Abstract
In this article, we present proliferation data from 10 years of the continuous in vitro incubation of cv. Solo papaya shoots and propose a reliable method for the long-term micropropagation of papaya, using microshoots developed from the axillary buds of papaya shoots as primary explants. Three different media were assayed. The proliferation medium (PPRM) allowed us to maintain papaya shoots under continuous proliferation for 20 years, maintaining consistent behavior. Most of the shoots developed in the PPRM rooted during the incubation and then acclimated easily, maintaining the ploidy and morphological characteristics of the parental plants, and flowering and setting fruits normally. The PPRM medium consisted of MS medium supplemented with naphthalene acetic acid (NAA) (0.1 mg L−1), benzyladenine (BA) (0.5 mg L−1), gibberellic acid (GA3) (0.5 mg L−1), and adenine hemisulphate (40 mg L−1). The average multiplication rate was higher than 20 shoots per explant during the long-term assay. The elongation medium (PELM) was designed to recover shoots with poor growth and allowed the development of high-quality shoots ready for rooting. It consisted of an MS basal medium supplemented with NAA (0.1 mg L−1), kinetin (KIN) (0.5 mg L−1), and GA3 (1 mg L−1). The rooting medium (PROM) was designed to induce high-quality roots from nonrooted shoots and consisted of a half-strength MS medium and indole-3-butiryc acid (IBA) (1 mg L−1). On PROM, agar can be exchanged for expanded vermiculite. Acclimation took place inside an acclimatization tunnel under progressive hydric stress. After 4 weeks, the plant recovery rate was 90% for plants maintained under continuous proliferation for ten years. The main objective of this work was to provide a micropropagation method which would maintain healthy elite genotypes of papaya for long periods of time and produce a high number of good quality plants.
1. Introduction
Papaya (Carica papaya L.) is an economically important fruit crop species belonging to the Caricaceae family which is cultured worldwide in tropical and subtropical areas because of the nutritive and medicinal value of its fruits. World production is estimated at 13,894,705 tonnes and India, Dominican Republic, Brazil, Mexico, Indonesia, and Nigeria are the major producers [1].
Papaya plants can be propagated vegetatively using classic methods such as cutting [2] and grafting [3], but these methods are inappropriate for large-scale production. Papayas are normally propagated from seeds, but this method is also problematic because of the high heterozygosis of the seeds and the dioecious character of the papaya, resulting in significant differences in yield, fruit quality, and pest and disease tolerance [4].
Micropropagation by tissue culture offers advantages over conventional propagation methods and has been widely applied to papaya species. Different approaches have been followed, such as axillary shoot culture [5,6,7,8] and plantlet recovery after shoot regeneration from somatic embryos [9,10,11,12,13,14,15,16]. This regeneration and plant recovery often occurs after genetic transformation procedures in this species [11,17,18,19,20,21,22,23,24], and it highlights the importance of developing reliable methods of preservation for long periods of time in order to finally obtain high-quality plants from selected lines of genetically modified papaya.
Reliable in vitro methods for the long-term micropropagation of papaya are also suitable for the in vitro preservation of germplasm from elite genotypes and mother plants (e.g., pathogen-free plants, elite genotypes selected through breeding), which provide protection from environmental stresses and are suitable for plant transportation across frontiers.
All the methods developed to date have been successful to varying degrees, depending on the papaya cultivars and the physiological conditions of the plants, but generally these protocols do not work properly when clonal lines need to support continuous culture for long periods of time, as is normally the case with transgenic or elite cell lines. After some months of incubation, there is a progressive decay of the in vitro shoots of the papaya, resulting in limited or null growth, dwarfing, defoliation, abnormal callus proliferation, loss of rooting capabilities, apical necrosis, degeneration, and, finally, death of the shoots.
Studies [25,26,27] confirm that somaclonal variation always occurs to varying degrees as the number of subcultures in vitro increases, depending on the plant genotype, the culture approach, and conditions. These somaclonal changes occurring during the long incubation period in vitro can severely affect development of the plant and the final product at the end of the micropropagation process, generating plants with undesirable, or sometimes useful, somaclonal variations, which are capable of producing morphological or functional changes at different levels (flowers, fruits, size, shape, color). Micropropagation through development of axillary buds is considered to be the in vitro plant regeneration system with the least genetic instability and somaclonal variation [28].
There are few studies concerning long-term in vitro assays in plant species: [29] worked with Ipecac (Cephaelis ipecacuanha) for 12 years to maintain mother plants under conditions of reduced growth and succeeded in regenerating viable and stable plants from this medicinal species. Other species such as cherimoya (Annona cherimola) have been also maintained in vitro for 250 subcultures (more than 20 years) in our laboratory, maintaining the shoot multiplication rate but losing most of their rooting capabilities (Encina, unpublished results). To date, even in the most recent reviews [30,31], we have found no references to long-term cultures focused on the proliferation and preservation of papaya in vitro over long periods of time.
The purpose of this work is to solve the problems of lack of proliferation, elongation/development, and rooting of shoots developed from nodal explants of selected papaya genotypes when maintained in vitro for long periods of time (e.g., in vitro collections of selected genotypes for plant breeders).
In this article, we propose a reliable method which overcomes the problems indicated above, allowing in vitro cultures of papaya to be maintained in active proliferation and full development for long time periods in order to produce and recover a high number of good quality papaya plants.
2. Materials and Methods
2.1. Plant Material and Disinfection
Micropropagation of papaya (Carica papaya L.) was initiated from axillary shoot tip explants from a 2-year-old mature papaya plant of the cv. “Solo” obtained from a seed. The mother plant was cultivated in a glasshouse at the IHSM “La Mayora” (CSIC-UMA, in Málaga, Spain). The axillary nodal sections used as explants were obtained in late spring from actively growing young shoots. These were induced by local application of an agar gelled solution of BA (100 mg L−1) in the form of sticky semisolid drops over the axillary bud areas of the main shoot, following the elimination of the main apical meristem of the mother plant. Nodal explants were surface sterilized for 20 min by immersion in 0.5% sodium hypochlorite solution containing a few drops of Tween-20, rinsed three times with sterile distilled water (5 min each), and cultured under aseptic conditions in different media (PPRM, PELM, PROM) for 8 weeks before being transferred to fresh medium. The survival rate of the explants was 98%.
Following several preliminary assays, three different media, papaya proliferation medium (PPRM), papaya elongation medium (PELM), and papaya rooting medium (PROM), were designed to improve shoot proliferation, elongation, and rooting, respectively, in the long-term culture of papaya. These three media were not designed to be used in sequence.
This long-term micropropagation study started in 1998, and the papaya cultures were maintained under continuous proliferation for 20 years (120 subcultures). Data were recorded for 13 years (1998–2010) and a plant stock was maintained in proliferation in PPRM medium until 2017.
2.2. Basal Nutrient Media and Culture Conditions
All media consisted of a formulation of MS salts [32] supplemented with (mg L−1): thiamine-HCl (0.1), pyridoxine-HCl (0.5), nicotinic acid (0.5), glycine (2), myo-inositol (100), sucrose (SUC) (3%), and agar (0.8%) (Sigma, A-1296). The rooting medium also consisted of MS salts but at half strength.
2.2.1. Media Composition
Control 1: MS + NAA (0.2 mg L−1) + BA (0.2 mg L−1) [7,14].
Control 2: MS + NAA (0.1 mg L−1) + KIN (0.5 mg L−1) [33].
PPRM: MS + NAA (0.1 mg L−1) + BA (0.5 mg L−1) + GA3 (0.5 mg L−1) + ADE (40 mg L−1).
PELM: MS + NAA (0.1 mg L−1) + KIN (0.5 mg L−1) + GA3 (1 mg L−1).
PROM: 0.5 × MS + NAA (0.2 mg L−1) + IBA (1 mg L−1) + ADE (10 mg L−1) + SUC (4%).
2.2.2. Culture Conditions
The pH of all the culture media was adjusted to 5.7 before autoclaving. For all experiments, 25 mL aliquots of medium were distributed into 150 × 25 mm test tubes, covered with polypropylene tops, and autoclaved for 20 min at 121 °C and 1.05 Kg cm−2. One explant was cultured per tube. Cultures were incubated at 25 ± 1 °C under a 16 h photoperiod with an irradiance of 45 µmol m−2 s−1 (400–700 nm) PAR (Photosynthetic Active Radiation) provided by cool white fluorescent tubes (F40 tubes Gro-Lux, Sylvania).
2.3. Culture Initiation and In Vitro Behavior during the First 2 Years
Shoot explants, which were obtained from axillary nodal sections, were cultured for two years on five media based on an MS mineral formulation and differing according to the supplements added (Control 1 (C1), Control 2 (C2), PPRM, PELM, and PROM). One hundred explants were cultured in each medium. The media were designed to encourage active growth of the papaya shoots in vitro, seeking different balances between axillary proliferation, shoot elongation, and rooting. Shoot sprouting of axillary buds was induced using the PPRM medium. Shoot proliferation and elongation were regarded as quality parameters.
2.4. Long-Term Shoot Proliferation (Years 3 to 10)
One hundred apical shoots (1.5–2 cm long) obtained from axillary shoots in the stock were subcultured every two months for 10 years, on the same media used in culture initiation. Data, including main shoot length, number of leaves, number and length of axillary shoots, number and length of roots, and rooting percentage, were collected from the shoot cultures in each subculture 6 times per year. The results achieved in both types of shoot explants and each media were compared.
2.4.1. Foliar Mineral Composition Analysis
The leaves for mineral analysis were obtained from 10-year-old in vitro rooted plantlets developed in PPRM. Mineral composition values for chlorotic and nonchlorotic papaya leaves were determined following the MAPA protocols [34]: total phosphorus through spectrophotometry using Vanadate–Molybdate reactive; boron via spectrophotometry using Azomethine H; total nitrogen via digestion and distillation; and calcium, magnesium, potassium, iron, copper, zinc, and manganese using atomic absorption spectrophotometry. The chlorotic and nonchlorotic leaves were collected from shoots growing in PPRM medium. The values obtained for nonchlorotic and chlorotic leaves were compared with normal values for mineral composition of papaya leaves in vivo.
2.4.2. Root System Morphology Analysis
The root system morphology of plantlets rooted in vitro in each medium (PPRM, PELM, and PROM, with five plantlets per medium) was analyzed using a developmental model [35]. Roots produced directly from the base of the plants were referred to as primary or first-order roots, those arising from the first-order as second-order roots, and so on. Roots were removed from the substrate by soaking them in water, after which they were gently washed, and excess water was removed with paper towels. Detailed root length measurements were performed for the five plantlets rooted in each medium. Roots were separated into branching orders and the lengths of the roots in each order were measured using video images (digital image analysis system IBAS-2000 (IPS)). The following morphometric parameters were determined: number of adventitious roots, number of roots of each order, and length of the roots present in each order, and these were compared between media. The plantlets used in this analysis belonged to subculture number 62, two subcultures later than the final data recorded in the long-term micropropagation described in this work, because of the destructive nature of the method applied to analyze the root structure.
2.5. Rooting
Apical shoots (2 cm long) with a minimum of 3 fully expanded leaves were used as explants for rooting. Good quality shoots were obtained from actively growing in vitro shoots. In parallel with the long-term assay, we carried out additional studies using inert substrates placed in the bottom of test tubes to improve rooting in papaya,. Perlite (Merck), vermiculite (Merck), siliceous sand, and liquid medium (MS + 1 mg L−1 IBA without agar) were pipetted into the tubes, resulting in a new protocol for rooting with maximum rooting rates, which involves substituting agar with expanded vermiculite. The percentage of rooted shoots and the number and length of the roots were recorded every 8 weeks.
2.6. Plantlet Acclimatization
The micropropagated plantlets were thoroughly washed in tap water and transplanted into polyethylene seedbed trays with 51 cells (4 cm wide × 4 cm deep) containing a mixture of autoclaved peat: vermiculite (1:1). Potted plantlets were maintained inside a polyethylene tunnel at 90% initial relative humidity (RH). The average temperature was 25 °C. The plastic cover was then temporarily removed every day, following an open–close cycle which gradually increased the time that the plastic cover was open each day, drastically reducing the RH for a limited period of time and then recovering the initial RH by closing the tunnel again. During this acclimatization process, plantlets were subject to controlled shocks of hydric stress to activate growth and development of roots and shoots. At the end of the experiment, the %RH was about 70%. During the experiment, plants were periodically watered and fertilized with ®Ficote (Scott O.M., Spain) containing 15% N, 6% P, 12% K, 2% Mg, 0.22% Fe, 0.08% Mn, 0.03 Zn, and 0.01 Cu. During this period, the PAR (400–700 nm) at the top of the plants was on average 200–300 µmol m−2 s−1 and the photoperiod was 12/12. A total of 30 plantlets from each medium were acclimatized after ten years of incubation in PPRM, PELM, and PROM media. Survival rate, shoot length, and number of leaves were recorded after 2 months of acclimation and comparisons made between the plantlets from each medium were obtained. Only ten acclimatized plants from each medium were grown until flowering and fruit setting to confirm the normal morphology of papayas after ten years in vitro.
2.7. Analysis of Genetic Stability
2.7.1. Ploidy Analysis Using Flow Cytometry
Papaya shoots grown in vitro with 20 years of incubation in PPRM medium were used for ploidy stability studies. The ploidy levels of twenty micropropagated papayas were determined by estimating the relative DNA content using flow cytometry (Ploidy Analyser PA-I; Partec GmbH, Münster, Germany). For analysis, 0.5 cm2 of young leaves or tips of papaya shoots were chopped using a razor blade for 30–60 s in a Petri dish containing 0.4 mL of nuclei isolation buffer (commercial Partec CyStain UV precise P, high resolution DNA staining kit 05-5002, extraction buffer) to release nuclei. The homogenate was filtered through a 50 lm nylon mesh (Partec 50 lm CellTrics disposable filter), and then the nuclei were stained with fluorescent dye (commercial Partec CyStain UV precise P, high resolution DNA staining kit 05-5002, staining buffer, about 1.6 mL). Finally, after 30 s of incubation, the samples were analyzed. Carica papaya cv. “Solo” (2n = 2x = 18) was used as an external standard. The nuclear DNA ploidy level of the samples was determined using channel values corresponding to the average G0/G1 peaks of the sample and standard plants. The peak relative to the standard nuclei was set to channel 50. Three independent repetitions were performed, with over 4000 nuclei being analyzed in each.
2.7.2. Somaclonal Variation Analysis
Because of the early loss of the original mother plant, verification of the “trueness-to-type” of the papaya progenies after 120 subcultures could not be carried out; however, the ploidy level, morphology, and flowering of the acclimated plants were evaluated to detect occurrence of possible anomalies.
2.8. Statistical Analysis
All data were analyzed using SPSS software (version 19.0; SPPS INC., Chicago, IL, USA). Normally distributed variables were analyzed using one-way ANOVA, and an HSD-Tukey test was used in the post hoc analysis for comparisons between the different culture media. Plant survival percentages were analyzed using generalized linear models with logit as the link function and binomial as the probability distribution.
3. Results
Papaya nodal explants were established in vitro under our culture conditions without problems: 98% survival was achieved, with a low level of contamination by microorganisms, and bud sprouting of the nodal explants in PPRM was over 85%.
3.1. Culture Initiation and In Vitro Behavior during the First Two Years
The results obtained during the first two years of incubation showed a clear and progressive loss of quality in both media used as the controls, C1 and C2 (Table 1). These two treatments were eliminated after 2 and 3 years of incubation, respectively, because the shoots and plantlets showed progressively clear signs of degeneration (defoliation, lack of growth, necrosis, and death), and finally, we were forced to discard both controls because of the minimal number of explants surviving in the culture. The other media, PPRM, PELM, and PROM, did not exhibit the growth and development problems which occurred in the control media (Table 1).

Table 1.
Comparison between micropropagation data obtained for nodal segments issued from axillary shoots of mature papayas. Average data corresponding to the first 2 years (12 subcultures). Data recorded every 8 weeks of incubation.
3.2. Long-Term Culture Assay (Years 3 to 10)
Throughout the ten years of culture, the data obtained for all proliferation parameters showed an acceptable homogeneity during incubation in PPRM, PELM, and PROM media, with the exception of the 4th and 8th years, in which all the cultures and parameters, and in particular the number of axillary shoots promoted in PPRM and PELM, decreased significantly for unknown reasons. Figure 1 shows the results (number of axillary shoots, length of axillary shoots, and number of leaves) obtained throughout the long-term micropropagation.
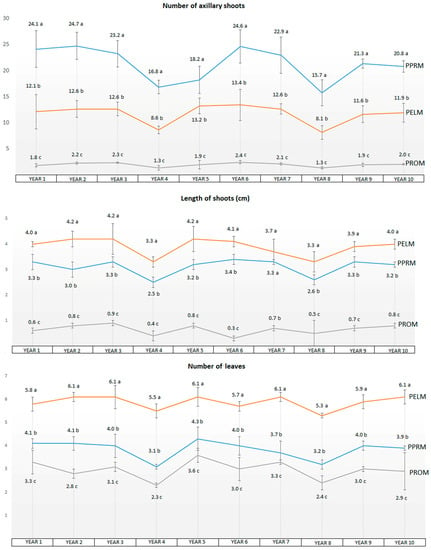
Figure 1.
Average data after an incubation of 10 years (60 subcultures, subculturing every 8 weeks) obtained for the different media assayed (PPRM, PELM, and PROM): no. of axillary shoots, length of axillary shoots, no. of leaves. Different letters indicate significant differences for each parameter between the various culture media using HSD-Tukey at α = 0.05.
3.2.1. In Vitro Behavior
Proliferation
As we can see in Table 1, the best results for proliferation of axillary shoots corresponded clearly to PPRM, which showed significant differences versus the PELM and PROM media, with the average number of axillary shoots ranging from 12.3 in PELM and 2.0 in PROM to 24.4 in PPRM to (Figure 1 and Figure 2). The most notable effects were the high number of axillary shoots obtained in PPRM and the almost total lack of shoot proliferation in PROM. With regard to the average length of shoots, the best values corresponded to PELM (4.1 cm) and to PPRM (3.2 cm), and the worst values to PROM (0.7 cm). Average values for the number of leaves were higher for PELM (5.9) and PPRM (4.1) and lower for PROM (3.0). The PROM medium showed the highest average values for rooting rate (74.8%) in comparison with PPRM (69.6%) and PELM (38.3%). With regard to the root length, small differences could be detected between treatments, with the best average values corresponding to the PPRM medium (3.1 cm), and almost identical values for the PELM and PROM media (2.6 and 2.7 cm, respectively).

Figure 2.
In vitro papaya plantlets growing in different media: (A) PPRM, (B) PELM, and (C) PROM.
Elongation
The PELM medium was designed to recover and grow the small axillary shoots obtained from proliferating clusters to an adequate size for rooting.
In PELM, a percentage of shoots (35.7%) developed roots, producing high-quality plantlets suitable for transplanting and acclimation.
The morphology of the shoots in explants incubated and developed in PELM was also different from that obtained when explants were incubated in PPRM. In general, PELM shoots grown in vitro were longer and stronger, generally without axillary bud development and growing as individual shoots, in contrast with the rosette pattern of growth shown by the shoots incubated in PPRM.
Rooting
The rooting percentage in PPRM fluctuated over time between 38% and 84%, with the lowest values corresponding to the years in which the general growth and development was poor (years 4 and 8). On average, 63.1% of the shoots developed roots (Figure 3). In the case of PELM, the rooting ranged from 15% to 60% and on average 35.7% of shoots rooted. In the PROM medium, the rooting rate ranged from 52% to 84%, with an average 70.3% success rate. In general, the root induction and development was better in the PROM medium but the values corresponding to PPRM were also good enough to compete with those obtained using PROM.
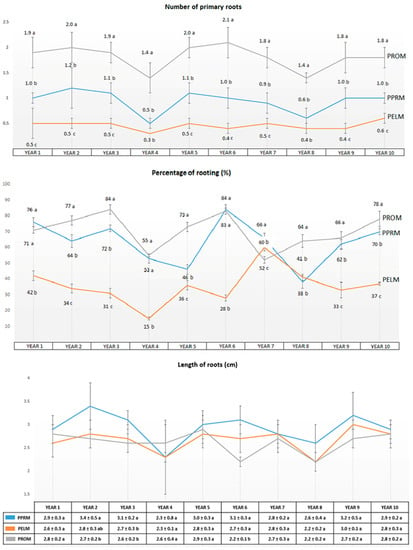
Figure 3.
Average data on rooting after an incubation of 10 years (60 subcultures, subculturing every 8 weeks) obtained for the different media assayed (PPRM, PELM, and PROM): rooting percentage, no. of roots, and length of roots. Different letters indicate significant differences for each parameter between the various culture media using HSD-Tukey at α = 0.05.
Analysis of the root system structure (Table 2) revealed that the higher numbers of roots for first- and second-order roots corresponded to the proliferation medium (PPRM), significantly higher than for PELM. No differences between PPRM and PROM were detected regarding the number of first- and third-order roots. PROM obtained the best results for root length, with the global growth for all orders of roots scoring clearly higher compared with the results obtained from the other media assayed (PPRM and PELM).

Table 2.
Results of analysis of papaya root morphology and development in vitro using the digital image analysis system. Data taken after 62 subcultures.
3.2.2. Foliar Mineral Composition Analysis
Throughout the long-term assays, some chlorosis problems of unknown origin were detected in the leaves of the papaya when incubated in the PPRM medium (Figure 4).

Figure 4.
Chlorotic leaves on in vitro papaya plantlets after incubation for 4 weeks in PPRM.
Some authors, such as Hidaka et al. [36], also detected chlorosis in leaves when papaya explants were incubated in media with high doses of BA and NAA (4 mg L−1), but this cause can be discarded in our studies because of the low levels (≤0.5 mg L−1) of these growth regulators supplementing the PPM medium. To explain this chlorosis, we carried out foliar analysis of in vitro nonchlorotic and chlorotic leaves collected from shoots growing in the PPRM medium and compared these with the normal values for mineral composition of papaya leaves in vivo (Table 3).

Table 3.
Data obtained from foliar mineral composition analysis of chlorotic and nonchlorotic papaya leaves obtained from in vitro plantlets grown for 4 weeks in PPRM. Normal values for the mineral composition of in vivo papaya leaves [37] are shown as a control.
3.3. Rooting
When the shoots were rooted in a simplified rooting medium consisting of an MS liquid medium supplemented with 1 mg L−1 IBA added over a base of expanded vermiculite, the rooting induction reached 100% in all of the experiments carried out.
3.4. Plantlet Acclimatization
No differences were detected in survival rate, which ranged around 90% in all the media assayed. The best results for development corresponded to plantlets obtained from explants developed in the PPRM and PROM media. These plants showed significant differences in the average shoot length and number of leaves compared with plants incubated in PELM (Table 4).

Table 4.
Average data corresponding to 30 plantlets obtained from in vitro plants 5 cm long developed on PPRM, PELM, and PROM after acclimation for 2 months. Data on survival rate, average length, and leaf number were recorded.
3.5. Analysis of Genetic Stability: Ploidy Analysis and Morphological Evaluation of Flowers
Using flow cytometry, we detected that the diploid level (2n = 2x = 18) of the mother plant remained diploid throughout the long-term culture. Analysis of the in vitro plantlets obtained at the end of the micropropagation process showed that these were also diploid (2n = 2x = 18), confirming the ploidy stability of papaya plants after 120 subcultures in vitro (Figure 5).
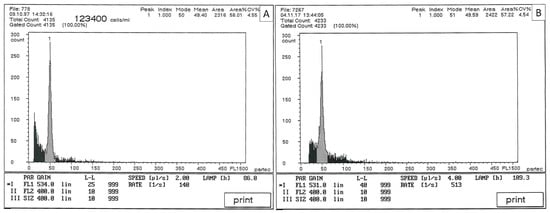
Figure 5.
Flow cytometry traces for: (A) Carica papaya cv. “Solo” used as mother plant (2n = 2x = 18) and (B) micropropagated papaya after 120 subcultures (2n = 2x = 18).
4. Discussion
4.1. Culture Initiation and In Vitro Behavior during the First 2 Years
It is clear that the proliferation medium (PPRM) with a molar ratio (MR) of BA/NAA: 4.13 promoted a good proliferation rate, significantly higher (24 shoots/explant) than the other media assayed. This is consistent with the findings from other research: Reuveni et al. [38] working with MS 0.5 mg L−1 BA + 1 mg L−1 NAA + 160 mg L−1 ADE (MR:12); Rajeevan and Pandey [39] with MS + 2 µM BA (MR: 15.8); McCubbin and van Staden [40] (2003) using DS salts and vitamins + 1 mg L−1 NAA + 1 mg L−1 BA + 3 g L−1 activated charcoal (MR: 3.5); and Shivayogi et al. [41] using MS + 2.0 mg L−1 BA + 1.0 mg L−1 NAA (MR: 19.9). The average results for PPRM of >24 shoots/explant were quite close to the 22 shoots/explant obtained by Anandan et al. [42] and the 25 shoots/explant obtained by Mumo et al. [43], using MS supplemented with 1 mg L−1 BA plus 0.01 mg L−1 NAA (MR: 82.6) or MS supplemented with 0.5 mg L−1 BA plus 0.1 mg L−1 NAA (MR: 4.13). Other authors, such as Roy et al. (2012) [44], also obtained high multiplication rates (33.8, 25.2, and 22.4 shoots/explant) using MS plus BA (0.5, 1.0, 1.5 mg L−1) and 0.1 mg L−1 NAA with MRs of 4.13, 8.2, and 12.4, respectively. When MS supplemented with 1 mg L−1 Zeatin (ZEA) plus 0.2 mg L−1 NAA was used, the multiplication rate increased to 34.2 shoots/explant, reaching the highest values (50) when casein hydrolysate was added to this medium. Unfortunately, all these protocols also require additional steps for shoot elongation and rooting to obtain full plantlets of papaya. Veena et al. [45] achieved the highest recorded number of shoots per explant (70) using MS medium plus 2.0 mg L−1 BA and 0.1 mg L−1 NAA (MR: 16.5).
A study of the MR cytokinins/auxin of the above micropropagation protocols showed that a MR BA/NAA = 4.13 and a MR ZEA/NAA = 4.25 provided the highest multiplication rates (24–34 shoots/explant). Veena et al. [45] achieved 70 shoots/explant with a higher MR BA/NAA = 16.5.
The elongation medium PELM offered a good rate of axillary shoot development and also ranked high for leaf growth, which provided very good quality cultures. The rooting medium PROM gave the best results for induction and development of roots (Table 1).
4.2. Long-Term Culture Assay (Years 3 to 10)
4.2.1. In Vitro Behavior
Proliferation
In general, the PROM medium was better for root induction and development, whereas the PPRM and PELM media were better for shoot development (Figure 2). The advantage of PPRM is that in just one step, it can regenerate plantlets of good quality, reducing costs and shortening the time in vitro. These results make the PPRM medium an excellent option for the maintenance of active collections of elite cell lines of papaya (e.g., transgenic new genotypes, selected breeding lines, mother plants, classic or endangered genotypes).
Elongation
Roy et al. [44] also developed an elongation method to improve the length of shoots before the rooting step by supplementing the MS medium with urea and activated charcoal plus zeatin (ZEA) and NAA, but the article provided no data regarding increases in shoot length or rooting rate obtained with this treatment. Other authors, such Wu et al. [46], used GA3 to obtain shoot elongation using an MS medium containing a low dose of BA (0.25 mg L−1).
Rooting
For most authors, IBA was the auxin choice for rooting papaya shoots. Rajeevan and Pandey [39] recorded 90% rooting success using MS + IBA 10 µM and Reuveni et al. [38] obtained a rooting rate close to 100% using MS + 1 mg L−1 IBA. Roy et al. [44] applied high concentrations of IBA (4 or 5 mg L−1) and obtained rooting of up to 90%. McCubbin and van Staden [40] combined a 1 h pulse in a 5 mg L−1 IBA solution with a second phase in DS salts and vitamins + 5.3 g L−1 PEG-6000 + 3 g L−1 Activated Charcoal in a vermiculite substrate and achieved rooting rates of 80%. Mumo et al. [43] applied 2.5 mg L−1 of IBA and achieved up to 83% rooting success. Hidaka et al. [36] and Van-Hong et al. [47] applied 2 mg L−1 IBA and obtained rooting rates of 85% and 100%, respectively. In the PROM medium, we combined lower doses of IBA (1 mg L−1) and NAA (0.2 mg L−1) in an agar substrate and the average rooting rate for our papaya line was 70.3%, with peaks of 84%, which is a similar range to those achieved by the abovementioned authors. Perez et al. [48] used ½ MS + 79 µM Phloroglucinol + 9.8 µM IBA + zeolite as the substrate and achieved 100% rooting with roots of good quality (1.76 roots/plant, 2.4 cm in length). Caple and Cheah [49], working with MS + 1 mg L−1 IBA + 4% sucrose, obtained a high rate of multiplication (65–95%). Shivayogi et al. [41] used MS + 3 mg L−1 NAA and achieved 80% rooting. However, even in the PPRM medium supplemented exclusively with NAA, we obtained an average rooting of 63.1% with peaks as high as 84%. Authors such as Fhaizal et al. [50], Anandan et al. [42], and Wu et al. [46] also applied a low level of auxin for rooting (0.5 mg L−1 IBA) and obtained results of around 75%, 60%, and 90%, respectively, which were close to our rooting rates.
Rooting in PPRM produced a mix of stumpy and normal roots, consistent with previous results obtained by Yu et al. [7], who rooted papaya shoots in MS culture media gelled with agar. The abnormal roots appear to result from the negative effect of the gelling agent on root development. Rooting on PROM, even in a gelled support, resulted in better quality roots. There were small differences in the results with regard to root number between the PPRM and PROM media. In PPRM, the number of second-order roots was higher than in PROM, but when the root length is considered, the PROM medium is clearly superior to the PPRM medium. Although the rooting induction rates are similar for both media, differences in root quality (root length) are important for plant recovery. Surprisingly, however, we did not detect differences in the acclimation and survival rates between these two treatments when transplanting. Apparently, both types of roots were functional and allowed the plantlets to grow and develop normally after transplanting. During the first month of growth in a glasshouse, the plantlets obtained from PROM seemed to develop faster than the ones originating from PPRM, but by the end of the second month these differences disappeared and both types of plants showed similar size and development.
4.2.2. Foliar Mineral Composition Analysis
The analysis showed that manganese, phosphorus, boron, and sodium values appeared to fall within the normal ranges for papaya leaves. There were low levels of calcium and magnesium and almost normal levels of potassium for nonchlorotic and chlorotic papaya leaves, by reference to the values accepted as adequate for papaya leaves by Tamimi et al. [37]. These low levels of calcium and magnesium are quite common in plants growing in vitro and sometimes create problems in micropropagation by causing apical necrosis, as in avocado [51]. Copper also appears at a low concentration in nonchlorotic papaya leaves and at even lower concentrations in chlorotic leaves. High levels of nitrogen are also frequent in vitro, probably due to the enormous amount of N available in the culture medium.
Iron was present in leaves with high values at the beginning of the incubation period and then decreased dramatically at the end of the incubation period, when the leaves started to fade, showing discoloration and chlorosis. We do not know the reason for this decrease in iron, but perhaps it could be due to one or more of the following factors: the exhaustion of the iron reserves in the culture medium, the huge uptake of iron for these fast growing clusters of papaya plantlets, and/or the light degradation of the iron chelate, making it difficult for the plantlets to absorb or some kind of interaction or inhibition caused by the very high level of zinc found in nonchlorotic in vitro leaves. Castillo et al. [52] indicated that a mix of iron chelates (EDDHA plus EDTA) improved shoot proliferation in papaya. After some assays in which we changed the type of iron chelate, we were not able to confirm this increase in shoot proliferation, possibly because we did not supplement the culture medium with both types of iron chelates together (EDTA and EDDHA). We only observed that when we switched between EDTA and EDDHA in the MS mineral formulation, the EDDHA–Ferric form delayed the discoloration and chlorosis of papaya leaves for some time compared with the EDTA–Ferric form, which is normally used in MS formulation. Another possible reason for the discoloration and chlorosis could be a magnesium deficiency, which can be inferred from the low levels detected in nonchlorotic and chlorotic leaves.
4.3. Rooting
Our results are completely consistent with those obtained by Yu et al. [7] and Panjaitan et al. [8], who achieved 94.5% and 90% rooting in an almost identical set of rooting assays, also using 1 mg L−1 IBA and vermiculite substrate but with a shorter incubation period. Using vermiculite as the substrate, Suksa-Ard et al. [53] and Caple and Cheah [49] achieved only limited rooting rates of good quality roots (56% and 54%, respectively).
These results suggest that an inert substrate such as expanded vermiculite is a very good option for growth and development of roots in papaya shoots in vitro, but rooting in PPRM in just one step also appears to be a very good method for obtaining plantlets that can be successfully acclimatized, because of the shorter incubation time and lower costs. The vermiculite protocol seems more appropriate for rooting recalcitrant shoots or shoots lacking roots after incubation in PPRM. Both rooting systems are clearly complementary and can be used together to obtain the maximum percentage of plantlets ready for acclimatization (Figure 6).

Figure 6.
Rooting results for papaya shoots over a physical support of expanded vermiculite with liquid MS medium supplemented with 1 mg L−1 IBA: (A) test tube with rooted papaya shoot, (B) papaya plantlets obtained from test tubes, and (C) detail of a papaya plantlet rooted in expanded vermiculite.
4.4. Plantlet Acclimatization
Different levels of success in acclimatization have been reported by various authors. Rajeevan and Pandey [39] recorded a 77.7% survival rate, Reuveni et al. [38] achieved an 85% acclimatization rate, and other reports have claimed 100% acclimatization success [5]. Thick, short, and stumpy roots as well as yellowing of leaves have frequently been reported in plantlets grown on agar-supplemented media [7,54,55,56]. We also reported some thick roots, but in general, for all media studied, normal well-branched roots were prevalent (see Table 3). This was true even in a medium with agar substrate, and the survival rate was high (90%) when plants with these roots were acclimatized and transplanted. This survival rate was higher than the 72%, 80%, and 40% recorded by Anandan et al. [42], Hidaka et al. [36], and Setargie et al. [57], respectively, and similar to the survival values recorded by Wu et al. [46] and Yu et al. [7] which ranged from 87% to 94.5%. The good quality of the roots obtained by Perez et al. [48] produced a high survival rate (96.5%). McCubbin and van Staden [40] and Shivayogi et al. [41] indicated success with acclimatization but without data regarding the percentage.
When we changed the rooting medium and the substrate to MS liquid plus 1 mg L−1 IBA in expanded vermiculite, we achieved 100% acclimatization success, even with shoots maintained for years in vitro.
Acclimatization finally allowed the plants to recover after long-term incubation in vitro, showing a normal morphology and development, flowering and fruiting normally after one year (Figure 7).

Figure 7.
Rooting and acclimatization of papaya explants and plantlets. (A) In vitro rooting of papaya shoots, (B) acclimatization of potted in vitro plantlets, and (C) field growth of acclimatized in vitro papaya plant.
4.5. Analysis of Genetic Stability: Ploidy Analysis and Morphological Evaluation of Flowers
After flow cytometry analysis, we can confirm that the initial diploid level (2n = 2x = 18) detected in the mother plant at the beginning of the culture was maintained by their progeny throughout the long-term incubation in vitro and after 120 subcultures (Figure 5). Additionally, the morphology of the plants and flowers after the acclimation and development of the papayas micropropagated in vitro for 10 years appeared to be normal in all the plants evaluated, with no morphological malformations or anomalies.
5. Conclusions
In this article, we present a reliable method for the maintenance and development of papaya plantlets in vitro which is valid for shoots obtained from any explant, which enables survival for more than twenty years in vitro while actively growing papaya cell lines and maintaining the original ploidy level of progenies. To achieve this, shoot explants were incubated in PPRM medium, and we also offer alternative methods for the efficient control of the morphogenesis (elongation, rooting, acclimatization) of papaya plants over long periods of time. We suggest the use of PPRM medium to obtain a continuous proliferation of papayas and also as a one-step method to produce full plantlets with good rates of success.
Author Contributions
J.J.R., formal analysis, experimental work, writing—original draft, and visualization; M.L.G., experimental work and writing—original draft; C.L.E., conceptualization, methodology, experimental work, writing—review and editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
Authors are grateful to J.R. Botella (University of Queensland, Australia) for providing papaya seeds and to Carlos Arana for English editing of the manuscript. This work has been partially supported by a postdoctoral grant of the Spanish Ministry of Education and Science.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agricultural Organization, United Nations, Roma. FAOSTAT Database. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 May 2022).
- Reuveni, O.; Shlesinger, D.R. Rapid vegetative propagation of papaya plants by cuttings. Acta Hortic. 1990, 275, 301–306. [Google Scholar] [CrossRef]
- Allan, P.; Clark, C.; Laing, M. Grafting papayas (Carica papaya L.). Acta Hortic. 2010, 851, 253–258. [Google Scholar] [CrossRef]
- Saker, M.M.; Bekheet, S.A.; Taha, H.S.; Reda, A.A. In vitro propagation of papaya (Carica papaya L.). Arab J. Biotech. 1999, 2, 235–244. [Google Scholar]
- Drew, R.A. Rapid clonal propagation of papaya in vitro from mature field-grown trees. HortScience 1988, 23, 609–611. [Google Scholar] [CrossRef]
- Drew, R.A. Micropropagation of Carica papaya and related species. In Micropropagation of Woody Trees and Fruits; Jain, S.M., Ishii, K., Eds.; Forestry Sciences; Springer: Dordrecht, The Netherlands, 2003; Volume 75, pp. 543–564. [Google Scholar] [CrossRef]
- Yu, T.-A.; Yeh, S.-D.; Cheng, Y.-H.; Yang, J.-S. Efficient rooting for establishment of papaya plantlets by micropropagation. Plant Cell Tissue Organ Cult. 2000, 61, 29–35. [Google Scholar] [CrossRef]
- Panjaitan, S.B.; Aziz, M.A.; Rashid, A.A.; Saleh, N.M. In-Vitro Plantlet Regeneration from Shoot Tip of Field-grown Hermaphrodite Papaya (Carica papaya L. cv. Eksotika). Int. J. Agric. Biol. 2007, 6, 827–832. [Google Scholar]
- Litz, R.E.; Conover, R.A. Effect of sex type, season, and other factors on in vitro establishment and culture of Carica papaya L. expiants. J. Am. Soc. Hortic. Sci. 1981, 106, 792–794. [Google Scholar] [CrossRef]
- Jordan, M.; Cortes, I.; Montenegro, G. Regeneration of plantlets by embryogenesis from callus cultures of Carica candamarcensis. Plant Sci. Lett. 1983, 28, 321–326. [Google Scholar] [CrossRef]
- Fitch, M.M.M.; Manshardt, R.M.; Gonsalves, D.; Slightom, J.L. Transgenic papaya plants from Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep. 1993, 12, 245–249. [Google Scholar] [CrossRef]
- Magdalita, P.M.; Persley, D.M.; Godwin, I.D.; Drew, R.A.; Adkins, S.W. Screening Carica papaya × C. cauliflora hybrids for resistance to papaya ringspot virus-type P. Plant Pathol. 1997, 46, 837–841. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Khuspe, S.S.; Renukdas, N.N.; Rawal, S.K. Somatic embryogenesis and plant regeneration from immature embryo explants of papaya cv. Washington and Honey Dew. Indian J. Exp. Biol. 2003, 40, 624–627. [Google Scholar]
- Fitch, M.M. Carica papaya Papaya. In Biotechnology of Fruits and Nut Crops; Litz, R.E., Ed.; Nº 29; CAB International: Wallingford, UK, 2005; pp. 17–207. [Google Scholar] [CrossRef]
- Anandan, R.; Sudhakar, D.; Balasubramanian, P.; Gutierrez-Mora, A. In vitro somatic embryogenesis from suspension cultures of Carica papaya L. Sci. Hortic. 2012, 136, 43–49. [Google Scholar] [CrossRef]
- Anandan, R.; Deenathayalan, T.; Nukala Sumanth Kumar Deepak, K.V. An alternative in vitro plant regeneration system in papaya (Carica papaya L.) through callus derived nodular cultures. Meta Gene 2018, 17, 147–152. [Google Scholar] [CrossRef]
- Fitch, M.M.M.; Pang, S.-Z.; Slightom, J.L.; Lius, S.; Tennant, P.; Manshardt, R.M.; Gonsalves, D. Genetic Transformation in Carica papaya L. (Papaya). In Plant Protoplasts and Genetic Engineering V; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1994; Volume 29. [Google Scholar] [CrossRef]
- Cai, W.; Gonsalves, C.; Tennant, P.; Fermin, G.; Souza, M., Jr.; Sarindu, N.; Jan, F.-J.; Zhu, H.-Y.; Gonsalves, D. A protocol for efficient transformation and regeneration of Carica papaya L. Vitr. Cell. Dev. Biol.-Plant 1999, 35, 61–69. [Google Scholar] [CrossRef]
- Magdalita, P.M.; Laurena, A.C.; Yabut-Perez, B.M.; Zaporteza, M.M.; Tecson-Mendoza, E.M.; Villegas, V.N.; Botella, J.R. Towards transformation, regeneration and screening of papaya containing antisense ACC synthase gene. In Plant Biotechnology 2002 and Beyond; Vasil, I.K., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 323–327. [Google Scholar] [CrossRef]
- Magdalita, P.M.; Laurena, A.C.; Perea, M.T.M. Cloning and characterization of partial 1-aminicyclopropane-1-carboxilate oxidase-gene and anti-sense transformation into yellow ‘Solo’ papaya via Agrobacterium tumefaciens. J. ISSAAS Int. Soc. Southeast Asian Agric. Sci. 2013, 19, 63–76. [Google Scholar]
- Kung, Y.-J.; Bau, H.-J.; Wu, Y.-L.; Huang, C.-H.; Chen, T.-M.; Yeh, S.-D. Generation of transgenic papaya with double resistance to Papaya ringspot virus and Papaya leaf-distortion mosaic virus. Phytopathology 2009, 99, 1312–1320. [Google Scholar] [CrossRef]
- Kung, Y.-J.; Yu, T.-A.; Huang, C.-H.; Wang, H.-C.; Wang, S.-L.; Yeh, S.-D. Generation of hermaphrodite transgenic papaya lines with virus resistance via transformation of somatic embryos derived from adventitious roots of in vitro shoots. Transgenic Res. 2010, 19, 621–635. [Google Scholar] [CrossRef]
- Fitch, M.M.M. Papaya ringspot virus (PRSV) resistance in papaya: Update on progress worldwide. Transgenic Plant J. 2010, 4, 16–28. [Google Scholar]
- Jia, R.; Zhao, H.; Huang, J.; Kong, H.; Zhang, Y.; Guo, J.; Huang, Q.; Guo, Y.; Wei, Q.; Zuo, J.; et al. Use of RNAi technology to develop a PRSV-resistant transgenic papaya. Sci. Rep. 2017, 7, 12636. [Google Scholar] [CrossRef]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation—A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Nebauer, S.G.; Arrillaga, I.; Segura, J. Assessments of somaclonal variation in micropropagated shoots of Cedrus: Consequences of axillary bud breaking. Tree Genet. Genomes 2005, 1, 3–10. [Google Scholar] [CrossRef]
- Jin, S.; Mushke, R.; Zhu, H.; Tu, L.; Lin, Z.; Zhang, Y.; Zhang, X. Detection of somaclonal variation of cotton (Gossypium hirsutum) using cytogenetics, flow cytometry and molecular markers. Plant Cell Rep. 2008, 27, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Clarindo, W.R.; Carvalho CR de Araujo, F.S.; Abreu IS de Otoni, W.C. Recovering polyploid papaya in vitro regenerants as screened by Flow cytometry. Plant Cell Tissue Organ Cult. 2008, 92, 207–214. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Jha, T.B. Conservation and Production of Ipecac (Cephaelis ipecacuanha Rich.) Plants from Long Term Shoot Cultures. Plant Tissue Cult. Biotechnol. 2008, 18, 157–164. [Google Scholar] [CrossRef]
- Al-Shara, B.; Mat Taha, R.; Rashid, K. Biotechnological methods and limitations of micropropagation in papaya (Carica papaya L.) production: A review. J. Anim. Plant Sci. 2018, 28, 1208–1226. [Google Scholar]
- da Costa, A.d.F.S.; Abreu, E.F.M.; Schmildt, E.R.; da Costa, A.N.; Schmildt, O. Advances observed in papaya tree propagation. Rev. Bras. Frutic. 2019, 41, 1–15. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mondal, M.; Gupta, S.; Mukherjee, B.B. In vitro propagation of shoot buds of Carica papaya L. (Caricaceae) var. Honey Dew. Plant Cell Rep. 1990, 8, 609–612. [Google Scholar] [CrossRef]
- MAPA. Métodos Oficiales de Análisis de Suelos, III; MAPA: Madrid, Spain, 1986; 166p. [Google Scholar]
- Rose, D.A. The description of the growth of root systems. Plant Soil 1983, 75, 405–415. [Google Scholar] [CrossRef]
- Hidaka, T.; Komori, S.; Yamada, M.; Fukamachi, H. Mass-production of papaya (Carica papaya L.) cv Shahi samplings using shoot-tip culture for commercial use. S. Pac. Stud. 2008, 28, 87–95. [Google Scholar]
- Tamimi, Y.N.; Silva, J.A.; Yost, R.S.; Hue, N.V. Adequate Nutrient Levels in Soils and Plants in Hawaii (General Guide). In Agronomy & Soils; AS-3; CTAHR: Honolulu, HI, USA, 1997; 2p. [Google Scholar]
- Reuveni, O.; Shlesinger, D.R.; Lavi, U. In vitro clonal propagation of dioecious Carica papaya. Plant Cell Tissue Organ Cult. 1990, 20, 41–46. [Google Scholar] [CrossRef]
- Rajeevan, M.S.; Pandey, R.M. Propagation of papaya through tissue culture. Acta Hortic. 1983, 131, 131–140. [Google Scholar] [CrossRef]
- McCubbin, M.J.; van Staden, J.; Debergh, P. A modified technique for in vitro propagation of papaya (Carica papaya L.). S. Afr. J. Bot. 2003, 69, 287–291. [Google Scholar] [CrossRef]
- Ryavalad, S.; Malabasari, T.A.; Shantappa, T.; Uppar, D.S.; Biradar, B.D.; Mantur, S.M. Micropropagation Studies in Papaya (Carica papaya L.) cv. ‘Surya’. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2362–2367. [Google Scholar] [CrossRef]
- Anandan, R.; Thirugnanakumar, S.; Sudhakar, D.; Balasubramanian, P. In vitro organogenesis and plantlet regeneration of Carica papaya L. J. Agric. Technol. 2011, 7, 1339–1348. [Google Scholar]
- Mumo, N.N.; Rimberia, F.K.; Mamati, G.E.; Kihurani, A.W. In vitro regeneration of selected Kenyan papaya (Carica papaya L.) lines through shoot tip culture. Afr. J. Biotechnol. 2013, 12, 6826–6832. Available online: https://www.ajol.info/index.php/ajb/article/view/130491 (accessed on 30 May 2022).
- Roy, P.K.; Roy, S.K.; Hakim, M.L. Propagation of papaya (Carica papaya L.) cv. Shahi through in vitro culture. Bangladesh J. Bot. 2012, 41, 191–195. [Google Scholar] [CrossRef]
- Veena, G.L.; Dinesh, M.R.; Kumar, R.A. Axillary bud culture in papaya. Bioinfolet 2015, 12, 147–149. [Google Scholar]
- Wu, K.; Zeng, S.; Chen, Z.; Duan, J. In vitro mass propagation of hermaphroditic Carica papaya cv. Meizhonghong. Pak. J. Bot. 2012, 44, 1669–1676. [Google Scholar]
- Nguyen, V.-H.; Yen, C.-R.; Hsieh, C.-H. Effects of nutritional and growth hormonal factors on in vitro regeneration of papaya (Carica papaya L. cv. Red Lady). J. Natl. Sci. Found. Sri Lanka 2018, 46, 559–568. [Google Scholar] [CrossRef]
- Pérez, L.P.; Montesinos, Y.P.; Olmedo, J.G.; Barbon Rodriguez, R.; Sánchez, R.R.; Montenegro, O.N.; Escriba, R.C.R.; Daniels, D.; Gómez-Kosky, R. Effect of phloroglucinol on rooting and in vitro acclimatization of papaya (Carica papaya L. var. Maradol Roja). Vitr. Cell. Dev. Biol.-Plant 2016, 52, 196–203. [Google Scholar] [CrossRef]
- Caple, A.D.; Cheah, K.T. Micropropagation of Hermaphrodite Carica papaya L. ‘Rainbow’ Seedlings via Axillary Bud Pathway. Biotechnology 2016, 12, 1–5. [Google Scholar]
- Fhaizal, M.B.; Vilasini, P.; Noorsaadah, A.R.; Norzulaani, K. Effect of carbenicillin on somatic embryos formation of papaya (Carica papaya L. var Eksotika I). Malays. J. Sci. 2006, 25, 47–54. [Google Scholar]
- Pliego-Alfaro, F.; López-Encina, C.; Barceló-Muñoz, A. Propagation of avocado rootstocks by tissue culture. S. Afr. Avocado Grow. Assoc. Yearb. 1987, 10, 36–39. [Google Scholar]
- Castillo, B.; Smith, M.A.L.; Madhavi, D.L.; Yadava, U.L. Interactions of irradiance level and iron chelate source during shoot tip culture of Carica papaya L. HortScience 1997, 32, 1120–1123. [Google Scholar] [CrossRef]
- Suksa-Ard, P.; Kataoka, I.; Beppu, K.; Fujime, Y.; Subhadrabandhu, S. Root Development of Tissue-Cultured Papaya Shoots in Several Rooting Substrates. Environ. Control Biol. 1998, 36, 115–120. [Google Scholar] [CrossRef]
- Drew, R.A. The effects of medium composition and cultural conditions on in vitro root initiation and growth of papaya (Carica papaya L.). J. Hortic. Sci. 1987, 62, 551–556. [Google Scholar] [CrossRef]
- Kataoka, I.; Inoue, H. Studies on the clonal propagation for tropical and subtropical fruit trees by tissue culture. In In Vitro Propagation of Papaya; Technical Bulletin of Faculty of Agriculture, Kagawa University: Kagawa, Japan, 1987; Volume 38, pp. 7–10. [Google Scholar]
- Teo, C.K.H.; Chan, L.K. The effects of agar content, nutrient concentration, genotype and light intensity on the in vitro rooting of papaya microcuttings. J. Hortic. Sci. 1994, 62, 267–273. [Google Scholar] [CrossRef]
- Setargie, A.; Mekbib, F.; Abraha, E. In vitro propagation of papaya (Carica papaya L.). World J. Agric. Res. 2015, 11, 84–88. Available online: https://www.idosi.org/wjas/wjas11(2)15/4.pdf (accessed on 30 May 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).