) for the growing period. Transgression by root mass was observed in plants of three new forms and amounted to 40.7–63.0% in small radish and 40.3% in radish. The breeding of new genotypes of R. sativus is aimed at expanding the range of crops cultivated in plant factories, and ultimately, at a better and more balanced nutrition for the population.
1. Introduction
The relevance of the research is associated with the growing role of artificial climate facilities in crop production and the need for the best adaptation of grown crops to new technologies. The demand of improvement of existing and integrate new technologies in agriculture is conditioned by the development of human society, including population growth and large-scale urbanization, as well as global climate change, which has affected all regions of the world in recent decades. The threat of climate risks associated with crop loss is increasing everywhere [1,2,3], and, according to analysts, can lead to a significant reduction of crop quantity and quality. Due to this, technologies associated with crop production in fully controlled conditions, isolated from the influence of the external environment, are among the most dynamically developing [4,5].
Currently, automated plant factories of various types are used for plant cultivation and crop production in many countries. At the same time, production under the controlled environment with artificial light (CEAL, light culture) is constantly developing and improving, both in the field of technology and the range of cultivated crops [6,7,8,9]. However, significant capital and operating costs are still the main obstacle to the rapid spread and development of plant factories. The production of different crops in CEAL requires the modern high-tech and expensive equipment for control and monitoring, as well as significant electricity costs for illumination and air conditioning [7,10]. This is one of the reasons why the range of crops grown in CEAL is still quite limited: it is determined primarily by economic efficiency. These are predominantly fast-growing green leafy vegetables, herbs, microgreens, and some fruiting crops [11,12,13,14]. The development and improvement of CEAL technologies requires the expansion of the range of crops and an increase in their adaptation to the controlled environmental conditions. This can be achieved through suitable selection and breeding of relevant varieties to perform in the artificial light culture [12,15,16].
Traditionally, the breeding of cultivars and hybrids available on the seed market was carried out for open or protected ground, under conditions that are very different from those in CEAL. So, in the open field, plants need resistance to the action of various abiotic and biotic stressors, and it is one of the target traits in breeding programs; however, controlled conditions require other traits and properties for selection and breeding. This is probably the reason for the decrease in potential yield and quality among many of these cultivars in plant factories [15,16,17]. Breeding and genetic studies are particularly relevant today, as they are aimed at creating new forms of agricultural crops for CEAL, taking into account its characteristics and the needs of producers [15,16]. For this purpose, phenotyping of collections, qualitative and quantitative assessment of the accessions, and identification of breeding-valuable traits sources are currently being carried out in light culture [5,18,19,20].
Currently, under controlled conditions, using specially selected growing regimes that accelerate the generative development of plants, genetic breeding studies of various crops (Cereals, Oilseeds, Legumes, and Vegetable and Fruit Crops) are being carried out. CAEL allows researchers to conduct a number of key breeding stages regardless of the season, while significantly reducing the amount of breeding material being worked. Moreover, the time required to create new lines and cultivars is reduced by two or more times [21,22,23,24,25]. Under controlled conditions of light culture, it is possible to most accurately identify the key QTL responsible for the manifestation of economically valuable traits as well as the change in their localization in the genome under changing growing conditions [19,26,27]. Thus, the use of CEAL in the breeding process now serves as a powerful tool for its increasing speed and efficiency.
Radish and small radish (Raphanus sativus L.) belong to the extremely diverse and numerous Brassicaceae family, different forms of which are used in the food and pharmaceutical industries. Crops of the R. sativus are grown in large areas in Asia, Europe, America, and Australia, mainly on open ground. Small radish, a mutant dwarf form of radish, is also commercially grown in greenhouses [20,28]. Radish and small radish are important components of a balanced healthy diet of the human population. Radish is consumed almost all over the world because of its fleshy edible roots that are used in fresh salads, as well as salted, pickled, canned, and dried, and added to soups and sauces or stewed. In addition, the young leaves are cooked and used as leafy greens [29]. The roots of the R. sativus have a high antioxidant potential and contain a significant amount of biologically active compounds: vitamins, polyphenols, terpenoids, glucosinolates (GLSs), and breakdown products GLSs, as well as mineral elements (potassium, calcium, etc.) [20,30,31]. Usually, their content in leaves significantly exceeds that in roots [31,32]. It has been shown that young leaves of small radish and radish are rich in protein, amino acids, fiber, fatty acids, ascorbic acid, calcium, magnesium, and potassium. The leaves also contain a large number of compounds with antioxidant properties: polyphenols such as phenolic acids, flavonoids (isoflavones, flavonones, anthocyanins), as well as glucosinolates and phytosterols [30,32,33,34]. It is well known that such compounds have hepatoprotective, antitumor, and hypoglycemic properties [33,35,36,37]. It was demonstrated that the antioxidant activity of red radish leaves was three times higher than that of its roots [34]. Due to their valuable biochemical composition, young radish leaves can serve as components of a healthy diet in fresh salads and other culinary products, as well as raw materials for the production of nutraceuticals [29,30,33,38].
The morphological characteristics of the radish vary greatly between different accessions. R. sativus is extremely diverse in terms of root shape, weight, and color, as a size and characteristic of leaf rosette [29]. It is used in research programs for genetic improvement and adaptation of new accessions to the formed environmental conditions [29,39]. Small radish and compact, early ripening forms of radish are of great interest for production under light culture conditions. They have all the necessary characteristics that are required for crops grown in CEAL on multitiered growing systems: a short crop season, a compact plant size, and high nutritional properties. These reasons led to the choice of R. sativus as the object of our investigation, aimed at creating new forms of plants in order to expand the range of crops cultivated in CEAL. One of the possible directions of radish breeding is the creation of productive forms with a glabrous leaf, which makes it possible to use plants as food as a whole. It can significantly increase the productivity and profitability of cultivating of the crop, especially in controlled conditions.
The aim of the presented work was to evaluate the collection of accessions of R. sativus under artificial light conditions according to physiological and morphological traits, to search for sources of economically valuable traits, and to breed on their basis new highly productive forms of small radish and radish intended for cultivation in CEAL. For this purpose, the original author’s accelerated breeding methodology was used, as described below.
2. Materials and Methods
2.1. Using the Methodology of Accelerated Transgressive Breeding in the Creating of New Forms of Small Radish and Radish for Controlled Conditions of Light Culture
The methodology of accelerated transgressive breeding was the basis for creating new accessions of R. sativus for CEAL. The primary task in R. sativus breeding was to obtain early ripe lines of small radish and radish, transgressive in terms of root weight. It was planned to obtain a positive transgression by weight by crossing early ripe parental varieties that contrasted in shape with a round and long cylindrical root.
The methodology of accelerated transgressive breeding new lines and cultivars of plants with a predictable complex of economically valuable traits and a high degree of adaptation to the agroecological conditions of their cultivation, on the basis of which new accessions were created, included the following elements:
- -
- taking into account the ecological and genetic organization of quantitative traits;
- -
- taking into account the modular structure of the targeted traits, represented by the complex of the resulting trait and its components;
- -
- taking into account the independence of inheritance of modular components of selected traits;
- -
- the use of growing regimes, which ensure a decrease in the modification and an increase in the clarity of the manifestation of genotypic variability, as well as regimes that accelerate the development of plants in CEAL;
- -
- assessment of the degree of manifestation of the components of the targeted traits in the modeling in CEAL of the main limiting environmental factors operating in the growing region;
- -
- selection of parental pairs based on the principle of favorable complementarity of different components of selectable traits determined by genes or blocks of genes that provide transgressions due to complementary interactions;
- -
- carrying out and studying hybrids F1 and F2, comparing them with parental forms and among themselves under controlled conditions, in order to obtain and identify transgressive genotypes with a predictable complex of economically valuable traits;
- -
- obtaining of stable lines according to the studied traits from selected transgressive genotypes by inbreeding and stabilizing the selection using growing regimes that accelerate generative development [22].
2.2. Growing Plants under Controlled Conditions
A total of 118 accessions of small radish and radish from the collection of the N. I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR) and Russian breeding seed company were evaluated in order to search and select sources of breeding valuable traits under conditions of light culture (Table S1, Supplementary Materials). The studies were carried out under controlled conditions of the agrobiopolygon of the Agrophysical Research Institute (AFI) (St. Petersburg, Russia). Plants were grown in a thin layer of organo-mineral substrate on the original layered vegetation and irradiation equipment with an area of one layer of 3 m2 (Figure 1) [40]. Illumination in the experiment was 20–25 klx, photoperiod 12 h, temperature 24 ± 2 °C during the day and 18 ± 2 °C at night. The light source was high-pressure sodium lamps DNaZ-400 (“Reflax” LLC, Moscow, Russia). Accessions of R. sativus were sown with dry seeds in a growing medium based on high-moor peat (manufacturer–LLC Pindstrup, Russia) with the addition of Cambrian clay and chalk [41], substrate pH 6.0–6.2. The thickness of the root layer was 3–4 cm, the sowing scheme for small radish was 10 × 5 cm, and for daikon 12 × 10 cm. Watering was carried out daily: with water, alternating with fertilizing (Knop’s solution with the addition of ammonium nitrate 0.2 g/liter) 3 times a week. The harvesting of small radish plants was carried out on the 21st–30th day from sowing, and daikon on 25th–45th day from sowing. Three replicates and five plants per replicate for each accession were used.

Figure 1.
Plants of R. sativus grown under light culture conditions AFI.
Based on this assessment, at the first stage of the work, parental pairs were selected for crosses in order to obtain transgressions by root weight, as described below in 3.1. After that, transgressive small radish cultivar and line for light culture were obtained–‘Peterburgskiy fioletovyy’ [42] and ‘Peterburgskiy krasniy’, respectively, in the progeny of a crossing combination of small radish ‘Viola’ (k-3161) × small radish ‘Pernot’ (k-2466). It was made by stabilizing the selection based on an improved methodology of accelerated breeding. The objects of the current study were the parent cultivars of small radish—‘Viola’, ‘Pernot’, and ‘Octava’ (Poisk Agroholding, Russia), the daikon cultivar ‘Peterburgskiy’ bred by the Federal State Budgetary Scientific Institution AFI in 2010 [43], and the two new lines (F8) of radish obtained by the authors through the hybridization of small radish ‘Viola’ × daikon ‘Peterburgskiy’ and small radish ‘Octava’ × daikon ‘Peterburgskiy’.
Growing conditions were fully consistent with those described above. The sowing scheme for hybrids and lines from crossing small radish × daikon was 10 × 6 cm for small radish and daikon cultivars, as described above. The harvesting of small radish plants was carried out on the 25th day from sowing, hybrids of small radish × daikon and daikon selectively on the 25th and completely on the 45th day from sowing. Additionally, three replicates and five plants per replicate for each accession were used.
To stimulate and accelerate the flowering and maturation of seeds, the following cultivation regime was used: the roots of mother plants were kept in a thermostat at 5 degrees for 15 days after harvesting. After that, they were grown in 2 L containers with a 16 h photoperiod. The plants were pollinated by hand. The growing medium, illumination, temperature, and irrigation scheme were used as described above.
When harvesting, the main biometric indicators of plants were measured—plant, root and leaf weight, rosette height and diameter, number of leaves, length and width of leaf and petiole, and length and diameter of root, as a number of bolted plants and degree of pubescence of leaves [44]. The yield of roots during the growing season (kg/m2) was calculated by multiplying the average weight of the root by the number of marketable roots taking into account percent of germination.
Based on the measurement of the mass-metric parameters of plants for both growing conditions, the following coefficients and indices were calculated:
1. Ei—index of economic efficiency:
Ei = Weight of the root/weight of the whole plant
2. Ai—attraction index:
Ai = Mass of root/mass of leaves
3. Ri—Root index:
Ri = Root length/root diameter
2.3. Statistical Analysis
The variability of the structure of feature relationships was evaluated using principal component analysis (PCA). Factor loads were expressed in correlation coefficients with the factor. In addition, the eigenvalues for each factor, the share of factors in the total variance, and the cumulative share of extracted factors were calculated. The selection of the number of optimal factors was carried out using the Scree test [45].
Statistical assessment of the obtained data was carried out by calculating the main descriptive characteristics: mean, standard deviation (SD), coefficient of variation (CV). The Tukey HSD (honestly significant difference) post hoc test was used to identify the differences between the means for each characteristic. A p-value < 0.05 (error probability 5%) was considered an acceptable limit of statistical significance. Data analysis was performed using the software Microsoft Office Excel 2019 and Statistica v. 13.3 (StatSoft Inc., Tulsa, OK, USA). Assessment of the reliability of differences between actual and theoretical expected data was carried out by using the χ2 method [46].
3. Results
The main target trait in our research on the breeding of new forms of R. sativus for controlled conditions was the ability to form a high yield of marketable roots in a short time, under artificial light conditions, when using energy- and resource-saving growing technologies. Promising areas of modern breeding are genomic selection and genome editing, however, traditional breeding methods, including transgressive breeding, are also quite effective in many cases [22,47]. When breeding R. sativus, we used the transgressive breeding methodology described above [22] in order to obtain positive transgressions in productivity (root weight, plant weight). By the term “transgression”, we mean a stable, hereditarily fixed increase (positive transgression) or decrease (negative transgression) of the value of a trait in individual F2 genotypes (or subsequent generations) compared to the extreme (+ or −) values of this trait in parental forms. Transgression is formed due to all types of interallelic and intergenic interactions, as well as genotype–environment interactions (epigenetic effect) [22].
3.1. Principal Component Analysis (PCA)
In breeding, the correlated inheritance of quantitative and qualitative traits of leaves, rosettes, and roots is interesting.
Based on the evaluation results, 112 accessions of small radish were grouped by type. As a result of the analysis of 12 phenotypical traits according to the method of principal component analysis (PCA), it was found that their variability is determined by six factors. Together, they determine 92.1% of the total variance. At the same time, the first component that characterizes the parameters of the rosette and the leaf (the height and diameter of the rosette, the length and width of the leaf) and the weight of the plant determines 45.9% (Table 1).

Table 1.
Factor structure of variability of features of 112 accessions of small radish.
The second component determines the length, diameter, and index of the root. The third determines the degree of pubescence, the fourth determines the root weight, the fifth determines the duration of the vegetative phase, and sixth determines number of bolted plants.
Thus, the analysis demonstrates the relationship between the characteristics of the leaf and the rosette with the signs of weight plant. The length root and root index are negatively related to the root diameter (Table 1).
Since the first two components characterize the largest part of the variability of features, we considered the location of accessions in their space. As a result, the accessions were divided into four groups (Figure 2). The first group includes 11 accessions of the While Icicle and French Breakfast types, which are characterized by a large rosette of leaves, a long root, and an average duration of vegetative phase. These are mainly accessions from Europe, Russia, Canada, Chile, and Turkey.
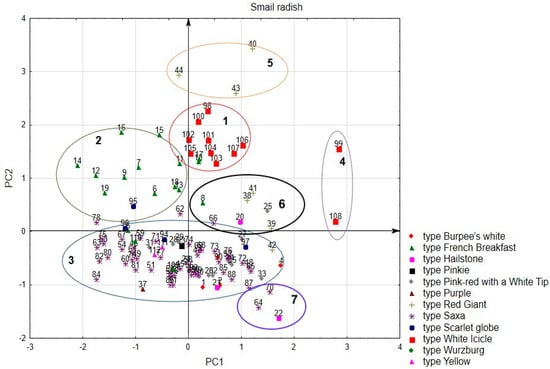
Figure 2.
Distribution of accessions for small radish in the space of the first two components. The color indicates the type of accessions.
The second group includes 11 accessions of the French Breakfast type and 1 accession of the Scarlet Globe type. The accessions are characterized by a cylindrical shape of the root, small size of the rosette of leaves, and medium pubescence of the leaves.
The accessions of the third group were characterized by a large variability in the combination of the studied features and formed a rather large and sparse cloud in the factor space. This group includes accessions of the Saxa type (47 acc.), Burpee’s White (4 acc.), Pink-Red with a White Tip (12 acc.), Scarlet Globe (3 acc.), Würzburg (2 acc.), Yellow (2 acc.), French Breakfast (1 acc.), Hailstone (1 acc.), Pinkie (1 acc.), Purple (1 acc.), and Red Giant (1 acc.).
Generally, of particular interest are the samples of groups 4, 5, 6, and 7 with extreme values of individual traits that exceed the established limits of these traits for the types to which they belong. However, it should be noted that the identified cultivar ‘Viola‘, source of selected trait “root diameter”, was in the 3rd group, and cv. ‘Pernot‘, the source of the trait “root length”, was in the 2nd group. Cv. ‘Octava‘—source of the trait “root diameter”—belonged to the 7th group. We took into account the presence of a complex of economically valuable traits when selecting accessions for subsequent crossing, which will be discussed below. Based on this, the most promising parent varieties belonged to the groups 2, 3, and 7.
3.2. Breeding under Artificial Light Culture Conditions and Obtaining Transgressions by the Mass of the Root of Raphanus sativus L.
The total number of studied accessions of small radish and radish was 118 (Table S1, Supplementary Materials). The aim was to initially assess and identify the sources of the characteristics “length” and “diameter” of the root components of the shape and weight of the root. We took into account the degree of manifestation of economically valuable traits which are realized when grown under conditions of artificial light culture. Along with the evaluation of the components of the root shape, the degree of manifestation of other economically valuable traits that are important for crop production in light culture was also investigated. This will be discussed below.
Our research has shown that a significant part of the accessions was characterized by low yield; some of them are not resistant or barely resistant to bolting; a number of accessions had a low percentage of marketable roots. Additionally, for many cultivars, there was a significant difference of roots within the same cultivar in shape, size, and weight (Figure 3).

Figure 3.
Differences in the size and shape of the root in the small radish cultivars under conditions of artificial light culture. Cultivars: (A) ‘Pernot’, (B) ‘Mohovskoy’, (C) ‘Pharaon’.
It is well known that various environmental factors can cause the formation of defects in radish roots, negatively affecting their commercial qualities. Abiotic factors, such as lack of moisture, temperature fluctuations, the quality of the growing medium, nutrient imbalance, plant density, and harvesting time cause a violation of the metabolic activity of root tissues, which leads to the formation of nonmarketable root deformation, cracking, hollowness, pithiness, branching, premature bolting, etc. In this regard, Manzoor et al. [48] conclude that, in order to obtain a high-quality crop, it is necessary to correctly select specific cultivars for specific conditions in order to minimize the manifestation of these disorders.
In our opinion, the reason for the low yield and marketability of many R. sativus accessions under controlled conditions is the discrepancy between the conditions of breeding and reproduction. The breeding of currently existing lines, cultivars, and hybrids of small radish and radish was carried out under conditions different from those observed in light culture. Most of the quantitative traits are ecologically dependent; the genotype-environment interaction has a significant impact on their manifestation. Therefore, with a significant change in growing conditions, the genetic heterogeneity of varietal populations was revealed, expressed in the appearance of nonmarketable and noncharacteristic roots. The findings of other researchers confirm this assumption. Sharath Kumar et al. [15] report that, despite the existence of an “ideotype” of a plant for CEAL, not a single cultivar has yet been created for controlled conditions. The authors concluded that the conditions for growing plants must correspond to the conditions in which their breeding and selection are carried out. The investigation by K. M. Folta [16] was also devoted to substantiating the need for breeding in controlled conditions. The author focused on those features that are of priority importance in plant breeding and selection for artificial light culture, such as rapid growth, reduced plant stature, ease of harvest, high crop value, lower startup costs, and less energy use. In contrast, the target traits of plant breeding for field are yield, disease resistance, tolerance to abiotic and biotic stress, and postharvest quality [16]. Thus, the goals and objectives of breeding for different conditions differ significantly. This confirms the need for genetic breeding research directly under the conditions for which new cultivars, hybrids, and lines will be created.
In view of the above, accessions were identified that exhibited traits targeted for breeding for productivity under light culture conditions: the short period of vegetation (ultra-ripe or early-ripe); the maximum length or diameter of the root; the small size of the leaf rosette; the resistance to bolting; the glabrous edible leaves; the different color of root. The idea of this work was to obtain a set of accessions of R. sativus with different growing periods, ultra-early or early-ripe, that are able to form a crop of marketable roots 20–25 days from sowing (small radish), and later-ripening forms with a more extended vegetative season: 40–45 days from sowing (radish). At the same time, later-ripening accessions should have been characterized by higher productivity and resistance to bolting, and retained the quality of root pulp at all harvesting times. It was also planned to obtain accessions with different shapes (elliptical, round) and colors of the root (white, red, purple).
The shape of the root directly affects the yield and marketability of radish. It is an important target trait in small radish breeding [49]. Usually, to evaluate it, the shape index used is the ratio of the length of the root to the diameter (Ri). The characteristic “root shape” refers to quantitative traits. The thickening of small radish roots occurs due to a combination of cell division in the cambium and an increase in their size during differentiation in the xylem and phloem [50]. Differences in the shape of the roots in various cultivars arise due to the difference in the number and size of these cells [51]. Zaki et al. [52] attempted to identify the genes involved in controlling the shape and size of the radish root. The authors showed that many of these genes were associated with the regulation of the activity of enzymes, transport systems, molecular structure, binding and transport of proteins and nucleic acids, etc., while the expression of these genes differed significantly in radish cultivars contrasting in shape and size of the root [52].
Makarova and Ivanova [53] showed that the characteristics “length” and “diameter” of the root in radish are inherited independently when crossing parental forms with rounded (Ri = 1.3) and cylindrical (Ri = 7.7) root. This allowed the authors to obtain heterotic hybrids in F1, while the length of the root was close to that of the long root parent, and the diameter was close to the diameter of the round root parent. The weight of heterotic hybrids was 163.45% higher compared to the best parent cultivar. Subsequently, the authors obtained a constant transgressive line of small radish, which had Ri intermediate between the parental cultivars (2.5–3.3) and exceeded the root weight of the best parent by 126.4% [53].
Currently, there are no reliable QTL associated with the shape of the radish root, including its constituent components—length and diameter, for different growing conditions. This can be explained by the genotype–environment interaction, which makes it impossible to clearly identify the number and localization of genes that affect these traits under changing environmental conditions. Recent results of QTL analysis and search for genetic components determined the shape of the radish root revealed the presence of at least seven QTLs on five radish chromosomes (R1, R2, R4, R5, and R7) [54]. Earlier research by Yu at al. (2016) and Tsuro at al. (2008) confirmed the data obtained by Makarova and Ivanova [53] and showed that the probability of independent inheritance of such components (length and diameter) in radish is quite high [55,56]. However, the influence of growing conditions on the manifestation of the components of the root shape should be taken into account when selecting parental forms to obtain transgressions in terms of root weight. This will guarantee the creation of new highly productive accessions of small radish and radish, transgressive in the shape and weight of the root, by combining in the progeny the components of the root form from parental cultivars that are contrasting for these traits under specific conditions.
Parental cultivars, contrasting in the color and shape of the root, were included in the crossing combination to obtain transgressive high-yield accessions. They had the maximum expression of one of the characteristics (length or diameter) (Figure 4a,d,e). It was supposed that transgressions can be obtained by combining (partially or completely) in the hybrid progeny the maximum expression of the components (length and diameter) of contrasting parental cultivars.
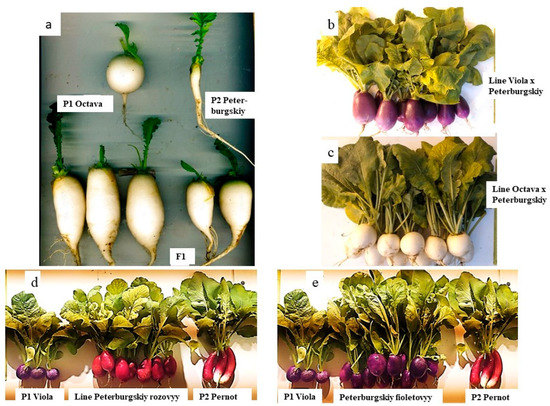
Figure 4.
Parental cultivars and heterotic and transgressive accessions of R. sativus obtained by breeding under light culture conditions. (a) Heterosis in F1 in the crossing combination of small radish ‘Octava’ × daikon ‘Peterburgskiy’; (b,c) transgressive lines of radish; (d,e) transgressive line and cultivar of small radish. The age of the plants in the photos is 25 days from sowing (a,d,e) and 45 days from sowing (b,c).
In our studies, F1 hybrids had an intermediate root shape close to elliptical (Ri = 1.75–2.42) in all selected crossing combinations (Figure 4a,d,e). The root length of hybrids is closed to the length of the long-rooted parent and the diameter is closed to the diameter of the round-rooted cultivar (as shown in Figure 4a), which confirms the data obtained earlier by Makarova and Ivanova [53]. Heterosis was observed in plant weight and root weight in all F1 hybrids of small radish and radish, in all the crossing combinations studied earlier [57,58]. In the combination ‘Viola’ × ‘Pernot’, the weight of the root of small radish F1 hybrids over the best parent was exceeded by 155.09%; in F1 hybrids in the combination of crossing small radish × daikon (‘Viola’ × ‘Peterburgskiy’ and ‘Oktava’ × ‘Peterburgskiy’) was 123.51% and 69.18%, respectively (Table 2 and Table 3).

Table 2.
Characteristics of small radish accessions (parent cultivars, F1, and new accessions) by morphological characteristics and productivity under controlled conditions of artificial light culture.

Table 3.
Characteristics of small radish and radish accessions (parent cultivars, F1, new lines) by morphological characteristics and productivity under controlled conditions of artificial light culture.
Additionally, heterosis was observed in plant weight, leaf rosette size (height, diameter), and linear leaf size (length and width of the leaf, length and diameter of the petiole). When crossing small radish and daikon, heterosis in F1 was observed only by root weight. The weight of leaves and their size did not exceed the best parent value in this combination (daikon cv. ‘Peterburgskiy’).
In the investigated crossing combinations, one of the parents (P1) (small radish cv. ‘Viola’ or ‘Octava’) had a rounded root (Ri = 1.19–1.38). Both of the second parents (P2), small radish cv. ‘Pernot’ and daikon cv. ‘Peterburgskiy’, had a cylindrical root (Ri = 4.27–4.36) (Table 2 and Table 3). In the heterotic F1, the root index varied within the range of 1.75–2.42. Then, plants were identified in segregated F2 and subsequent hybrid populations that were transgressive at the weight of the root. A stabilizing selection was carried out for a complex of breeding traits, as a result of which new hereditarily fixed transgressive forms of R. sativus were obtained (Table 2 and Table 3; Figure 4). It should be noted that, as a result of the self-pollination of the plants selected in F2 and F3, which maximally corresponded to the ideal type (significant weight of the root combined with a compact leaf rosette, glabrous leaves, and high resistance to bolting), a decrease in the weight of the root was observed in all combinations compared to F1 heterotic hybrids. At the same time, the marketability of the roots of new accessions reached 90–95%, in contrast to the lower values for parental cultivars of 75–80%.
3.3. Resistance to Premature Bolting
Resistance to premature bolting is an important indicator that affects yield and quality of radish roots. Premature bolting is a serious problem that limits the production of radish and small radish in the world, due to a decrease in the weight and quality of the roots [59]. It is known that small radish and radish are long day plants, and day length of more than 14 h can cause bolting and flowering in most cultivars. Flowering initiation is controlled by complex genetic networks and may depend on the action of various environmental factors (light, temperature, and day length) that affect the hormonal status of the plants. Under different environmental conditions, the same result (bolting and flowering) can be stimulated or inhibited by the action of various genes, the expression of which depends on various external factors. Many of them are not always available for control [60]. Our studies in controlled conditions at a 12 h photoperiod showed a high variability between accessions for the trait “bolting resistance” (Table S1, Supplementary Materials) and made it possible to identify accessions—the sources of that economically valuable trait. However, cultivars that have shown resistance to bolting in short day artificial light culture may show a different reaction in open field conditions. There, bolting can be provoked by low or high temperatures, suboptimal humidity conditions, and other factors. Therefore, it is highly likely that the results of evaluation of the same accessions in the field would not match those obtained in CEAL. This requires further experimental investigation.
A set of accessions of small radish and radish was evaluated at a 12 h photoperiod under artificial light culture conditions, and 59.5% of the accessions showed bolting within 4–100% of the total number of plants (Table S1, Supplementary Materials). The accessions selected for breeding (with a contrasting shape of the root) were characterized by high and very high resistance to bolting. So, in the cultivars of small radish ‘Octava’ and ‘Viola’, as well as the daikon ‘Peterburgskiy’, bolting was not observed under these conditions, and in the cultivar ‘Pernot’, the number of bolted plants was no more than 10% when the plants were harvested. This trait was hereditarily fixed by stabilizing the selection in hybrid progeny.
3.4. The Compactness of a Leaf Rosette and Other Economically Valuable Traits
The parent small radish cv. ‘Viola’, the source of the “root diameter” trait (mean is 3.29 cm), is characterized by a whole range of valuable breeding traits. So, in addition to 100% resistance to bolting, it has a compact leaf rosette and glabrous leaves. Another source of the “root diameter” trait, cv. ‘Octava’ (mean is 3.17 cm), was also characterized by 100% resistance to bolting and glabrous leaves. However, the leaf rosette of cv. ‘Octava’ was larger in size in comparison with cv. ‘Viola’ (Table 3): rosette height and diameter were larger at 11.9% and 18.3%, respectively; the number of leaves was 14.2% higher than that for cv. ‘Viola’; leaf length and width were larger at 24.7% and 41.9%, respectively. Differences between the height and diameter of the rosette, as well as the length and width of the leaves, were significant (p < 0.05). Small radish cv. ‘Pernot’, the source of the trait “length of the root” (mean is 8.29 cm), was characterized by a compact leaf rosette and medium-pubescent leaves. Another source of the trait “length of the root”, the daikon ‘Peterburgskiy’ (mean is 11.15 cm), is generally adapted to light culture. Its breeding was carried out for the open ground conditions of the Northwestern region of Russia, but with partial use of controlled conditions [43]. Along with the early start of root growth (the average weight of roots is about 50 g on the 40th–45th day of vegetation), it has a leaf rosette that is quite compact for daikon, and low-pubescent leaves. It should be noted that the daikon has a larger rosette with more leaves compared to the small radish (Table 3). The maximum differences were observed by traits “diameter of the rosette” and “number of leaves”, the excess over the small radish cultivars were 46.5–73.4% and 69.3–93.3%, respectively, differences are significant (p < 0.05). Therefore, a lower sowing density was used—80 seeds per 1 m2 (for small radish—200 seeds per m2). In addition, in low-volume soilless cultivation technologies (substrate layer 3–4 cm), a long daikon root often turned out to be branched and curved and the crop was not marketable.
The compactness of the leaf rosette was another selected trait. A large leaf is a rather negative quality for a radish in a protected ground, as it prevents the cultivation of plants in close planting. Plants with a compact leaf rosette with a similar weight of the root can be grown at a higher plant density, which allows growers to obtain a greater yield of roots per 1 sq. m. [20,28].
One of the most important goals in creating new accessions of R. sativus for protected ground, and CEAL in particular, is the breeding of accessions with limited leaf growth and intensive roots, since the combination of light, heat, and humidity, typical for cultivation facilities with controlled environments, promotes enhanced leaf growth. Therefore, of particular interest are highly productive forms with a small number of leaves and a compact rosette. It can reduce the competition between plants for light. This is especially important for reducing energy costs per unit of production under artificial light culture conditions. The coefficient of economic efficiency (Ei) along with the attraction index (Ai) reflect the ability of accessions to intensively form roots and indirectly characterize the intensity of the photosynthetic apparatus and the redistribution of assimilates between the aboveground and underground parts of the plants. At the same time, both of these indices also characterize the compactness of the leaf rosette: the higher values of Ei and Ai match the lower proportion of leaves in the plant mass. The cultivars of small radish ‘Viola’ and ‘Pernot’ had high values of these indices—0.57 and 0.63 (Ei) and 1.42 and 1.80 (Ai), respectively (Table 2 and Table 3).
Heterosis was observed in small radish F1 hybrids not only by root weight, but also by leaf size and weight (Table 2). The weight of the plants, roots, and leaves of the hybrids was 160.1%, 155.1%, and 171.4% higher compared to the best parent cultivar, respectively. In contrast to this, the size and weight of the leaves in F1 hybrids did not exceed those of the daikon in the small radish × daikon combination (Table 3). In the process of selection in populations F2–F7, the compactness of the leaf rosette was hereditarily fixed (Table 2 and Table 3): the values of index of economic efficiency in all new forms are 0.64–0.69, and 1.79–2.34 for attraction index. At the same time, all the created forms of small radish and radish did not begin bolting during the growing season (25 days for small radish and 40–45 days for radish). A comparison of plants for selected valuable traits was carried out under standardized environmental conditions, without exposure to environmental fluctuations typical of open ground. This made it possible to clearly identify breeding-valuable genotypes for subsequent selection stages.
Daikon cv. ‘Peterburgskiy’ is characterized by a longer growing season compared to small radish. For the formation of marketable roots, it needs a more developed leaf apparatus than that of small radish. However, plants of the daikon ‘Peterburgskiy’ are able to form roots of considerable length (11.15 ± 2.57 cm) with an average weight of 47.18 g under the controlled conditions on the 40th–45th day from sowing. This circumstance allowed us to use it as a parent cultivar for obtaining of new R. sativus forms that are superior to small radish cultivars by weight of the roots and characterized by denser and juicier pulp. The Ei and Ai for daikon in the early stages of development (up to 40–45 days of age) had significantly lower values compared to small radish—0.47 and 0.99, respectively, especially in open ground conditions (data not provided), due to the physiology of this crop. However, as a result of selection by root weight and compactness of the leaf rosette, stable inbred lines were obtained in the crossing combinations ‘Viola’ × ‘Peterburgskiy’ and ‘Octava’ × ‘Peterburgskiy’. Their Ei and Ai values are close to and even exceed those of the parent small radish cultivars—0.64–0.69 and 1.93–2.34, respectively (Table 3). The created lines are adapted to growing at a higher plant density—twice as large as those for daikon (140–160 plants/m2).
The use in this experiment of cultivars with a difference in root color (white, red, purple) as parental forms allowed us to obtain diversity for this trait in the created accessions. It is known that flavonoids are the major pigments responsible for the color of R. sativus. Pelargonidin-based anthocyanins (callistephin and pelargonin) are mainly found in red radishes, and acylated cyanidin and its derivatives have been identified in purple ones [37,61]. It has been shown that, when radish cultivars with purple and red roots are crossed, the color of edible root is usually inherited as a monohybrid inheritance with the purple color dominating [53,62]. Our results support this conclusion. When purple (cv. Viola) and red (cv. Pernot) small radish were crossed, F1 hybrids had the purple roots. The segregation by the root color in the F2 corresponded to 3 (purple): 1 (red) (p < 0.05, χ2 = 0.008). Accordingly, in F2, the ancestors of the purple and red lines were selected. These plants were transgressive in terms of root weight (Figure 4d,e). In crossing combination Viola × Peterburgskiy, F1 hybrids also had purple roots. In their F2 segregated population, the dark purple plant transgressive by root weight was selected as an ancestor, and based on it, a line with purple roots was obtained (Figure 4b). In crossing combination Octava × Peterburgskiy, all the hybrids (F1–F7) were white (Figure 4a,c).
It is known that radish accessions differ in color of the root have significant differences in the components of the biochemical composition. In particular, it has been shown that the content of flavonoids in colored roots (red and purple) is significantly higher than in white ones [63]. Flavonoids (pelargonidin and cyanidin), which determine the color of the root, are important components in the complex of biologically active compounds responsible for the antioxidant properties of various plants, and the use of plants rich in flavonoids in food has a positive effect on human health [63]. The higher content of anthocyanins in colored roots was also confirmed by our results (data not provided). In general, small radish and radish both with pigmented and uncolored roots are characterized by high dietary properties. In addition to anthocyanins, they contain a large number of other compounds with high biological activity [20,30,31]. Therefore, accessions with white roots also have a high consumer value.
It should be noted that some reduction in total plant weight and root weight were observed in plants F3–F6 in the crossing combination of small radish ‘Viola’ × ‘Pernot’ compared to F1 as a result of inbreeding. However, a significant increase in the number of leaves in the rosette, while maintaining its compactness in plants of stabilized lines, increased the leaf-assimilating surface (data not provided), which probably contributed to higher rates of root formation compared to the parental cultivars.
In contrast to the investigations presented by Makarova and Ivanova [53] we could not achieve a combination of the maximum root length of the one parent and the diameter of the other parent in the hybrid progeny, except for heterotic F1. Therefore, we did not obtain the maximum transgressive effect. The probable reason is that the selection in our studies was carried out according to a whole complex of economically valuable traits, and not only according to the size and weight of the root. The length of the root of newly created accessions had an intermediate value between the parental cultivars. The average yield of the radish cultivar ‘Peterburgskiy fioletovyy’ and the inbred line ‘Peterburgskiy krasniy’ (taking into account germination and marketability) was 4.98 and 4.30 kg/m2 for 23–25 days of the growing season, which is 78.4–106.6% higher compared to the best parental cultivar ‘Pernot’ (2.41 kg kg/m2), which corresponds to 50–60 kg/m2 of roots per year. The positive transgression by root weight was 63.0% and 40.7%, respectively.
In another series of experiments, when crossing small radish and daikon, a positive transgression by root weight (40.3%) and Ei and Ai indices (21.0% and 64.8%, respectively) was obtained only in the crossing combination of small radish ‘Viola’ × daikon ‘Peterburgskiy’. At the same time, heterosis in F1 was obtained in both combinations: ‘Viola’ × ‘Peterburgskiy’ and ‘Octava’ × ‘Peterburgskiy’ (Table 3). In the combination of ‘Octava’ × ‘Peterburgskiy’, transgression was obtained only for indices Ei and Ai (25.5% and 73.9%, respectively). Transgression by root weight was not fixed in this crossing combination. A plant with a compact rosette and a rounded root was selected in F2 as the ancestor of the new line in order to obtain the variability in shape of roots in the set of new accessions. The root weight of that plant was lower than that in transgressive plants with an elliptical root. Later, in the progeny of this plant, segregation in shape was not observed, and the roots of the stabilized line F8 had a rounded shape and Ri = 1.19, while the F1 roots were elliptical and had intermediate Ri between the parents, equal to 2.42 (Table 3, Figure 4a,c). Despite the absence of transgression in the mass of the root, this approach made it possible to create a new highly productive form of radish that is resistant to close planting, with a compact rosette and a longer growing season compared to small radish. Its roots had high marketability, are close in quality to daikon pulp, and do not have hollowness, pithiness, or fibrosis at 45 days of vegetation.
The radish lines in both crossing combinations have a denser and more uniform pulp compared to small radish and good palatability traits. The yield of new lines is 5.46–7.66 kg/m2 for 40–45 days of vegetation—about 40–60 kg/m2 per year, depending on the number of growing seasons (7 or 8 per year), which is comparable to the yield of small radish. It should also be noted that the line ‘Viola’ × ‘Peterburgskiy’ is early-ripe, similar to small radish accessions. The average root weight of this line was 30.08 g on the 25th day of cultivation, while the average weight of the ‘Peterburgskiy’ daikon roots was equal to 10.18 g. It can be recommended as an accession with an extended period of harvesting, and harvested selectively, discharging dense sowing, from 20 to 45 days of vegetation. At the same time, the weight of roots increases without loss of commercial qualities (Figure 5).

Figure 5.
Radish roots in the crossing combination of small radish ‘Viola’ × daikon ‘Peterburgskiy’ (F8) on the 25th and 45th days from sowing.
All new accessions of R. sativus (small radish and radish) obtained as a result of the our breeding research under artificial light culture conditions are characterized by a glabrous leaf type, which corresponds to the goal: the plant can be used as food in fresh salads as a whole and to increase the profitability of growing crops in CEAL.
4. Conclusions
The genetic diversity and breeding potential of cultural forms of R. sativus with different lengths of the growing period (small radish, daikon) were studied under controlled conditions with artificial light. Accessions were identified—sources of valuable traits for CEAL. New accessions of R. sativus with a complex of economically valuable traits were created that are transgressive in terms of root weight. The yield of the roots of new small radish forms was 4.30–4.98 kg/m2 for 25 days of cultivation under light culture conditions. Radish lines from the crossing of small radish and daikon had a longer growing period of 40–45 days, and their yield was 5.46–7.66 kg/m2 per growing season under light culture conditions. All created accessions of R. sativus are characterized by a complex of economically valuable traits: a compact leaf rosette, glabrous leaves, high values of the index of economic efficiency and the index of attraction, and resistance to premature bolting.
All selection and genetic studies were conducted under CEAL conditions. It significantly reduced the amount of material involved in the research, as well as the time spent on creating new forms of R. sativus (up to 4–5 years).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9060678/s1, Table S1: Evaluation of radish accessions by morphological characteristics and productivity when growing under controlled conditions of artificial light.
Author Contributions
Conceptualization, N.G.S. and A.A.K.; methodology, N.G.S. and A.A.K.; software, N.V.K.; validation, N.V.K., A.B.K. and Y.V.C.; formal analysis, K.V.E. and G.G.P.; investigation, N.G.S. and A.A.K.; resources, A.B.K.; data curation, N.V.K. and A.B.K.; writing—original draft preparation, N.G.S.; writing—review and editing, N.G.S., A.A.K. and Y.V.C.; visualization, N.G.S.; project administration, G.G.P. and Y.V.C. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by the Ministry of Education and Science of the Russian Federation (agreement no. 075-15-2020-805 from 2 October 2020).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hansen, J.; Hellin, J.; Rosenstock, T.; Fisher, E.; Cairns, J.; Stirling, C.; Campbell, B. Climate risk management and rural poverty reduction. Agric. Syst. 2019, 172, 28–46. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Dinesh, D.; Howden, S.M.; Cramer, L.; Thornton, P.K. Transformation in practice: A review of empirical cases of transformational adaptation in agriculture under climate change. Front. Sustain. Food Syst. 2018, 2, 65. [Google Scholar] [CrossRef]
- Ksenofontov, M.Y.; Polzikov, D.A. On the issue of the impact of climate change on the development of Russian agriculture in the long term. Stud. Russ. Econ. Dev. 2020, 31, 304–311. [Google Scholar] [CrossRef]
- Vyas, S.; Khatri-Chhetri, A.; Aggarwal, P.; Thornton, P.; Campbell, B.M. Perspective: The gap between intent and climate action in agriculture. Glob. Food Sec. 2022, 32, 100612. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Martin, M.; Weidner, T.; Gullström, C. Estimating the potential of building integration and regional synergies to improve the environmental performance of urban vertical farming. Front. Sustain. Food Syst. 2022, 6, 109. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Role of the plant factory with artificial lighting (PFAL) in urban areas. In Plant Factory, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 7–34. ISBN 978-0-12-816691-8. [Google Scholar]
- Shamshiri, R.; Kalantari, F.; Ting, K.C.; Thorp, K.R.; Hameed, I.A.; Weltzien, C.; Shad, Z.M. Advances in greenhouse automation and controlled environment agriculture: A transition to plant factories and urban agriculture. Int. J. Agric. Biol. Eng. 2018, 11, 1–22. [Google Scholar] [CrossRef]
- Saito, K.; Ishigami, Y.; Goto, E. Evaluation of the light environment of a plant factory with artificial light by using an optical simulation. Agronomy 2020, 10, 1663. [Google Scholar] [CrossRef]
- Gómez, C.; Currey, C.J.; Dickson, R.W.; Kim, H.J.; Hernández, R.; Sabeh, N.C.; Burnett, S.E. Controlled environment food production for urban agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Tong, Y.; Li, L.; Sahari, S.Q.; Almogahed, A.M.; Cheng, R. Integrative effects of CO2 concentration, illumination intensity and air speed on the growth, gas exchange and light use efficiency of lettuce plants grown under artificial lighting. Horticulturae 2022, 8, 270. [Google Scholar] [CrossRef]
- O'sullivan, C.A.; Bonnett, G.D.; McIntyre, C.L.; Hochman, Z.; Wasson, A.P. Strategies to improve the productivity, product diversity and profitability of urban agriculture. Agric. Syst. 2019, 174, 133–144. [Google Scholar] [CrossRef]
- Lubna, F.A.; Lewus, D.C.; Shelford, T.J.; Both, A.J. What you may not realize about vertical farming. Horticulturae 2022, 8, 322. [Google Scholar] [CrossRef]
- Van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Marcelis, L.F.M. Current status and future challenges in implementing and upscaling vertical farming systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef] [PubMed]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F. Vertical farming: Moving from genetic to environmental modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef]
- Folta, K.M. Breeding new varieties for controlled environments. Plant Biol. 2019, 21, 6–12. [Google Scholar] [CrossRef]
- Awika, H.O.; Mishra, A.K.; Gill, H.; DiPiazza, J.; Avila, C.A.; Joshi, V. Selection of nitrogen responsive root architectural traits in spinach using machine learning and genetic correlations. Sci. Rep. 2021, 11, 9536. [Google Scholar] [CrossRef]
- Artemyeva, A.M.; Sinyavina, N.G.; Panova, G.G.; Chesnokov, Y.V. Biological features of Brassica rapa L. vegetable leafy crops when growing in an intensive light culture. Agric. Biol. 2021, 56, 103–120. [Google Scholar] [CrossRef]
- Egorova, K.V.; Sinyavina, N.G.; Artemyeva, A.M.; Kocherina, N.V.; Chesnokov, Y.V. QTL Analysis of the Content of Some Bioactive Compounds in Brassica rapa L. Grown under Light Culture Conditions. Horticulturae 2021, 7, 583. [Google Scholar] [CrossRef]
- Kurina, A.B.; Kornyukhin, D.L.; Solovyeva, A.E.; Artemyeva, A.M. Genetic diversity of phenotypic and biochemical traits in VIR radish (Raphanus sativus L.) germplasm collection. Plants 2021, 10, 1799. [Google Scholar] [CrossRef]
- Samantara, K.; Bohra, A.; Mohapatra, S.R.; Prihatini, R.; Asibe, F.; Singh, L.; Varshney, R.K. Breeding More Crops in Less Time: A Perspective on Speed Breeding. Biology 2022, 11, 275. [Google Scholar] [CrossRef]
- Kochetov, A.A.; Mirskaya, G.V.; Sinyavina, N.G.; Egorova, K.V. Transgressive Breeding: A methodology for accelerated creation of new forms of plants with a predictable complex of economically valuable traits. Russ. Agric. Sci. 2021, 47, 40–50. [Google Scholar] [CrossRef]
- Wanga, M.A.; Shimelis, H.; Mashilo, J.; Laing, M.D. Opportunities and challenges of speed breeding: A review. Plant Breed. 2021, 140, 185–194. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.D.; Hickey, L.T. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Chesnokov, Y.V.; Kanash, E.V.; Mirskaya, G.V.; Kocherina, N.V.; Rusakov, D.V.; Lohwasser, U.; Börner, A. QTL mapping of diffuse reflectance indices of leaves in hexaploid bread wheat (Triticum aestivum L.). Russ. J. Plant Physiol. 2019, 66, 77–86. [Google Scholar] [CrossRef]
- Chesnokov, Y.V.; Mirskaya, G.V.; Kanash, E.V.; Kocherina, N.V.; Rusakov, D.V.; Lohwasser, U.; Börner, A. QTL identification and mapping in soft spring wheat (Triticum aestivum L.) under controlled agroecological and biological testing area conditions with and without nitrogen fertilizer. Russ. J. Plant Physiol. 2018, 65, 123–135. [Google Scholar] [CrossRef]
- Yanaeva, D.A. Cultivars and hybrids of the garden radish for the growing according to cassette technology. Potato Veg. 2015, 2, 19–21. (In Russian) [Google Scholar]
- Singh, B.K. Radish (Raphanus sativus L.): Breeding for higher yield, better quality and wider adaptability. In Advances in Plant Breeding Strategies: Vegetable Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Cham, Switzerland, 2021; Volume 8, pp. 275–304. ISBN 978-3-030-66964-5. [Google Scholar]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P.; Perez, R.L. Raphanus sativus (Radish): Their chemistry and biology. Sci. World J. 2004, 4, 811–837. [Google Scholar] [CrossRef]
- Keyata, E.O.; Tola, Y.B.; Bultosa, G.; Forsido, S.F. Proximate, mineral, and anti-nutrient compositions of underutilized plants of Ethiopia: Figl (Raphanus sativus L.), Girgir (Eruca sativa L) and Karkade (Hibiscus sabdariffa): Implications for in-vitro mineral bioavailability. Food Res. Int. 2020, 137, 109724. [Google Scholar] [CrossRef]
- Manivannan, A.; Kim, J.H.; Kim, D.S.; Lee, E.S.; Lee, H.E. Deciphering the nutraceutical potential of Raphanus sativus—A comprehensive overview. Nutrients 2019, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Roy, S.; Chakraborty, R.; Rebezov, M.; Shariati, M.A.; Rengasamy, K.R.R. Underutilized green leafy vegetables: Frontier in fortified food development and nutrition. Crit. Rev. Food Sci. Nutr. 2022, 11, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Noman, O.M.; Nasr, F.A.; Alqahtani, A.S.; Al-zharani, M.; Cordero, M.A.W.; Alotaibi, A.A.; Daoud, A. Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia. Open Chem. 2021, 19, 408–416. [Google Scholar] [CrossRef]
- Koley, T.K.; Khan, Z.; Oulkar, D.; Singh, B.K.; Maurya, A.; Singh, B.; Banerjee, K. High resolution LC-MS characterization of phenolic compounds and the evaluation of antioxidant properties of a tropical purple radish genotype. Arab. J. Chem. 2020, 13, 1355–1366. [Google Scholar] [CrossRef]
- Jahangir, M.; Abdel-Farid, I.B.; de Vos, C.H.R.; Jonker, H.H.; Choi, Y.H.; Verpoorte, R. Metabolomic variation of Brassica rapa var. rapa (var. raapstelen) and Raphanus sativus L. at different developmental stages. Pak. J. Bot. 2014, 46, 1445–1452. [Google Scholar]
- Li, X.; Wang, J.; Qiu, Y.; Wang, H.; Wang, P.; Zhang, X.; Li, X. SSR-sequencing reveals the inter-and intraspecific genetic variation and phylogenetic relationships among an extensive collection of Radish (Raphanus) germplasm resources. Biology 2021, 10, 1250. [Google Scholar] [CrossRef]
- Panova, G.G.; Chernousov, I.N.; Udalova, O.R.; Alexandrov, A.V.; Karmanov, I.V.; Anikina, L.M.; Yakushev, V.P. Scientific basis for large year-round yields of high-quality crop products under artificial lighting. Russ. Agric. Sci. 2015, 41, 335–339. [Google Scholar] [CrossRef]
- Ermakov, E.I.; Zheltov, Y.I.; Milto, N.E.; Kucherov, V.I. Soil for Growing Plants “Agrophyte”. Russian Federation Patent 2081555, 13 July 1997. (In Russian). [Google Scholar]
- Kochetov, A.A.; Sinyavina, N.G. Small Radish Raphanus sativus var. sativus Peterburgskiy Fioletovyy. Russian Federation Patent 11,518 (8,058,521), 25 March 2021. (In Russian). [Google Scholar]
- Kochetov, A.A.; Artem’eva, A.M. Daikon Peterburgskiy. Russian Federation Patent 6392 (8,953,530), 22 March 2012. (In Russian). [Google Scholar]
- Sazonova, L.V. Guidelines for the Study and Maintenance of the World Collection of Root Vegetables; VIR: Leningrad, Russia, 1989; p. 88. (In Russian) [Google Scholar]
- Cattell, R.B. The Scree test for the number of factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef]
- Dospekhov, B.A. Field Experience Methodology (with the Basics of Statistical Processing of Research Results), 5th ed., add and revised; Agropromizdat: Moscow, Russia, 1985; 351p. [Google Scholar]
- Benildo, G.D.L.R. Genomic and epigenomic bases of transgressive segregation–New breeding paradigm for novel plant phenotypes. Plant Sci. 2019, 288, 110213. [Google Scholar]
- Manzoor, A.; Bashir, M.A.; Naveed, M.S.; Cheema, K.L.; Cardarelli, M. Role of different abiotic factors in inducing pre-harvest physiological disorders in radish (Raphanus sativus). Plants 2021, 10, 2003. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Niikura, S.; Matsuura, S.; Takano, Y.; Ukai, Y. Interaction between genetic effects and soil type in diallel analysis of root shape and size of Japanese radish (Raphanus sativus L.). Breed. Sci. 2004, 54, 313–318. [Google Scholar] [CrossRef]
- Ting, F.S.-T.; Wren, M.J. Storage organ development in radish (Raphanus sativus L.). 1. A comparison of development in seedlings and rooted cuttings of two contrasting varieties. Ann. Bot. 1980, 46, 267–276. [Google Scholar] [CrossRef]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular organisation of the Arabidopsis thaliana root. Development 1993, 119, 71–84. [Google Scholar] [CrossRef]
- Zaki, H.E.; Yokoi, S.; Takahata, Y. Identification of genes related to root shape in radish (Raphanus sativus) using suppression subtractive hybridization. Breed. Sci. 2010, 60, 130–138. [Google Scholar] [CrossRef]
- Makarova, G.A.; Ivanova, T.I. Inheritance of root and leaf characteristics in radish plants. Genetiks 1983, 19, 304–311. (In Russian) [Google Scholar]
- Wei, Q.; Wang, J.; Wang, W.; Hu, H.; Yan, Y.; Bao, C.; Hu, T. Identification of QTLs controlling radish root shape using multiple populations. Horticulturae 2022, 8, 931. [Google Scholar] [CrossRef]
- Yu, X.; Choi, S.R.; Dhandapani, V.; Rameneni, J.J.; Li, X.; Pang, W.; Lim, Y.P. Quantitative trait loci for morphological traits and their association with functional genes in Raphanus sativus. Front. Plant Sci. 2016, 7, 255. [Google Scholar] [CrossRef]
- Tsuro, M.; Suwabe, K.; Kubo, N.; Matsumoto, S.; Hirai, M. Mapping of QTLs controlling root shape and red pigmentation in radish, Raphanus sativus L. Breed. Sci. 2008, 58, 55–61. [Google Scholar] [CrossRef]
- Sinyavina, N.G.; Kochetov, A.A.; Egorova, K.V.; Kocherina, N.V.; Chesnokov, Y.V. Genetic-Biochemical Studies and Morphobiological Assessment of Small Radish (Raphanus sativus L.) under Artificial Light Culture Conditions. Russ. J. Genet. 2022, 58, 662–670. [Google Scholar] [CrossRef]
- Kochetov, A.A.; Sinyavina, N.G. Creation of new forms of Raphanus sativus L. with the predicted complex of economically valuable traits using the methodology of accelerated plant breeding. Potato Veg. 2019, 10, 29–34, (In Russian with English Abstract). [Google Scholar]
- Nie, S.; Li, C.; Xu, L.; Wang, Y.; Huang, D.; Muleke, E.M.; Liu, L. De novo transcriptome analysis in radish (Raphanus sativus L.) and identification of critical genes involved in bolting and flowering. BMC Genom. 2016, 17, 389. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wei, Q.; Wang, W.; Hu, H.; Mao, W.; Zhu, Q.; Bao, C. Genome-wide identification and characterization of CONSTANS-like gene family in radish (Raphanus sativus). PLoS ONE 2018, 13, e0204137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S. A comparative metabolomics study of flavonoids in radish with different skin and flesh colors (Raphanus sativus L.). J. Agric. Food Chem. 2020, 68, 14463–14470. [Google Scholar] [CrossRef]
- Ugarova, S.V.; Zelenin, A.V. The use the genetically difficultly inherited trait of purple root color in breeding program for the complicated trait in radish. Veg. Crop Russ. 2017, 4, 66–69, (In Russian with English Abstract). [Google Scholar] [CrossRef]
- Wang, J.; Wei, Q.; Wang, W.; Hu, H.; Yan, Y.; Wang, Y.; Bao, C. Understanding the nutraceutical diversity through a comparative analysis of the taproot metabolomes of different edible radish types via UHPLC–Q–TOF–MS. Food Chem. 2023, 403, 134469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).