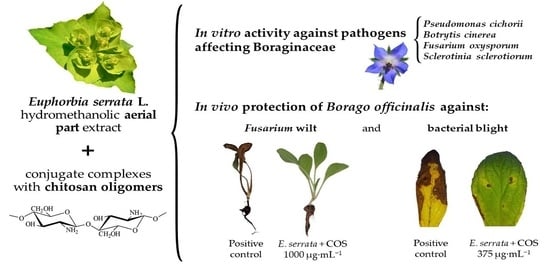
Phytochemical Constituents and Antimicrobial Activity of Euphorbia serrata L. Extracts for Borago officinalis L. Crop Protection
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Samples and Reagents
2.2. Phytopathogens
2.3. Preparation of E. serrata Extract, Chitosan Oligomers, and Their Conjugate Complex
2.4. Physicochemical Characterization
2.5. In Vitro Antimicrobial Activity Assessment
2.6. In Vivo Tests on Borage
3. Results
3.1. Infrared Spectra
3.2. GC−MS Characterization of the Extract
3.3. In Vitro Antimicrobial Activity
3.3.1. In Vitro Antibacterial Activity
3.3.2. In Vitro Antifungal Activity
3.4. In Vivo Antimicrobial Activity
3.4.1. In Vivo Antibacterial Activity against P. cichorii
3.4.2. In Vivo Antifungal Activity against F. oxysporum
4. Discussion
4.1. On the Phytochemical Profile of the Extract
4.2. On the Antimicrobial Activity in Comparison with Other Euphorbia spp. Extracts
4.3. On the Efficacy of Other Natural Compounds against the Phytopathogens under Study
4.4. Comparison with Synthetic Fungicides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, R.J. Euphorbia serrata latex. In Sax’s Dangerous Properties of Industrial Materials, 11th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; p. 1737. [Google Scholar] [CrossRef]
- Upadhyay, R.R.; Ansarin, M.; Zarintan, M.H.; Shakui, P. Tumor promoting constituent of Euphorbia serrata L. latex. Experientia 1976, 32, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.V.; da Silva, L.S.; Faleiros, C.A.; Silva, V.A.O.; Reis, R.M. The role of ingenane diterpenes in cancer therapy: From bioactive secondary compounds to small molecules. Nat. Prod. Commun. 2022, 17, 1–30. [Google Scholar] [CrossRef]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry 2006, 67, 1977–1984. [Google Scholar] [CrossRef]
- Panzu, P.; Inkoto, C.; Ngbolua, K.; Mukeba, F.; Kitadi, J.M.; Taba, K.; Mbala, B.; Tshilanda, D.; Kayembe, J.P.K.; Pius Mpiana, T. Review on the phytochemistry, toxicology and bioactivities of Euphorbia hirta L.: A potential antisickling medicinal plant species. J. Med. Plant Herb. Ther. Res. 2020, 7, 8–18. [Google Scholar]
- Rajeh, M.A.B.; Zuraini, Z.; Sasidharan, S.; Latha, L.Y.; Amutha, S. Assessment of Euphorbia hirta L. leaf, flower, stem and root extracts for their antibacterial and antifungal activity and brine shrimp lethality. Molecules 2010, 15, 6008–6018. [Google Scholar] [CrossRef]
- Mekam, P.N.; Martini, S.; Nguefack, J.; Tagliazucchi, D.; Stefani, E. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. S. Afr. J. Bot. 2019, 127, 319–332. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.W. Chemical studies on the constituents of the chinese medicinal herb Euphorbia helioscopia L. Chem. Pharm. Bull 2006, 54, 1037–1039. [Google Scholar] [CrossRef]
- Alghazeer, R.; El-Saltani, H.; Saleh, N.; Al-Najjar, A.; Hebail, F. Antioxidant and antimicrobial properties of five medicinal Libyan plants extracts. Nat. Sci. 2012, 4, 324–335. [Google Scholar] [CrossRef]
- Azimova, S.S.; Glushenkova, A.I. Euphorbia serrata L. In Lipids, Lipophilic Components and Essential Oils from Plant Sources; Azimova, S.S., Glushenkova, A.I., Eds.; Springer: London, UK, 2012; p. 373. [Google Scholar] [CrossRef]
- Zahid, K. Evaluation of antifungal activity of nine members of family Euphorbiaceae of Lahore Region against Aspergillus niger, Rhizopus oryzae & Alternaria solani. Am. J. Biomed. Sci. Res. 2019, 6, 216–220. [Google Scholar] [CrossRef]
- Smaili, A.; Mazoir, N.; Rifai, L.A.; Koussa, T.; Makroum, K.; Benharref, A.; Faize, L.; Alburquerque, N.; Burgos, L.; Belfaiza, M.; et al. Antimicrobial activity of two semisynthetic triterpene derivatives from Euphorbia officinarum latex against fungal and bacterial phytopathogens. Nat. Prod. Commun. 2017, 12, 331–336. [Google Scholar] [CrossRef]
- González, V.; Aguado, A.; Garcés-Claver, A. First report of Fusarium oxysporum causing wilt and root rot in common borage (Borago officinalis) in Spain. Plant Dis. 2019, 103, 1774. [Google Scholar] [CrossRef]
- Bertetti, D.; Pensa, P.; Saracco, P.; Gullino, M.; Garibaldi, A. Recent disease observed on ornamental species bred in Liguria. Prot. Delle Colt. 2009, 2, 58–59. [Google Scholar]
- Garibaldi, A.; Pensa, P.; Bertetti, D.; Gullino, M.L. First report of white mold caused by Sclerotinia sclerotiorum on Borago officinalis in Italy. Plant Dis. 2008, 92, 1711. [Google Scholar] [CrossRef]
- Bradley, C.A.; del Río, L.E.; Chesrown, C.D.; Johnson, B.L. First report of soft rot caused by Sclerotinia sclerotiorum on borage in North Dakota. Plant Dis. 2005, 89, 208. [Google Scholar] [CrossRef]
- Cambra, M.A.; Palacio-Bielsa, A.; López, M.M. Borage (Borago officinalis) is a new host of Pseudomonas cichorii in the Ebro Valley of Spain. Plant Dis. 2004, 88, 769. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.; Ballesteros, A.; Morales, J.; Kidibule, P.; Fernandez-Lobato, M. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2018, 36, 57–67. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Sánchez-Hernández, E.; Baquero-Foz, R.; Martín-Ramos, P.; Martín-Gil, J.; Torres-Sánchez, S.; Casanova-Gascón, J. Chitosan-based bioactive formulations for the control of powdery mildew in viticulture. Agronomy 2022, 12, 495. [Google Scholar] [CrossRef]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; EUCAST-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The joint action of fungicides in mixtures: Comparison of two methods for synergy calculation. Bull. OEPP 1986, 16, 651–657. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; González-García, V.; Palacio-Bielsa, A.; Lorenzo-Vidal, B.; Buzón-Durán, L.; Martín-Gil, J.; Martín-Ramos, P. Antibacterial activity of Ginkgo biloba extracts against Clavibacter michiganensis subsp. michiganensis, Pseudomonas spp., and Xanthomonas vesicatoria. Horticulturae 2023, 9, 461. [Google Scholar] [CrossRef]
- Günzler, H.; Gremlich, H.-U. IR-Spektroskopie: Eine Einführung, 4th ed.; John Wiley & Sons: Weinheim, Germany, 2012; p. 352. [Google Scholar]
- Klesk, K.; Qian, M.; Martin, R.R. Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington. J. Agric. Food. Chem. 2004, 52, 5155–5161. [Google Scholar] [CrossRef] [PubMed]
- Mozūraitis, R.; Apšegaitė, V.; Radžiutė, S.; Aleknavičius, D.; Būdienė, J.; Stanevičienė, R.; Blažytė-Čereškienė, L.; Servienė, E.; Būda, V. Volatiles produced by yeasts related to Prunus avium and P. cerasus fruits and their potentials to modulate the behaviour of the pest Rhagoletis cerasi fruit flies. J. Fungi 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.I.; Córdoba, M.G.; Casquete, R.; Serradilla, M.J.; Martín, A. Selection and application of antifungal VOCs-producing yeasts as biocontrol agents of grey mould in fruits. Food Microbiol. 2020, 92, 103556. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef]
- Arrarte, E.; Garmendia, G.; Rossini, C.; Wisniewski, M.; Vero, S. Volatile organic compounds produced by Antarctic strains of Candida sake play a role in the control of postharvest pathogens of apples. Biol. Control 2017, 109, 14–20. [Google Scholar] [CrossRef]
- Åkesson, C.; Lindgren, H.; Pero, R.W.; Leanderson, T.; Ivars, F. Quinic acid is a biologically active component of the Uncaria tomentosa extract C-Med 100®. Int. Immunopharmacol. 2005, 5, 219–229. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022, 1–30. [Google Scholar] [CrossRef]
- Bai, J.; Wu, Y.; Zhong, K.; Xiao, K.; Liu, L.; Huang, Y.; Wang, Z.; Gao, H. A comparative study on the effects of quinic acid and shikimic acid on cellular functions of Staphylococcus aureus. J. Food Prot. 2018, 81, 1187–1192. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, Y.; Yi, G.; Li, M.; Liao, L.; Yang, C.; Cho, C.; Zhang, B.; Zhu, J.; Zou, K.; et al. Quinic acid: A potential antibiofilm agent against clinical resistant Pseudomonas aeruginosa. Chin. Med. 2021, 16, 72. [Google Scholar] [CrossRef]
- Peng, W.X.; Wu, F.J.; Wu, Y.Q.; Zhang, Z.F. Study on biomedical resources of benzene/alcohol extractives of Eucalyptus leaves by GC/MS. Adv. Mater. Res. 2010, 97–101, 2231–2236. [Google Scholar] [CrossRef]
- Budhiraja, H.; Gupta, R.K.; Nand, P. Formulation and characterization of Cucumis sativus extract in the treatment of acne. World J. Pharm. Pharm. Sci. 2014, 3, 1043–1057. [Google Scholar]
- Ma, Q.-Z.; Wu, F.-J.; Zhang, D.-Q.; Zhang, M.-L.; Liu, Q.-M. Study on risk management of investment on food and drug safety of fiddlehead. In Proceedings of the 2010 International Conference on Management and Service Science, Wuhan, China, 24–26 August 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, M.; Liu, F.; Zeng, S.; Hu, J. Identification of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose–histidine Maillard reaction products. Food Res. Int. 2013, 51, 397–403. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Cuchí-Oterino, J.A.; Martín-Gil, J.; Lorenzo-Vidal, B.; Martín-Ramos, P. Dwarf pomegranate (Punica granatum L. var. nana): Source of 5-HMF and bioactive compounds with applications in the protection of woody crops. Plants 2022, 11, 550. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Balduque-Gil, J.; González-García, V.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Phytochemical profiling of Sambucus nigra L. flower and leaf extracts and their antimicrobial potential against almond tree pathogens. Int. J. Mol. Sci. 2023, 24, 1154. [Google Scholar] [CrossRef]
- Suman, T.Y.; Elumalai, D.; Kaleena, P.K.; Rajasree, S.R.R. GC−MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia baccifera aerial extract and its larvicidal activity against malaria and filariasis vectors. Ind. Crops Prod. 2013, 47, 239–245. [Google Scholar] [CrossRef]
- Thangavelu, S.; Balasubramanian, B.; Palanisamy, S.; Shanmugam, V.; Natchiappan, S.; Kalibulla, S.I.; Rathinasamy, B.; Arumugam, V.A. Characterization and phytoconstituents of Petroselinum crispum (Mill) and Coriandrum sativum (Linn) and their impacts on inflammation—An in vitro analysis against human adenocarcinoma cells with molecular docking. S. Afr. J. Bot. 2022, 146, 776–788. [Google Scholar] [CrossRef]
- Flouzat, C.; Bresson, Y.; Mattio, A.; Bonnet, J.; Guillaumet, G. Novel nonopioid non-antiinflammatory analgesics: 3-(aminoalkyl)-and 3-[(4-aryl-1-piperazinyl) alkyl] oxazolo [4, 5-b] pyridin-2 (3H)-ones. J. Med. Chem. 1993, 36, 497–503. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Martín-Ramos, P.; Navas Gracia, L.M.; Martín-Gil, J.; Garcés-Claver, A.; Flores-León, A.; González-García, V. Armeria maritima (Mill.) Willd. flower hydromethanolic extract for cucurbitaceae fungal diseases control. Molecules 2023, 28, 3730. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Balduque-Gil, J.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; González-García, V.; Cuchí-Oterino, J.A.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Holm oak (Quercus ilex subsp. ballota (Desf.) Samp.) bark aqueous ammonia extract for the control of invasive forest pathogens. Int. J. Mol. Sci. 2022, 23, 11882. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical characterization and antimicrobial activity against Erwinia amylovora, Erwinia vitivora, and Diplodia seriata of a light purple Hibiscus syriacus L. cultivar. Plants 2021, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Ryder, N.S. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992, 126, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Jabbari, H.; Shendabadizad, R. GC−MS analysis of essential oils of Humulusn lupulus, Malva Sylvestris and thymus plants in water solvent. J. Adv. Pharm. Educ. Res. 2020, 10, 57. [Google Scholar]
- Sharma, P.; Shri, R.; Ntie-Kang, F.; Kumar, S. Phytochemical and ethnopharmacological perspectives of Ehretia laevis. Molecules 2021, 26, 3489. [Google Scholar] [CrossRef]
- Joshi, U.P.; Wagh, R.D. GC−MS analysis of phytochemical compounds present in the stembark extracts of plant Maytenus emarginata. Res. J. Pharm. Biol. Chem. Sci. 2019, 10, 159–165. [Google Scholar]
- Daud, M.; Sohail, S.; Nangyal, H.; Hidayatullah, M.M.; Akhtar, B.; Khattak, Z.F. Comparative in vitro study of Euphorbia cf Serrata stem extract against different pathogenic bacteria. Int. J. Biosci. 2014, 4, 182–186. [Google Scholar]
- Jayalakshmi, B.; Raveesha, K.; Amruthesh, K. Phytochemical analysis and antibacterial activity of Euphorbia cotinifolia Linn. leaf extracts against phytopathogenic bacteria. J. Pharm. Res. 2011, 4, 3759–3762. [Google Scholar]
- Shirazi, M.; Abid, M.; Hussain, F.; Abbas, A.; Sitara, U. Antifungal activity of some medicinal plant extracts against soil-borne phytopathogens. Pak. J. Bot. 2020, 52, 679–685. [Google Scholar] [CrossRef]
- Upadhyay, B.; Singh, K.; Kumar, A. Ethno-medicinal, phytochemical and antimicrobial studies of Euphorbia tirucalli L. J. Phytol. 2010, 2, 65–67. [Google Scholar]
- Salhi, N.; Mohammed Saghir, S.A.; Terzi, V.; Brahmi, I.; Ghedairi, N.; Bissati, S. Antifungal activity of aqueous extracts of some dominant Algerian medicinal plants. BioMed. Res. Int. 2017, 2017, 7526291. [Google Scholar] [CrossRef]
- Ashraf, A.; Sarfraz, R.A.; Rashid, M.A.; Shahid, M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J. Food Drug Anal. 2015, 23, 109–115. [Google Scholar] [CrossRef]
- Al-Mughrabi, K. Antimicrobial activity of extracts from leaves, stems and flowers of Euphorbia macroclada against plant pathogenic fungi. Phytopathol. Mediterr. 2003, 42, 245–250. [Google Scholar]
- Kirbag, S.; Erecevit, P.; Zengin, F.; Guvenc, A.N. Antimicrobial activities of some Euphorbia species. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 305–309. [Google Scholar] [CrossRef]
- Kamba, A.; Hassan, L. Phytochemical screening and antimicrobial activities of Euphorbia balsamifera leaves, stems and root against some pathogenic microorganisms. Afr. J. Pharm. Pharmacol. 2010, 4, 645–652. [Google Scholar]
- Awaad, A.S.; Alothman, M.R.; Zain, Y.M.; Zain, G.M.; Alqasoumi, S.I.; Hassan, D.A. Comparative nutritional value and antimicrobial activities between three Euphorbia species growing in Saudi Arabia. Saudi Pharm. J. 2017, 25, 1226–1230. [Google Scholar] [CrossRef]
- Mohamed, S.; Saka, S.; El-Sharkawy, S.H.; Ali, A.M.; Muid, S. Antimycotic screening of 58 Malaysian plants against plant pathogens. Pestic. Sci. 1996, 47, 259–264. [Google Scholar] [CrossRef]
- Rao, K.V.B.; Karthik, L.; Elumalai, E.; Srinivasan, K.; Kumar, G. Antibacterial and antifungal activity of Euphorbia hirta l. leaves: A comparative study. J. Pharm. Res. 2010, 3, 548. [Google Scholar]
- Singh, G.; Kumar, P. Phytochemical study and screening for antimicrobial activity of flavonoids of Euphorbia hirta. Int. J. Appl. Basic Med. Res. 2013, 3, 111. [Google Scholar] [CrossRef]
- De Araújo, K.M.; De Lima, A.; do N. Silva, J.; Rodrigues, L.L.; Amorim, A.G.; Quelemes, P.V.; Dos Santos, R.C.; Rocha, J.A.; De Andrades, É.O.; Leite, J.R.S.; et al. Identification of phenolic compounds and evaluation of antioxidant and antimicrobial properties of Euphorbia tirucalli L. Antioxidants 2014, 3, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Dou, J.; Xu, J.; Aisa, H.A. Chemical composition, antimicrobial and antitumor activities of the essential oils and crude extracts of Euphorbia macrorrhiza. Molecules 2012, 17, 5030–5039. [Google Scholar] [CrossRef] [PubMed]
- Swamy, K.M.; Pokharen, N.; Dahal, S.; Anuradha, M. Phytochemical and antimicrobial studies of leaf extract of Euphorbia neriifolia. J. Med. Plants Res. 2011, 5, 5785–5788. [Google Scholar]
- Waheed, K.; Muhammad, S.K.; Shomaila, A.; Muhammad, Z.; Izhar, U.; Ullah, S. Antimicrobial activity and phytochemical screening of Euphorbia helioscopia. Planta Daninha 2020, 38, e020213727. [Google Scholar] [CrossRef]
- Behiry, S.I.; Okla, M.K.; Alamri, S.A.; El-Hefny, M.; Salem, M.Z.M.; Alaraidh, I.A.; Ali, H.M.; Al-Ghtani, S.M.; Monroy, J.C.; Salem, A.Z.M. Antifungal and antibacterial activities of wood treated with Musa paradisiaca L. peel extract: HPLC analysis of phenolic and flavonoid contents. Processes 2019, 7, 215. [Google Scholar] [CrossRef]
- El-Hefny, M.; Salem, M.Z.M.; Behiry, S.I.; Ali, H.M. The potential antibacterial and antifungal activities of wood treated with Withania somnifera fruit extract, and the phenolic, caffeine, and flavonoid composition of the extract according to HPLC. Processes 2020, 8, 113. [Google Scholar] [CrossRef]
- Delisle-Houde, M.; Tweddell, R.J.; Beres, B. Sugar maple autumn-shed leaf extract: A potential antibacterial agent for the management of lettuce bacterial leaf spot (Xanthomonas campestris pv. vitians) and varnish spot (Pseudomonas cichorii). Can. J. Plant Sci. 2020, 100, 78–85. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Sánchez-Hernández, E.; Martín-Ramos, P.; Navas-Gracia, L.M.; García-González, M.C.; Oliveira, R.; Martín-Gil, J. Silene uniflora extracts for strawberry postharvest protection. Plants 2023, 12, 1846. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Martín-Ramos, P.; Martín-Gil, J.; Santiago-Aliste, A.; Hernández-Navarro, S.; Oliveira, R.; González-García, V. Bark extract of Uncaria tomentosa L. for the control of strawberry phytopathogens. Horticulturae 2022, 8, 672. [Google Scholar] [CrossRef]
- Boiteux, J.; Espino, M.; de los Ángeles Fernández, M.; Pizzuolo, P.; Silva, M.F. Eco-friendly postharvest protection: Larrea cuneifolia-NADES extract against Botrytis cinerea. Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo 2019, 51, 427–437. [Google Scholar]
- Yusoff, S.F.; Haron, F.F.; Tengku Muda Mohamed, M.; Asib, N.; Sakimin, S.Z.; Abu Kassim, F.; Ismail, S.I. Antifungal activity and phytochemical screening of Vernonia amygdalina extract against Botrytis cinerea causing gray mold disease on tomato fruits. Biology 2020, 9, 286. [Google Scholar]
- Gwiazdowska, D.; Uwineza, P.A.; Frąk, S.; Juś, K.; Marchwińska, K.; Gwiazdowski, R.; Waśkiewicz, A. Antioxidant, antimicrobial and antibiofilm properties of Glechoma hederacea extracts obtained by supercritical fluid extraction, using different extraction conditions. Appl. Sci. 2022, 12, 3572. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. Application of plant extracts to control postharvest gray mold and susceptibility of apple fruits to B. cinerea from different plant hosts. Foods 2020, 9, 1430. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Dambrauskienė, E.; Viškelis, P.; Valiuškaitė, A. Biocontrol of strawberry pathogen Botrytis cinerea using plant extracts and essential oils. Zemdirbyste 2020, 107, 147–152. [Google Scholar] [CrossRef]
- Righini, H.; Baraldi, E.; García Fernández, Y.; Martel Quintana, A.; Roberti, R. Different antifungal activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Mar. Drugs 2019, 17, 299. [Google Scholar] [CrossRef]
- Şesan, T.E.; Enache, E.; Iacomi, B.M.; Oprea, M.; Oancea, F.; Iacomi, C. Antifungal activity of some plant extracts against Botrytis cinerea Pers. in the blackcurrant crop (Ribes nigrum L.). Acta Sci. Pol. Hortorum Cultus 2015, 14, 29–43. [Google Scholar]
- Abou-Jawdah, Y.; Wardan, R.; Sobh, H.; Salameh, A. Antifungal activities of extracts from selected lebanese wild plants against plant pathogenic fungi. Phytopathol. Mediterr. 2004, 43, 377–386. [Google Scholar]
- Salamone, A.; Zizzo, G.V.; Scarito, G. The antimicrobial activity of water extracts from Labiatae. Acta Hortic. 2006, 723, 465–470. [Google Scholar] [CrossRef]
- Minova, S.; Sešķēna, R.; Voitkāne, S.; Metla, Z.; Daugavietis, M.; Jankevica, L. Impact of pine (Pinus sylvestris L.) and spruce (Picea abies (L.) Karst.) bark extracts on important strawberry pathogens. Proc. Latv. Acad. Sci. B Nat. Exact Appl. Sci. 2015, 69, 62–67. [Google Scholar] [CrossRef]
- Onaran, A.; Bayan, Y. Antifungal activity of Liquidambar orientalis L., and Myrtus communis L. against some plant pathogenic fungi. Sci. Pap. Ser. A Agron. 2016, 59, 360–364. [Google Scholar]
- Méndez-Chávez, M.; Ledesma-Escobar, C.A.; Hidalgo-Morales, M.; Rodríguez-Jimenes, G.d.C.; Robles-Olvera, V.J. Antifungal activity screening of fractions from Annona cherimola Mill. leaf extract against Fusarium oxysporum. Arch. Microbiol. 2022, 204, 330. [Google Scholar] [CrossRef] [PubMed]
- Cherkupally, R.; Kota, S.R.; Amballa, H.; Reddy, B.N. In vitro antifungal potential of plant extracts against Fusarium oxysporum, Rhizoctonia solani and Macrophomina phaseolina. Ann. Plant Sci. 2017, 6, 1676. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of pomegranate peel extract against fusarium wilt of tomato. Eur. J. Plant Pathol. 2016, 147, 229–238. [Google Scholar] [CrossRef]
- Al-Reza, S.M.; Rahman, A.; Ahmed, Y.; Kang, S.C. Inhibition of plant pathogens in vitro and in vivo with essential oil and organic extracts of Cestrum nocturnum L. Pestic. Biochem. Physiol. 2010, 96, 86–92. [Google Scholar] [CrossRef]
- Chakma, M.; Bashar, M.A. In vitro control of Fusarium solani and F. oxysporum the causative agent of brinjal wilt. Dhaka Univ. J. Biol. Sci. 2014, 23, 53–60. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Hernández-Castillo, D.; Angulo-Sánchez, J.L.; Rodríguez-García, R.; Villarreal Quintanilla, J.A.; Lira-Saldivar, R.H. Antifungal activity in vitro of Flourensia spp. extracts on Alternaria sp., Rhizoctonia solani, and Fusarium oxysporum. Ind. Crops Prod. 2007, 25, 111–116. [Google Scholar] [CrossRef]
- El-Mohamedy, R.S.R.; Abdalla, A.M. Evaluation of antifungal activity of Moringa oleifera extracts as natural fungicide against some plant pathogenic fungi in-vitro. Int. J. Agric. Technol. 2014, 10, 963–982. [Google Scholar]
- Chacón, C.; Bojórquez-Quintal, E.; Caamal-Chan, G.; Ruíz-Valdiviezo, V.M.; Montes-Molina, J.A.; Garrido-Ramírez, E.R.; Rojas-Abarca, L.M.; Ruiz-Lau, N. In vitro antifungal activity and chemical composition of Piper auritum Kunth essential oil against Fusarium oxysporum and Fusarium equiseti. Agronomy 2021, 11, 1098. [Google Scholar] [CrossRef]
- Satish, S.; Raghavendra, M.P.; Raveesha, K.A. Antifungal potentiality of some plant extracts against Fusarium sp. Arch. Phytopathol. Pflanzenschutz 2009, 42, 618–625. [Google Scholar] [CrossRef]
- Nelson, D.; Beattie, K.; McCollum, G.; Martin, T.; Sharma, S.; Rao, J.R. Performance of natural antagonists and commercial microbiocides towards in vitro suppression of flower bed soil-borne Fusarium oxysporum. Adv. Microbiol. 2014, 4, 151–159. [Google Scholar] [CrossRef]
- Al-Araji, A.M.Y.; Hasan, A.M.; Fradi, A.J.; Majeed, S.M.A.; Majeed, W.W.A.; Naji, E.T. Study of alkaloids, phenols and terpenes of Mentha spicata as a fungicide against Rhizoctonia solani, Sclerotinia sclerotiorum and Fusarium oxysporum. Int. J. Adv. Biol. Res. 2017, 7, 345–354. [Google Scholar]
- Singh, U.P.; Pathak, K.K.; Khare, M.N.; Singh, R.B. Effect of leaf extract of garlic on Fusarium oxysporum f. sp. ciceri, Sclerotinia sclerotiorum and on gram seeds. Mycologia 2018, 71, 556–564. [Google Scholar] [CrossRef]
- Ahmed, G.; Elsisi, A. Efficacy of compost and some essential oils alone or in combination in controlling cucumber white mould disease under protected house conditions. J. Plant Prot. Pathol. 2020, 11, 291–297. [Google Scholar] [CrossRef]
- Elbatawy, Y.M.; Mohamed, F.G.; Eisa, N.A.; El-Habbak, M.H. Evaluation of some biological agents and plant extracts for controlling cucumber white rot caused by Sclerotinia sclerotiorum (Lib) de Bary. Ann. Agric. Sci. Moshtohor 2020, 58, 351–364. [Google Scholar] [CrossRef]
- Hussein, K.A. Antifungal activity and chemical composition of ginger essential oil against ginseng pathogenic fungi. Curr. Res. Environ. Appl. Mycol. 2018, 8, 194–203. [Google Scholar] [CrossRef]
- Onaran, A.; Yılar, M. Antifungal and herbicidal activity of Trachystemon orientalis (L.) G. Don against some plant pathogenic fungi and Cuscuta campestris Yunck. Iğdır Univ. J. Inst. Sci. Tech. 2018, 8, 37–43. [Google Scholar]
- Goussous, S.J.; Mas’ad, I.S.; Abu El-Samen, F.M.; Tahhan, R.A. In vitro inhibitory effects of rosemary and sage extracts on mycelial growth and sclerotial formation and germination of Sclerotinia sclerotiorum. Arch. Phytopathol. Pflanzenschutz 2013, 46, 890–902. [Google Scholar] [CrossRef]



| Flowers | Leaves | Latex | Assignment |
|---|---|---|---|
| 3300 | 3297 | 3355 | Bonded O–H stretching (cellulose, hemicellulose, lignin) |
| 2917 | 2916 | 2916 | –CH2 asymmetric stretching of alkyls (cutine, wax, pectin) |
| 2849 | 2848 | 2848 | –CH2 symmetric stretching (cutine and wax); CH2–(C6)–bending (cellulose) |
| 1732 | 1732 | 1728 | C=O from esters |
| 1635 | 1635 | 1646 | C=O stretching of alkyl ester |
| 1598 | phenyl ring (aromatic skeletal vibration) | ||
| 1472 | 1472 | methyl C–H asymmetrical | |
| 1462 | 1462 | 1463 | scissoring mode of –CH in CH2 |
| 1416 | 1416 | 1417 | (CH)C=CH2 in plane scissoring |
| 1362 | 1375 | 1374 | C-C asymmetrical stretching phenolic hydroxyl groups |
| 1311 | 1315 | 1312 | C–H in-plane bending |
| 1243 | 1245 | 1243 | C–O stretching; amide N–H vibration |
| 1170 | 1169 | 1167 | C–O–C asymmetric stretching in cellulose; C–C in-plane |
| 1103 | 1101 | 1103 | C–O–C stretching in the pyranose skeletal ring |
| 1050 | 1062 | 1050 | stretching mode of C–O bond |
| 1019 | 1019 | 1019 | C–H bending; C–C stretching |
| 959 | 961 | C–H out-of-plane bending | |
| 719 | 719 | 719 | CH2 rocking vibrations |
| RT (min) | Area (%) | Assignment |
|---|---|---|
| 4.2767 | 1.8852 | 1,3-Dihydroxyacetone dimer |
| 4.3539 | 1.2792 | Dihydroxyacetone |
| 4.5853 | 0.8633 | Butyrolactone |
| 4.6625 | 0.9757 | Formic acid, pentyl ester |
| 4.7337 | 1.1785 | 2-Cyclopenten-1-one, 2-hydroxy- |
| 5.0602 | 0.7557 | 2H-Pyran-2-one |
| 5.5528 | 0.7291 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one |
| 5.7308 | 1.3414 | Formic acid, 2-propenyl ester |
| 5.7902 | 0.7461 | Cyclopropanecarboxamide |
| 5.8614 | 3.3739 | (E)-4-Oxohex-2-enal |
| 6.4609 | 0.6257 | Guanazine |
| 6.5499 | 3.1997 | Benzeneacetaldehyde |
| 6.728 | 0.8216 | 1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone |
| 7.3393 | 1.0234 | 2-Heptanamine, 5-methyl- |
| 7.5233 | 15.4575 | 1-Butanol, 3-methyl, formate |
| 7.9566 | 1.2667 | Ethanamine, N-ethyl-N-nitroso- |
| 8.0871 | 3.9711 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- |
| 8.3898 | 0.7566 | 2(3H)-Furanone, dihydro-4-hydroxy- |
| 8.4789 | 1.0122 | Bicyclo[4.2.0]octa-1,3,5-triene, 7-(2-propenyl)- |
| 8.9181 | 1.7303 | Catechol |
| 9.0487 | 1.6384 | 3-(α-Hydroxyethyl)-aniline |
| 9.1674 | 1.22 | Benzofuran, 2,3-dihydro- |
| 9.3573 | 0.5829 | 5-Hydroxymethylfurfural |
| 9.5888 | 0.6945 | Butanoic acid, 3-oxo-, hexyl ester |
| 9.8381 | 0.9602 | 1,2-Benzenediol, 3-methoxy- |
| 10.0636 | 0.4961 | Hydroquinone |
| 10.1645 | 0.5023 | Isosorbide |
| 10.2239 | 1.3233 | 2-Butanone, 4-hydroxy-3-methyl- |
| 10.5562 | 1.5294 | 2-Methoxy-4-vinylphenol |
| 11.4762 | 0.3876 | 1,4-Hexadiene, 2,3,4,5-tetramethyl- |
| 11.6543 | 0.4644 | N,N-Diethylaniline |
| 11.8679 | 0.2927 | 6-Methylthiocarbonyl-2,4-diamino-6,7-dihydro-5H-pyrrolo[3,4-d]pyrimidin |
| 12.4615 | 5.2679 | N1-(4-hydroxybutyl)-N3-methylguanidine acetate |
| 12.4852 | 3.2235 | Acetone, ethyl methyl acetal |
| 12.8235 | 0.8293 | Ethanone, 1-(3-hydroxy-4-methoxyphenyl)- |
| 12.8591 | 1.065 | Adamantane |
| 13.0491 | 1.0355 | Buformin |
| 13.1737 | 1.1752 | 2-Hydroxy-1-(1′-pyrrolidiyl)-1-buten-3-one |
| 13.9572 | 0.2278 | 3-Methyl-4-phenyl-1H-pyrrole |
| 14.4142 | 11.7827 | (1R,3R,4R,5R)-(-)-Quinic acid |
| 15.8802 | 3.9341 | 2-Amino-3-hydroxypyridine |
| 16.0286 | 0.7147 | d-Proline, N-allyloxycarbonyl-, allyl ester |
| 16.907 | 1.3827 | Tetradecanoic acid |
| 17.5896 | 0.5213 | Hexadecanoic acid, methyl ester |
| 17.7202 | 0.6145 | 9-Hexadecenoic acid |
| 17.916 | 1.9156 | n-Hexadecanoic acid |
| 19.2811 | 0.593 | Methyl 6,9,12-hexadecatrienoate |
| 19.4948 | 0.1775 | Heptadecanoic acid, 16-methyl-, methyl ester |
| 19.5898 | 0.4676 | 9-Octadecenoic acid, (E)- |
| 19.7856 | 0.3783 | Octadecanoic acid |
| 25.0918 | 2.7381 | Squalene |
| 25.5251 | 1.7309 | n-Tetracosanol-1 |
| 30.9203 | 1.7537 | N-Methyl-1-adamantaneacetamide |
| Bacteria | Strain | COS | E. serrata | COS–E. serrata | SF |
|---|---|---|---|---|---|
| P. cichorii | Pci-2 | 750 | 750 | 187.5 | 4.00 |
| Pci-3 | 750 | 750 | 187.5 | 4.00 | |
| Pci-4 | 500 | 750 | 187.5 | 3.20 | |
| Pci-5 | 500 | 750 | 187.5 | 3.20 |
| Treatment | EC | B. cinerea | F. oxysporum | S. sclerotiorum |
|---|---|---|---|---|
| COS | EC50 | 236.2 | 233.2 | 864.3 |
| EC90 | 1426.3 | 743.4 | 1344.8 | |
| E. serrata | EC50 | 402.2 | 402.1 | 408.7 |
| EC90 | 832.4 | 796.2 | 673.4 | |
| COS–E. serrata | EC50 | 140.0 | 142.4 | 282.0 |
| EC90 | 500.6 | 378.4 | 363.7 |
| Treatment | EC | B. cinerea | F. oxysporum | S. sclerotiorum |
|---|---|---|---|---|
| COS–E. serrata | EC50 | 2.13 | 2.07 | 1.97 |
| EC90 | 2.10 | 2.03 | 2.47 |
| Euphorbia spp. | Extraction Medium | Pathogens | Efficacy | Ref. |
|---|---|---|---|---|
| E. guyoniana | water (20%) | Fusarium graminearum Fusarium sporotrichioides | IZ = 27.3–47.85 mm | [57] |
| E. cotinifolia | ethyl acetate and methanol | Xanthomonas axonopodis pv. malvacearum Xanthomonas axonopodis pv. vesicatoria Xanthomonas oryzae Agrobacterium tumefaciens Xanthomonas campestris pv. campestris Pectobacterium carotovorum subsp. carotovorum Pseudomonas solanacearum Pseudomonas syringae | MIC = 300–1300 μg·mL−1 | [54] |
| E. royleana | methanol, hexane, and water (800 μg·mL−1) | Escherichia coli Bacillus subtilis Pasteurella multocida Aspergillus niger Fusarium solani | IZ = 5.7–14.0 mm | [58] |
| E. macroclada | butanol, chloroform, petroleum ether, and water extracts of flowers, stems, and leaves (1000 μg·mL−1) | Fusarium oxysporum Rhizoctonia solani Pythium sp. Verticillium dahliae Alternaria solani Stemphylium solani Rhizopus stolonifer Penicillium italicum Cladosporium sp. Mucor sp. | IR = 0.1–89.9% (pooled avg.) for flower extracts; 7–81.3% for stem extracts; 0–8.1% for leaf extracts | [59] |
| E. aleppica E. szovitsii E. falcata E. denticulata E. macroclada E. cheiradenia E. virgata E. petiolata | methanol | Staphylococcus aureus Bacillus megaterium Proteus vulgaris Klebsiella pneumoniae E. coli Pseudomonas aeruginosa Candida albicans Candida glabrata Candida tropicalis Trichophyton sp. Epidermophyton sp. | IZ = n.a.–25 mm | [60] |
| E. balsamifera | ethanol, petroleum ether, chloroform, and water | Salmonella typhimurium P. aeruginosa Klebsiella spp. E. coli C. albicans | MIC = 5000–6000 μg·mL−1 MBC = 4500–6000 μg·mL−1 | [61] |
| E. granulata E. helioscopia E. hirta | ethanol | E. coli K. pneumoniae P. vulgaris S. aureus Staphylococcus epidermidis Streptococcus pyogenes Aspergillus fumigatus C. albicans C. tropicalis Geotrichum candidum Microsporum canis Trichophyton mentagrophytes | MIC = 3.90–250 μg·mL−1 for Gram-negative bacteria; 1.95–15.62 μg·mL−1 for Gram-positive bacteria; 1.95–500 μg·mL−1 for fungi | [62] |
| E. hirta E. tirucalli | ethanol (concentration not available) | Colletotrichum capsicii Fusarium pallidoroseum Botryodiplodia theobromae Alternaria alternata Penicillium citrinum Phomopsis caricae-papayae A. niger | IZ = n.a.–9 mm | [63] |
| E. hirta | ethanol and water | Fusarium oxysporum f. sp. vasinfectum A. solani R. solani | IC50 = 2930–32,140 μg·mL−1 | [8] |
| water (5%) | R. solani F. oxysporum Macrophomina phaseolina | IR = 10–80% | [55] | |
| ethanol (concentration not available) | S. aureus Bacillus cereus Salmonella typhi K. pneumoniae P. aeruginosa A. niger A. fumigatus Aspergillus flavus Rhizopus oryzae | IZ = n.a.–13.90 mm | [64] | |
| methanol | S. aureus Bacillus thuringiensis B. subtilis Micrococcus sp. E. coli K. pneumoniae Proteus mirabilis S. typhi C. albicans | IZ = n.a.–29 mm, at 100,000 μg·mL−1 MIC = 3130–100,000 μg·mL−1 MBC/MFC = 3130–100,000 μg·mL−1 | [7] | |
| methanol, petroleum ether, ethyl ether, and ethyl acetate | E. coli P. aeruginosa P. mirabilis S. aureus A. flavus A. niger T. mentagrophytes C. albicans | MIC = n.a.–625 μg·mL−1 MBC/MFC = n.a.–1250 μg·mL−1 | [65] | |
| E. tirucalli | acetone, methanol, and water | S. aureus S. epidermidis Enterococcus faecalis E. coli P. aeruginosa | IZ = n.a.–16 mm, at 10,000 μg·mL−1 MIC = n.a.–400 μg·mL−1 | [66] |
| methanol | E. coli P. vulgaris Salmonella enteritidis B. subtilis S. aureus P. aeruginosa K. pneumoniae, C. albicans C. tropicalis A. niger A. fumigatus A. flavus F. oxysporum | IZ = 1–21.5 mm, at 7500 μg·mL−1 MIC = n.a.–>1000 μg·mL−1 | [56] | |
| E. macrorrhiza | butanol, chloroform, ethyl acetate, methanol, hexane | S. aureus E. coli C. albicans | MIC = 500–>2000 μg·mL−1 MBC/MFC = 1000–>2000 μg·mL−1 | [67] |
| E. neriifolia | butanol, chloroform, ethyl acetate, ethanol, and water (50 μg·mL−1) | S. aureus K. pneumoniae E. coli P. vulgaris Pseudomonas fluorescens | IZ = n.a.–8 mm | [68] |
| E. helioscopia | ethanol and water (1000 μg·mL−1) | Trichoderma harzianum Rhizopus nigricans A. niger | IZ = 32–33 mm IZ = 22–32 mm IZ = 24–34 mm | [69] |
| Commercial Fungicide | Pathogen | Mycelium Radial Growth (mm) | Inhibition (%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Control | Rd/10 | Rd * | Rd/10 | Rd * | |||
| Azoxystrobin | B. cinerea | 75.0 | 12 | 51 | 84 | 32 | [74] |
| F. oxysporum spp. | 75.0 | 45.0 | 40.0 | 40.0 | 46.7 | [44] | |
| S. sclerotiorum | 75.0 | 14.0 | 9.0 | 81.3 | 88.0 | ||
| Mancozeb | B. cinerea | 75.0 | 0 | 0 | 100 | 100 | [74] |
| F. oxysporum spp. | 75.0 | 0.0 | 0.0 | 100.0 | 100.0 | [44] | |
| S. sclerotiorum | 75.0 | 0.0 | 0.0 | 100.0 | 100.0 | ||
| Fosetyl-Al | B. cinerea | 75.0 | 38 | 0 | 49.3 | 100 | [74] |
| F. oxysporum spp. | 75.0 | 66.7 | 0.0 | 11.1 | 100.0 | [44] | |
| S. sclerotiorum | 75.0 | 75.0 | 13.3 | 0.0 | 82.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; González-García, V.; Palacio-Bielsa, A.; Casanova-Gascón, J.; Navas-Gracia, L.M.; Martín-Gil, J.; Martín-Ramos, P. Phytochemical Constituents and Antimicrobial Activity of Euphorbia serrata L. Extracts for Borago officinalis L. Crop Protection. Horticulturae 2023, 9, 652. https://doi.org/10.3390/horticulturae9060652
Sánchez-Hernández E, González-García V, Palacio-Bielsa A, Casanova-Gascón J, Navas-Gracia LM, Martín-Gil J, Martín-Ramos P. Phytochemical Constituents and Antimicrobial Activity of Euphorbia serrata L. Extracts for Borago officinalis L. Crop Protection. Horticulturae. 2023; 9(6):652. https://doi.org/10.3390/horticulturae9060652
Chicago/Turabian StyleSánchez-Hernández, Eva, Vicente González-García, Ana Palacio-Bielsa, José Casanova-Gascón, Luis Manuel Navas-Gracia, Jesús Martín-Gil, and Pablo Martín-Ramos. 2023. "Phytochemical Constituents and Antimicrobial Activity of Euphorbia serrata L. Extracts for Borago officinalis L. Crop Protection" Horticulturae 9, no. 6: 652. https://doi.org/10.3390/horticulturae9060652
APA StyleSánchez-Hernández, E., González-García, V., Palacio-Bielsa, A., Casanova-Gascón, J., Navas-Gracia, L. M., Martín-Gil, J., & Martín-Ramos, P. (2023). Phytochemical Constituents and Antimicrobial Activity of Euphorbia serrata L. Extracts for Borago officinalis L. Crop Protection. Horticulturae, 9(6), 652. https://doi.org/10.3390/horticulturae9060652