Selection of Salt-Tolerant Jojoba (Simmondisa chinensis L.) Cultivars via In Vitro Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Disinfection and In Vitro Germination of Jojoba Seeds
2.2. Multiple Shoots Induction
2.3. In Vitro Rooting
2.4. Acclimatization of Plantlets
2.5. Determination of Chlorophyll a, b, and Carotenoid Contents
2.6. Determination of Sodium (Na), Potassium (K), Magnesium (Mg), and Calcium (Ca) Compositions
2.7. Peroxidase (POD) Enzyme Assay
2.7.1. Preparation of Plant Extract
2.7.2. Composition and Condition of Enzymatic Reaction
2.8. Statistical Analysis
3. Results
3.1. The Effect of Salinity on Jojoba Seed Germination
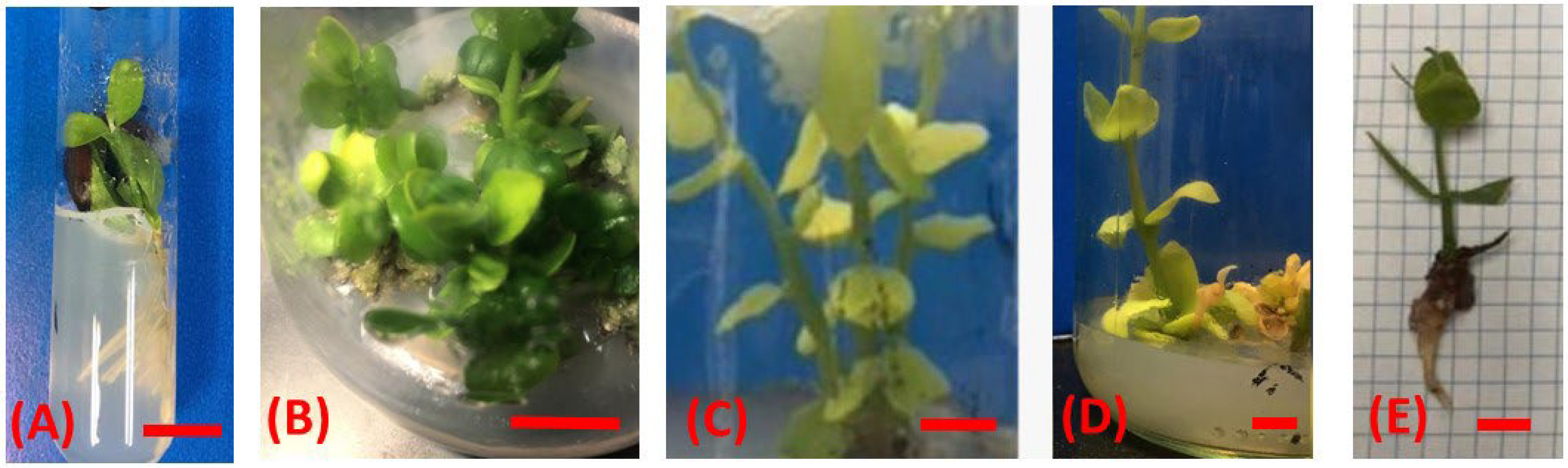
3.2. The Effect of Salinity on Jojoba Multiple-Shoot Induction
3.3. Effects of Salinity on Jojoba Rooting
3.4. Acclimatization
3.5. The Effect of Salinity on the Photosynthetic Pigments Content in Jojoba Shoots
3.5.1. The Effect of Salinity on the Photosynthetic Pigment Content in Jojoba (Simmondsia chinensis L.) Shoots Growing in the Multiple-Shoot Induction Media with Various Salinity Levels
3.5.2. The Effect of Salinity on the Photosynthetic Pigment Content in Jojoba (Simmondsia chinensis L.) Shoots Growing in the Rooting Media with Various Salinity Levels
3.6. The Effect of Salinity on the Mineral Content in Jojoba Shoots
3.7. The Effect of Salinity on the Peroxidase (POD) Activities in Jojoba Shoots
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bafeel, S.O.; Galal, H.K.; Basha, A.Z. Effect of Seawater Irrigation on Growth and Some Metabolites of Jojoba Plants. Am.-Eurasian J. Agric. Environ. Sci. 2016, 16, 49–59. [Google Scholar] [CrossRef]
- Al-Obaidi, J.R.H.; AlKhalifah, M.F.; Asanar, N.S.; Al-Soqeer, S.; Abdulrahman, A.; Attia, M.F. A review on plant importance, biotechnological aspects, and cultivation challenges of jojoba plant. Biol. Res. 2017, 50, 25. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Bashir, M.A.; Ahmad, M. Brackish water impact on growth of jojoba (Simmondsia chinensis). J. Agric. Res. 2011, 49, 591–596. Available online: http://agris.fao.org/agris-search/search/display.do?f=2012/PK/PK1204.xml;PK2012000565 (accessed on 1 January 2022).
- Abdel-Mageed, W.M.; Bayoumi, S.A.L.H.; Salama, A.A.R.; Salem-Bekhit, M.M.; Abd-Alrahman, S.H.; Sayed, H.M. Antioxidant lipoxygenase inhibitors from the leaf extracts of Simmondsia chinensis. Asian Pac. J. Trop. Med. 2014, 7, S521–S526. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Majeed, A.; Siyyar, S. Salinity Stress Management in Field Crops: An Overview of the Agronomic Approaches. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–16. [Google Scholar]
- Rai, M.K.; Kalia, R.K.; Singh, R.; Gangola, M.P.; Dhawan, A.K. An overview of the recent progress. Environ. Exp. Bot. 2011, 71, 89–98. [Google Scholar] [CrossRef]
- Jan, N.; Qazi, H.A.; Ramzan, S.; John, R. Developing Stress-Tolerant Plants Through In Vitro Tissue Culture: Family Brassicaceae. In Biotechnologies of Crop Improvement; Gosal, S., Wani, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 1. [Google Scholar] [CrossRef]
- Sahu, M.; Maurya, S.; Jha, Z. In vitro selection for drought and salt stress tolerance in rice: An overview. Plant Physiol. Rep. 2023, 28, 8–33. [Google Scholar] [CrossRef]
- Cha-Um, S.; Kirdmanee, C. Assessment of salt tolerance in Eucalyptus, rain tree and Thai neem under laboratory and the field conditions. Pak. J. Bot. 2008, 40, 2041–2051. [Google Scholar]
- Khasa, P.; Hambling, B.; Kernaghan, G.; Fung, M.; Ngimbi, E. Genetic variability in salt tolerance of selected boreal woody seedlings. For. Ecol. Manag. 2002, 165, 257–269. [Google Scholar] [CrossRef]
- Melgar, J.C.; Syvertsen, J.P.; García-Sánchez, F. Can elevated CO2 improve salt tolerance in olive trees? J. Plant Physiol. 2008, 165, 631–640. [Google Scholar] [CrossRef]
- Nguyen, N.; Moghaieb, R.; Seneoka, H.; Fujita, K. RAPD makers associated with Acacia auriculiformis and Acacia mangium. Plant Sci. 2004, 167, 797–805. [Google Scholar] [CrossRef]
- Tewary, P.K.; Sharma, A.; Raghunath, M.K.; Sarkar, A. In Vitro response of promising mulberry (Morus sp.) genotypes for tolerance to salt and osmotic stresses. Plant Growth Regul. 2000, 30, 17–21. [Google Scholar] [CrossRef]
- Llorente, B.E.; Apóstolo, N.M. In Vitro Propagation of Jojoba. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Lambardi, M., Ozudogru, E., Jain, S., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 994. [Google Scholar] [CrossRef]
- Reddy, M.P.; Chikara, J. Biotechnology Advances in Jojoba (Simmondsia chinensis). In Desert Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 407–421. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962, 13, 473–497. [Google Scholar] [CrossRef]
- Horwitz, W.; Chichilo, P.; Reynolds, H. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1970. [Google Scholar]
- Mazumdar, B.C.; Majumder, K. Methods of Physiochemical Analysis of Fruits; Daya Publisher: Delhi, India, 2003. [Google Scholar]
- Nasef, I.N. Short hot water as safe treatment induces chilling tolerance and antioxidant enzymes, prevents decay and maintains quality of cold-stored cucumbers. Postharvest Biol. Technol. 2018, 138, 1–10. [Google Scholar] [CrossRef]
- StatSoft STATISTICA for Windows; Version 6; 2300; StatSoft Inc.: Tulsa, OK, USA, 2001.
- Joshi, M.; Dhar, U. In Vitro propagation of Saussurea obvallata (DC.) Edgew.—An endangered ethnoreligious medicinal herb of Himalaya. Plant Cell Rep. 2003, 21, 933–939. [Google Scholar] [CrossRef]
- Benzioni, A.; Ventura, M.; Malach, Y.D. Long-term effect of irrigation with saline water on the development and productivity of jojoba steris. J. Hortic. Sci. Biotechnol. 2015, 71, 835–846. [Google Scholar]
- Taha, R.A. Effect of growth regulators and salinity levels on in vitro cultures of jojoba plants. World Appl. Sci. J. 2014, 31, 751–758. [Google Scholar] [CrossRef]
- Fayek, M.A.; Shaaban, E.A.; Zayed, N.S.; El-Obeidy, A.A.; Taha, R.A. Effect of salt stress on chemical and physiological contents of jojoba (Simmondsia chinensis (Link) Schneider) using in vitro culture. World J. Agric. Sci. 2010, 6, 446–450. [Google Scholar]
- Mohasseb, H.A.A.; Solliman, M.E.; Al-mssallem, I.S.; El-shemy, H.A. Salt-Tolerant Phenomena, Sequencing and Characterization of a Glyoxalase I (Jojo-Gly I) Gene from Jojoba in Comparison with Other Glyoxalase I Genes. Plants 2020, 9, 1285. [Google Scholar] [CrossRef]
- Roussos, P.A.; Vemmos, S.N.; Pontikis, C.A. The role of carbohydrates on the salt tolerance of jojoba [Simmondsia chinensis (Link)] explants in vitro. Eur. J. Hortic. Sci. 2005, 70, 278–282. [Google Scholar]
- Gao, S.; Ouyang, C.; Wang, S.; Xu, Y.; Tang, L.; Chen, F. Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings. Plant Soil Environ. 2008, 54, 374–381. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, Y.; Ji, Y.; Jiang, Z.; Wang, L. Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of Broussonetia papyrifera. S. Afr. J. Bot. 2013, 85, 1–9. [Google Scholar] [CrossRef]
- Alghamdi, B.A.; Bafeel, S.O.; Edris, S.; Atef, A.; Al-Matary, M.; Bahieldin, A. Molecular mechanisms underlying salt stress tolerance in jojoba (Simmondsia chinensis). BioSci Biotech Res. Asia 2021, 18, 37–57. [Google Scholar] [CrossRef]
- Alghamdi, B.A.; Bafeel, S.O.; Edris, S.; Atef, A.; Al-Matary, M.; Bahieldin, A. Physiological and molecular mechanisms underlying salt stress tolerance in jojoba (Simmondsia chinensis). Appl. Ecol. Environ. Res. 2021, 19, 1953–1982. [Google Scholar] [CrossRef]
| Salinity (ppm) | Shoot Length (cm) | Leaf Numbers (n) | Root Length (cm) | Root Numbers (n) | Fresh Weight of Whole Seedling (g) |
|---|---|---|---|---|---|
| Control | 4.57 ab ± 0.061 | 6.14 a ± 0.610 | 5.14 a ± 0.003 | 29.86 a ± 0.910 | 4.86 ab ± 0.008 |
| 2000 | 5.90 a ± 0.052 | 5.8 a ± 0.820 | 6.1 a ± 0.223 | 26.8 a ± 0.230 | 4.55 ab ± 0.021 |
| 3000 | 2.94 b ± 0.071 | 2.75 b ± 0.340 | 5.38 a ± 0.530 | 23.13 a ± 0.208 | 3.2 b ± 0.802 |
| 5000 | 4.0 ab ± 0.120 | 4.4 ab ± 0.240 | 6.4 a ± 0.210 | 18.8 a ± 0.304 | 7.0 a ± 0.504 |
| Subculture | Salinity (ppm) | Shoot Numbers (n) | Leaf Numbers (n) | Plant Length (cm) | Fresh Weight of Plantlet (g) |
|---|---|---|---|---|---|
| (A) Main effect of salinity | |||||
| Control | 3.67 b | 11.0 b | 3.08 b | 3.18 b | |
| 2000 | 30.04 a | 173.05 a | 17.66 a | 17.91 a | |
| 3000 | 14.17 ab | 67.72 ab | 8.22 ab | 6.88 ab | |
| 5000 | 7.73 b | 38.93 ab | 5.8 ab | 6.53 ab | |
| (B) Main effect of subculture | |||||
| Sub1 | 5.83 b | 21.75 b | 5.38 b | 3.13 b | |
| Sub2 | 12.09 b | 61.59 b | 7.95 b | 7.22 b | |
| Sub4 | 31.90 a | 184.05 a | 16.98 a | 20.0 a | |
| (C) The interaction effect of salinity and subculture | |||||
| Sub1 | Control | 3.8 c ± 0.002 | 9 d ± 0.010 | 3.8 bc ± 0.800 | 3.76 c ± 0.730 |
| Sub1 | 2000 | 5.5 bc ± 0.200 | 27.5 c ± 1.600 | 6.38 b ± 0.200 | 2.0 d ± 0.230 |
| Sub1 | 3000 | 5.33 bc ± 0.4 | 11.5 d ± 2.4 | 4.17 b ± 0.7 | 1.08 cd ± 0.52 |
| Sub1 | 5000 | 9 b ± 0.1 | 37.6 c ± 0.06 | 6.8 b ± 0.002 | 6.7 c ± 0.82 |
| Sub2 | Control | 3.5 c ± 0.8 | 11 d ± 0.5 | 3 bc ± 1.2 | 1.03 cd ± 0.90 |
| Sub2 | 2000 | 21.29 b ± 1.1 | 119.57 b ± 0.03 | 12.86 b ± 0.03 | 14.04 b ± 0.34 |
| Sub2 | 3000 | 10 b ± 0.6 | 40.67 c ± 0.9 | 7 b ± 0.5 | 4.08 c ± 1.03 |
| Sub2 | 5000 | 8.6 b ± 1.2 | 46 c ± 1.02 | 6.2 b ± 0.04 | 4.6 c ± 0.007 |
| Sub4 | Control | 3.7 c ± 0.8 | 14.3 d ± 0.04 | 2 b c ± 0.6 | 5.1 c ± 0.03 |
| Sub4 | 2000 | 66.86 a ± 1.03 | 392.85 a ± 0.001 | 35.36 a ± 1.2 | 39.96 a ± 0.62 |
| Sub4 | 3000 | 27.17 b ± 0.04 | 151 b ± 0.7 | 13.5 b ± 0.03 | 15.48 b ± 0.70 |
| Sub4 | 5000 | 5.6 bc ± 0.001 | 33.2 c ± 0.03 | 4.4 b ± 0.9 | 6.46 c ± 0.003 |
| Salinity (ppm) | Root Number (n) | Root Length (cm) | Leaves Number (n) | Shoot Length (cm) | Plantlet Fresh Weight (g) | Plantlet Dry Weight (g) |
|---|---|---|---|---|---|---|
| Control | 0.27 a ± 0.646 | 1.09 a ± 2.700 | 2.27 b ± 1.380 | 0.77 b ± 0.408 | 0.22 ab ± 0.869 | 0.063 ab ± 1.380 |
| 2000 | 0 b ± 0.000 | 0 b ± 0.000 | 3.92 a ± 0.428 | 1.31 ab ± 0.076 | 0.43 a ± 0.589 | 0.083 a ±0.301 |
| 3000 | 0.14 a ± 0.590 | 0.36 a ± 1.596 | 2.96 ab ± 0.135 | 1.52 a ± 0.038 | 0.32 ab ± 0.001 | 0.052 bc ± 1.002 |
| 5000 | 0 b ± 0.000 | 0 b ± 0.000 | 2.10 b ± 0.223 | 0.75 b ± 0.001 | 0.16 b ± 1.132 | 0.028 c ± 0.234 |
| Salinity (ppm) | Chlorophyll a (mg/100 g F.W) | Chlorophyll b (mg/100 g F.W) | Carotenoid (mg/100 g F.W) |
|---|---|---|---|
| Control | 18.69 ab ± 2.593 | 7.93 a ± 1.444 | 19.28 ab ± 1.593 |
| 2000 | 12.27 b ± 3.440 | 3.62 a ± 2.781 | 14.74 b ± 1.444 |
| 3000 | 30.44 a ± 3.311 | 5.35 a ± 1.605 | 33.84 a ± 0.897 |
| 5000 | 13.41 ab ± 2.441 | 3.26 a ± 3.363 | 15.96 ab ± 0.311 |
| Salinity (ppm) | Chlorophyll a (mg/100 g F.W) | Chlorophyll b (mg/100 g F.W) | Carotenoid (mg/100 g F.W) |
|---|---|---|---|
| Control | 17.42 a ± 0.084 | 15.98 a ± 0.776 | 21.57 a ± 1.135 |
| 2000 | 22.19 a ± 1.899 | 26.10 a ± 3.269 | 27.48 a ± 2.281 |
| 3000 | 19.16 a ± 1.583 | 19.56 a ± 1.399 | 23.41 a ± 1.429 |
| 5000 | 6.567 a ± 4.741 | 4.88 a ± 4.893 | 5.79 a ± 4.276 |
| Salinity (ppm) | Ca2+ (g/kg) | K+ (g/kg) | Na+ (g/kg) | Mg2+ (g/kg) |
|---|---|---|---|---|
| Control | 1.63 a ± 0.751 | 17.74 a ± 2.708 | 7.71 b ± 1.229 | 0.08 b ± 0.268 |
| 2000 | 1.61 a ± 0.623 | 12.28 a ± 1.727 | 26.99 ab ± 4.632 | 1.67 b ± 0.212 |
| 3000 | 3.08 a ± 1.829 | 17.05 a ± 2.851 | 46.78 a ± 1.771 | 2.14 ab ± 0.079 |
| 5000 | 2.43 a ± 1.032 | 8.82 a ± 1.455 | 72.87 a ± 1.378 | 4.75 a ± 0.973 |
| Salinity (ppm) | Enzyme Activity (unit/min/g) |
|---|---|
| Control | 0.147 a ± 0.0370 |
| 2000 | 0.054 b ± 0.0395 |
| 3000 | 0.052 b ± 0.0206 |
| 5000 | 0.06 b ± 0.0081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyousif, N.A.; El Sherif, F.; Yap, Y.-K.; Khattab, S. Selection of Salt-Tolerant Jojoba (Simmondisa chinensis L.) Cultivars via In Vitro Culture. Horticulturae 2023, 9, 675. https://doi.org/10.3390/horticulturae9060675
Alyousif NA, El Sherif F, Yap Y-K, Khattab S. Selection of Salt-Tolerant Jojoba (Simmondisa chinensis L.) Cultivars via In Vitro Culture. Horticulturae. 2023; 9(6):675. https://doi.org/10.3390/horticulturae9060675
Chicago/Turabian StyleAlyousif, Nouf Ali, Fadia El Sherif, Yun-Kiam Yap, and Salah Khattab. 2023. "Selection of Salt-Tolerant Jojoba (Simmondisa chinensis L.) Cultivars via In Vitro Culture" Horticulturae 9, no. 6: 675. https://doi.org/10.3390/horticulturae9060675
APA StyleAlyousif, N. A., El Sherif, F., Yap, Y.-K., & Khattab, S. (2023). Selection of Salt-Tolerant Jojoba (Simmondisa chinensis L.) Cultivars via In Vitro Culture. Horticulturae, 9(6), 675. https://doi.org/10.3390/horticulturae9060675





