Preharvest Applications of Oxalic Acid and Salicylic Acid Increase Fruit Firmness and Polyphenolic Content in Blueberry (Vaccinium corymbosum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of the Study Site and Experimental Design
2.2. Plant Physiological Parameters
2.3. Yield and Physical-Chemical Parameters of Fruit
2.4. Statistical Analysis
3. Results
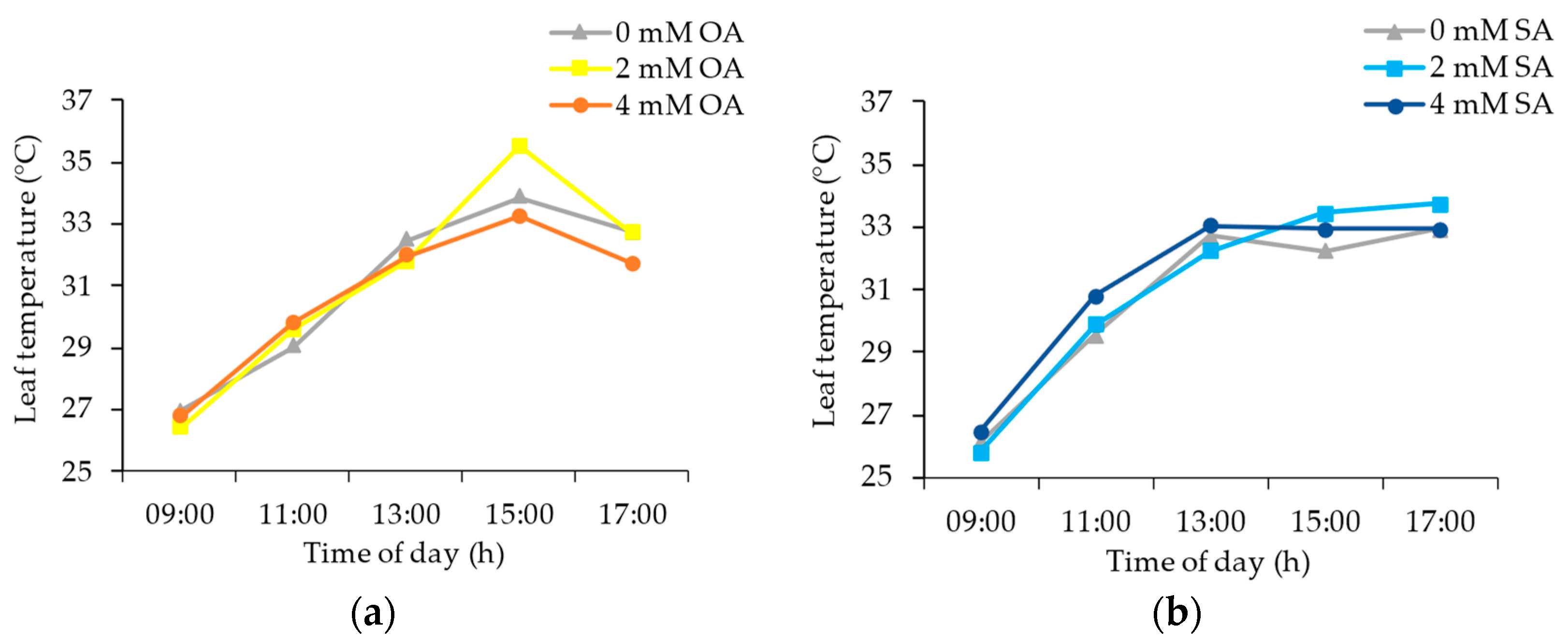
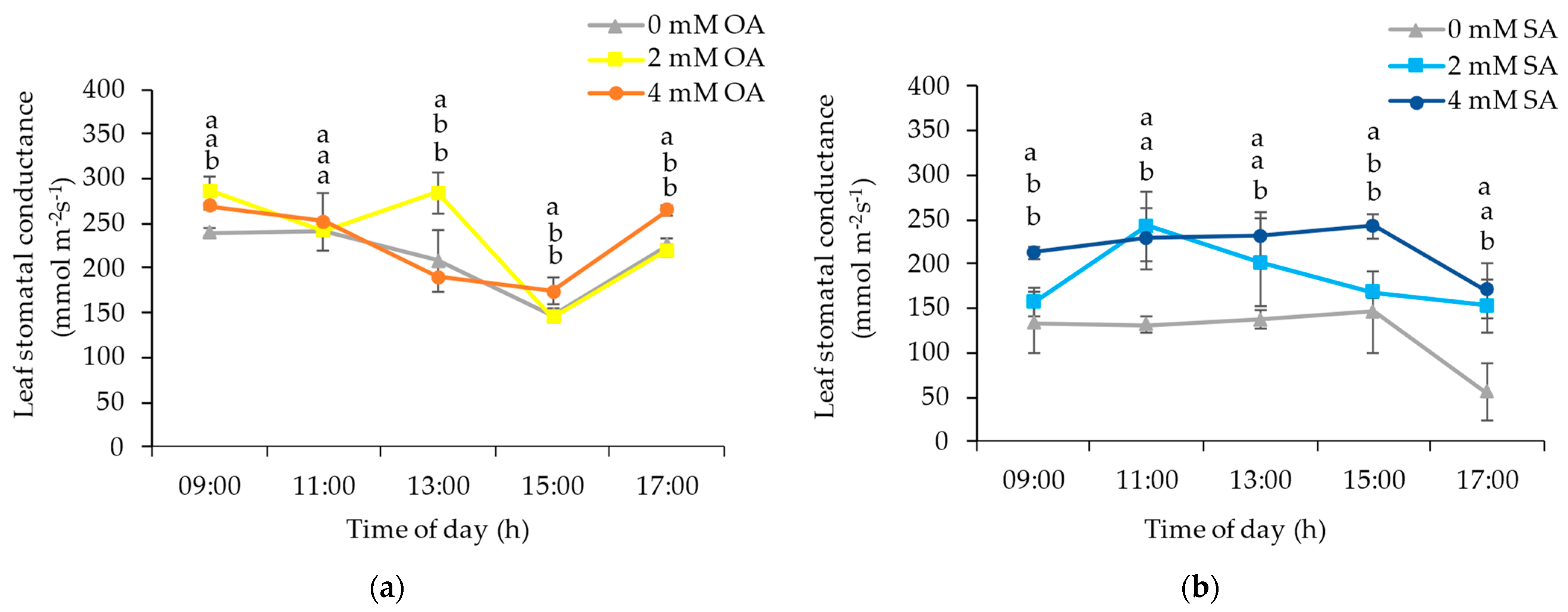
3.1. Environmental and Plant Physiological Parameters
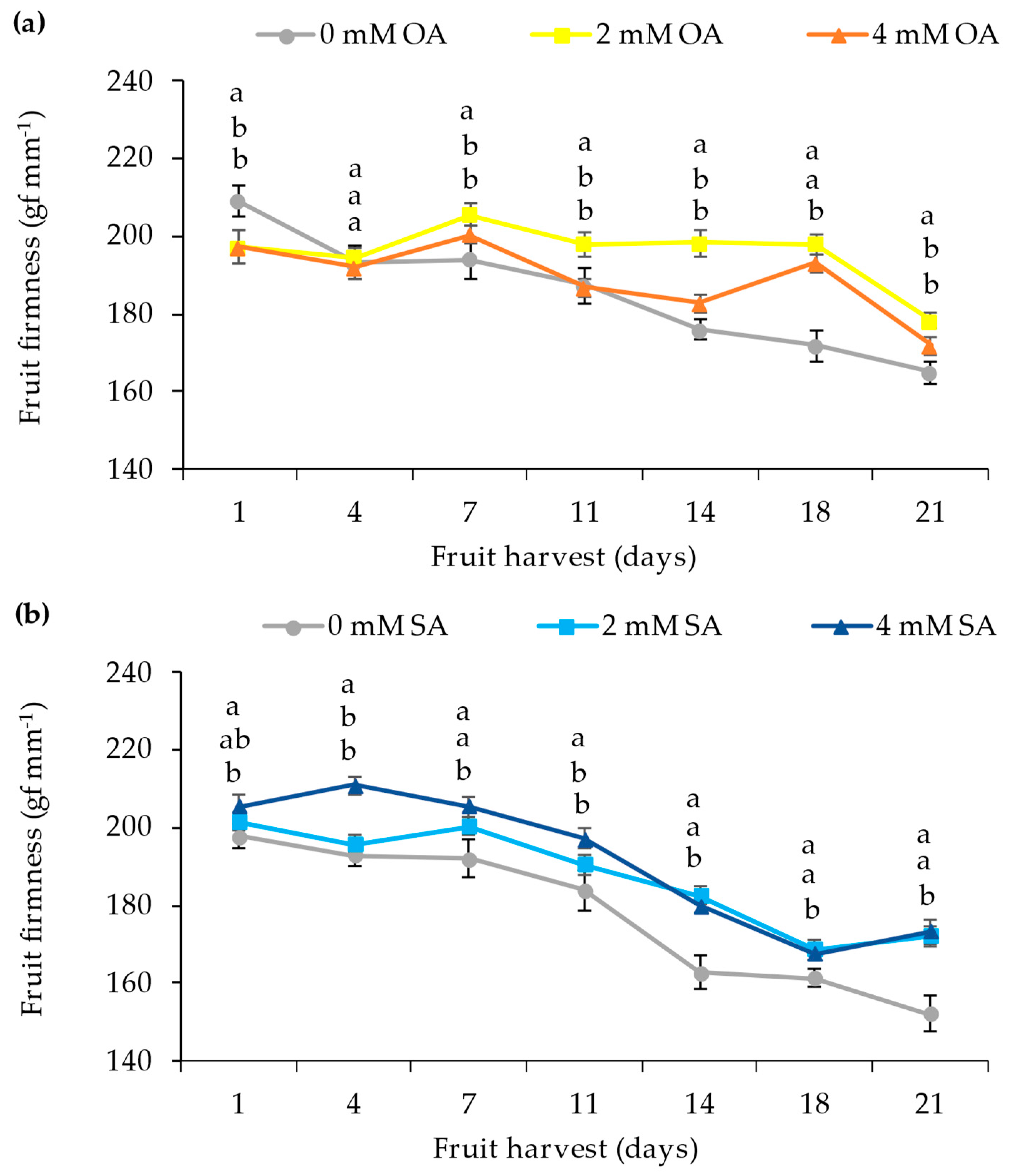
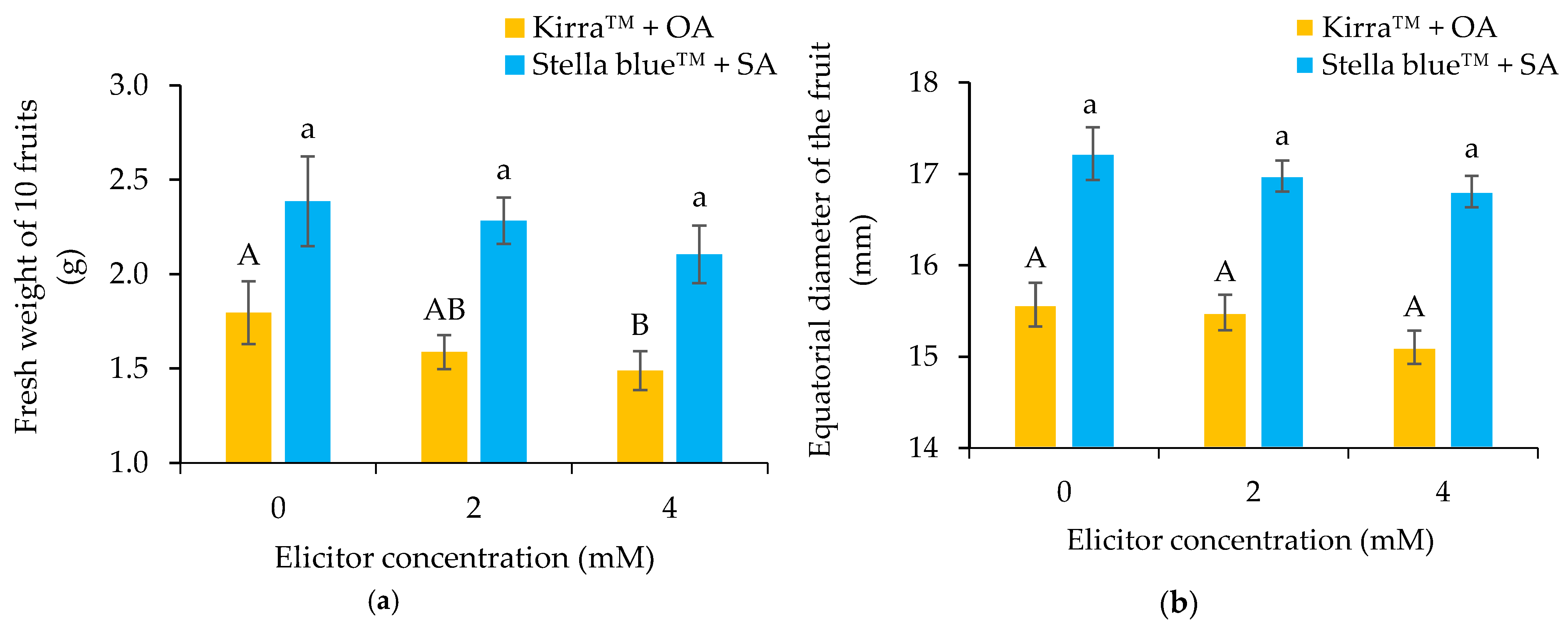
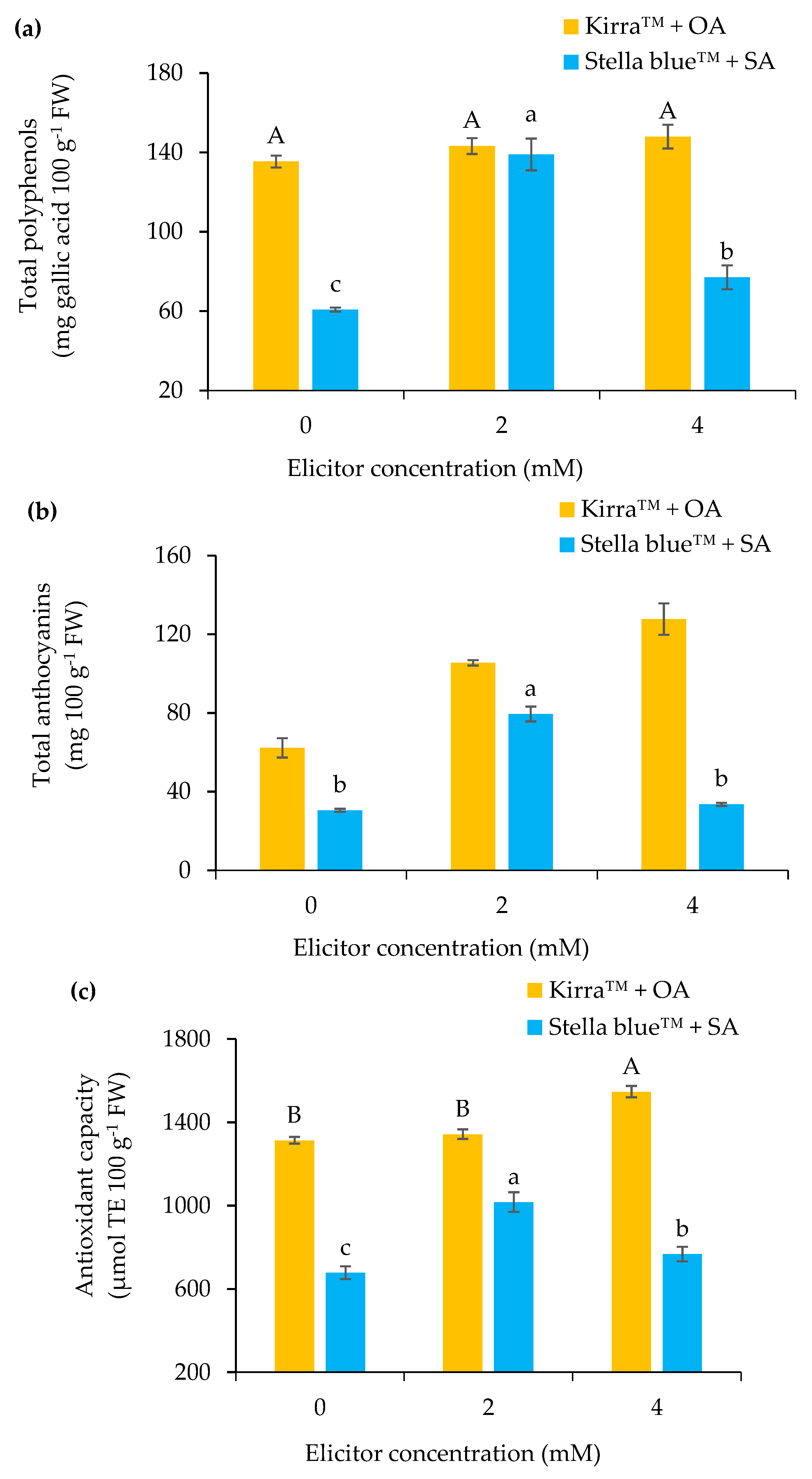
3.2. Physical–Chemical Parameters of Fruit
3.3. Correlations
4. Discussion
4.1. Environmental and Plant Physiological Parameters
4.2. Physical–Chemical Parameters of the Fruit
4.3. Influence of Variables
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerezo, A.B.; Cătunescu, G.M.; González, M.M.-P.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirilă, F.; Rotar, A.M.; Garcia-Parrilla, M.C.; Troncoso, A.M. Anthocyanins in Blueberries Grown in Hot Climate Exert Strong Antioxidant Activity and May Be Effective against Urinary Tract Bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Retamales, J.B.; Palma, M.J.; Morales, Y.A.; Lobos, G.A.; Moggia, C.E.; Mena, C.A. Blueberry Production in Chile: Current Status and Future Developments. Rev. Bras. Frutic. 2014, 36, 58–67. [Google Scholar] [CrossRef]
- Moggia, C.; Graell, J.; Lara, I.; González, G.; Lobos, G.A. Firmness at Harvest Impacts Postharvest Fruit Softening and Internal Browning Development in Mechanically Damaged and Non-Damaged Highbush Blueberries (Vaccinium corymbosum L.). Front. Plant Sci. 2017, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Eum, H.L.; Hong, S.C.; Chun, C.; Shin, I.S.; Lee, B.Y.; Kim, H.K.; Hong, S.J. Influence of Temperature during Transport on Shelf-Life Quality of Highbush Blueberries (Vaccinium corymbosum L. Cvs. Bluetta, Duke). Hortic. Environ. Biotechnol. 2013, 54, 128–133. [Google Scholar] [CrossRef]
- Lobos, G.A.; Hancock, J.F. Breeding Blueberries for a Changing Global Environment: A Review. Front. Plant Sci. 2015, 6, 782. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Preharvest Factors Influencing Bruise Damage of Fresh Fruits—A Review. Sci. Hortic. 2018, 229, 45–58. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, S.; Meng, X.; Li, B. Physiological and Biochemical Responses in Peach Fruit to Oxalic Acid Treatment during Storage at Room Temperature. Food Chem. 2007, 104, 156–162. [Google Scholar] [CrossRef]
- Majidi, H.; Minaei, S.; Almassi, M.; Mostofi, Y. Tomato Quality in Controlled Atmosphere Storage, Modified Atmosphere Packaging and Cold Storage. J. Food Sci. Technol. 2014, 51, 2155–2161. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Onrubia, M.; Moyano, E.; Bonfill, M.; Cusidó, R.M.; Goossens, A.; Palazón, J. Coronatine, a More Powerful Elicitor for Inducing Taxane Biosynthesis in Taxus Media Cell Cultures than Methyl Jasmonate. J. Plant Physiol. 2013, 170, 211–219. [Google Scholar] [CrossRef]
- Sharma, S.; Pareek, S.; Sagar, N.A.; Valero, D.; Serrano, M. Modulatory Effects of Exogenously Applied Polyamines on Postharvest Physiology, Antioxidant System and Shelf Life of Fruits: A Review. Int. J. Mol. Sci. 2017, 18, 1789. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A Tool for Improving Fruit Phenolic Content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, C.; Xie, J. Effect of Salicylic Acid Treatment on Alleviating Postharvest Chilling Injury of ‘Qingnai’ Plum Fruit. Postharvest Biol. Technol. 2011, 62, 115–120. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of Exogenous Salicylic Acid under Changing Environment: A Review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.A.; Plotto, A.; Luo, W.; Raithore, S.; Yu, Z.; Bai, J. Effect of Methyl Salicylate and Methyl Jasmonate Pre-Treatment on the Volatile Profile in Tomato Fruit Subjected to Chilling Temperature. Postharvest Biol. Technol. 2015, 108, 28–38. [Google Scholar] [CrossRef]
- Zavaleta-MejÃ-a, E.; Lagunes-FortÃ-z, E. FunciÃ3n de la Lignina en la InteracciÃ3n Planta-Nematodos Endoparásitos Sedentarios. Rev. Mex. De Fitopatol. Mex. J. Phytopathol. 2015, 34. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; García-Viguera, C.; Castillo, S.; Serrano, M. Preharvest Application of Oxalic Acid Increased Fruit Size, Bioactive Compounds, and Antioxidant Capacity in Sweet Cherry Cultivars (Prunus avium L.). J. Agric. Food Chem. 2014, 62, 3432–3437. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; García-Pastor, M.E.; Zapata, P.J.; Guillén, F.; Serrano, M.; Valero, D.; Gironés-Vilaplana, A. Preharvest Application of Oxalic Acid Improves Quality and Phytochemical Content of Artichoke (Cynara scolymus L.) at Harvest and during Storage. Food Chem. 2017, 230, 343–349. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Postharvest Treatment of Polyamines Maintains Quality and Extends Shelf-Life of Table Grapes (Vitis vinifera L.) Cv. Flame Seedless. Postharvest Biol. Technol. 2014, 91, 57–63. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Asghari, M.; Aghdam, M.S. Impact of Salicylic Acid on Post-Harvest Physiology of Horticultural Crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Serrano, M. Postharvest Treatments with Salicylic Acid, Acetylsalicylic Acid or Oxalic Acid Delayed Ripening and Enhanced Bioactive Compounds and Antioxidant Capacity in Sweet Cherry. J. Agric. Food Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jing, G.; Guo, L.; Zhang, D.; Yang, B.; Duan, X.; Ashraf, M.; Jiang, Y. Effect of Oxalic Acid on Ripening Attributes of Banana Fruit during Storage. Postharvest Biol. Technol. 2013, 84, 22. [Google Scholar] [CrossRef]
- Xu, R.; Takeda, F.; Krewer, G.; Li, C. Measure of Mechanical Impacts in Commercial Blueberry Packing Lines and Potential Damage to Blueberry Fruit. Postharvest Biol. Technol. 2015, 110, 103–113. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Mitra, S.; Hafiz Muhammad, R.; Debnath, B.; Lu, X.; Jian, H.; Qiu, D. Comparative Phytochemical Profiles and Antioxidant Enzyme Activity Analyses of the Southern Highbush Blueberry (Vaccinium Corymbosum) at Different Developmental Stages. Molecules 2018, 23, 2209. [Google Scholar] [CrossRef]
- Pinto-Poblete, A.; Retamal-Salgado, J.; López, M.D.; Zapata, N.; Sierra-Almeida, A.; Schoebitz, M. Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants. Plants 2022, 11, 536. [Google Scholar] [CrossRef]
- Pinto-Morales, F.; Retamal-Salgado, J.; Lopéz, M.D.; Zapata, N.; Vergara-Retamales, R.; Pinto-Poblete, A. The Use of Compost Increases Bioactive Compounds and Fruit Yield in Calafate Grown in the Central South of Chile. Agriculture 2022, 12, 98. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Bastías, R.M.; Wilckens, R.; Paulino, L. Influence of Microclimatic Conditions under High Tunnels on the Physiological and Productive Responses in Blueberry Cv. O´Neal. Chil. J. Agric. Res. 2015, 75, 291–297. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, J.; Brecht, J.K.; Jiang, T.; Zheng, X. Pre-Harvest Application of Oxalic Acid Increases Quality and Resistance to Penicillium Expansum in Kiwifruit during Postharvest Storage. Food Chem. 2016, 190, 537–543. [Google Scholar] [CrossRef]
- Cordon, G.; Lagorio, M.G.; Paruelo, J.M. Chlorophyll Fluorescence, Photochemical Reflective Index and Normalized Difference Vegetative Index during Plant Senescence. J. Plant Physiol. 2016, 199, 100–110. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Loor, B.; Hirzel, J.; López, M.D.; Undurraga, P.; Zapata, N.; Vergara-Retamales, R.; Olivares-Soto, H. Chlorophyll Fluorescence and Fruit Quality Response of Blueberry to Different Mulches. Agronomy 2022, 12, 1702. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Morales, F.; Retamal-Salgado, J.; López, M.D.; Zapata, N.; Vergara-Retamales, R.; Palma, D. Variation in Physical-Chemical Parameters and Phenolic Compounds in Fruits of Four Calafate Clones. Agronomy 2022, 12, 2146. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Vásquez, R.; Fischer, S.; Hirzel, J.; Zapata, N. Decrease in Artificial Radiation with Netting Reduces Stress and Improves Rabbit-Eye Blueberry ( Vaccinium Virgatum Aiton) Cv. Ochlockonee Productivity. Chil. J. Agric. Res. 2017, 77, 226–233. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of Antioxidant and Antimicrobial Activities of Rumex crispus L. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical Characterisation for Industrial Use of Pomegranate (Punica granatum L.) Cultivars Grown in Spain: Selection of Pomegranates for Juices. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Betancur, M.; Retamal-Salgado, J.; López, M.D.; Vergara-Retamales, R.; Schoebitz, M. Plant Performance and Soil Microbial Responses to Irrigation Management: A Novel Study in a Calafate Orchard. Horticulturae 2022, 8, 1138. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzales, L.; Tablada, M.; Robledo, C.W. InfoStat, Versión 2013; Grupo InfoStat, FCA; Universidad Nacional de Córdova: Córdova, Argentina, 2013; Available online: http://www.infostat.com.ar (accessed on 1 January 2021).
- Kahle, D.J.; Wickham, H. Ggmap: Spatial Visualization with Ggplot2. R J. 2013, 5, 144. [Google Scholar] [CrossRef]
- Chen, W.; Cen, W.; Chen, L.; Di, L.; Li, Y.; Guo, W. Differential Sensitivity of Four Highbush Blueberry (Vaccinium corymbosum L.) Cultivars to Heat Stress. Pak. J. Bot. 2012, 44, 853–860. [Google Scholar]
- Zheng, Y.; Li, R.; Sun, Y.; Xu, M.; Zhang, H.; Huang, L.; Zhu, Y.; Wang, H.; Li, G.; Liu, L.; et al. The Optimal Temperature for the Growth of Blueberry (Vaccinium corymbosum L.). Pak. J. Bot. 2017, 49, 965–979. [Google Scholar]
- Lobos, G.A.; Bravo, C.; Valdés, M.; Graell, J.; Lara Ayala, I.; Beaudry, R.M.; Moggia, C. Within-Plant Variability in Blueberry (Vaccinium corymbosum L.): Maturity at Harvest and Position within the Canopy Influence Fruit Firmness at Harvest and Postharvest. Postharvest Biol. Technol. 2018, 146, 26–35. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic Acid in Relation to Other Phytohormones in Plant: A Study towards Physiology and Signal Transduction under Challenging Environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Yu, X.; Cui, X.; Wu, C.; Shi, S.; Yan, S. Salicylic Acid Inhibits Gibberellin Signaling through Receptor Interactions. Mol. Plant 2022, 15, 1759–1771. [Google Scholar] [CrossRef]
- Sakhabutdinova, A.R.; Fatkhutdinova, D.R.; Bezrukova, M.V.; Shakirova, F.M. Salicylic Acid Prevents the Damaging Action of Stress Factors on Wheat Plants. Bulg. J. Plant Physiol. 2003, 21, 314–319. [Google Scholar]
- Hao, L.; Guo, L.; Li, R.; Cheng, Y.; Huang, L.; Zhou, H.; Xu, M.; Li, F.; Zhang, X.; Zheng, Y. Responses of Photosynthesis to High Temperature Stress Associated with Changes in Leaf Structure and Biochemistry of Blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2019, 246, 251–264. [Google Scholar] [CrossRef]
- Wang, L.-J.; Fan, L.; Loescher, W.; Duan, W.; Liu, G.-J.; Cheng, J.-S.; Luo, H.-B.; Li, S.-H. Salicylic Acid Alleviates Decreases in Photosynthesis under Heat Stress and Accelerates Recovery in Grapevine Leaves. BMC Plant Biol. 2010, 10, 34. [Google Scholar] [CrossRef]
- Bastam, N.; Baninasab, B.; Ghobadi, C. Improving Salt Tolerance by Exogenous Application of Salicylic Acid in Seedlings of Pistachio. Plant Growth Regul 2013, 69, 275–284. [Google Scholar] [CrossRef]
- Ali, M.; Liu, M.; Wang, Z.; Li, S.; Jiang, T.; Zheng, X. Pre-Harvest Spraying of Oxalic Acid Improves Postharvest Quality Associated with Increase in Ascorbic Acid and Regulation of Ethanol Fermentation in Kiwifruit Cv. Bruno during Storage. J. Integr. Agric. 2019, 18, 2514–2520. [Google Scholar] [CrossRef]
- Urbano Bron, I.; Vasconcelos Ribeiro, R.; Azzolini, M.; Pedro Jacomino, A.; Caruso Machado, E. Chlorophyll Fluorescence as a Tool to Evaluate the Ripening of ‘Golden’ Papaya Fruit. Postharvest Biol. Technol. 2004, 33, 163–173. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, D.; Zhang, H.; Jiang, G.; Su, X.; Qu, H.; Jiang, Y.; Duan, X. Physiological and Biochemical Response of Harvested Plum Fruit to Oxalic Acid during Ripening or Shelf-Life. Food Res. Int. 2011, 44, 1299–1305. [Google Scholar] [CrossRef]
- Jimenez, A.; Creissen, G.; Kular, B.; Firmin, J.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in Oxidative Processes and Components of the Antioxidant System during Tomato Fruit Ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, S. Maturation and Ripening of Fruit OfAmelanchier AlnifoliaNutt. Are Accompanied by Increasing Oxidative Stress. Ann. Bot. 1998, 81, 203–211. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, S.; Gidley, M.J.; Yue, H.; Li, B. Effects of Exogenous Oxalic Acid on Ripening and Decay Incidence in Mango Fruit during Storage at Room Temperature. Postharvest Biol. Technol. 2007, 45, 281. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Zapata, P.J.; Castillo, S.; Serrano, M. Postharvest Methyl Salicylate Treatments Delay Ripening and Maintain Quality Attributes and Antioxidant Compounds of ‘Early Lory’ Sweet Cherry. Postharvest Biol. Technol. 2016, 117, 102–109. [Google Scholar] [CrossRef]
- Liang, C.; Cui, X.; Sun, C.; Ye, S.; Huang, N.; Chen, R.; Zhang, A.; Yang, Y.; Gong, H.; Sun, S.; et al. Synergistic and Antagonistic Effects of Preharvest Salicylic Acid and Postharvest 1-Methylcyclopropene Treatments on the Storage Quality of Apricot. Food Chem. 2023, 405, 134764. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Xu, J.; Fu, L. Influence of 1-Methylcyclopropene (1-MCP) Combined with Salicylic Acid (SA) Treatment on the Postharvest Physiology and Quality of Bananas. J. Food Process. Preserv. 2019, 43, e13880. [Google Scholar] [CrossRef]
- Ortiz-Delvasto, N.; Garcia-Ibañez, P.; Olmos-Ruiz, R.; Bárzana, G.; Carvajal, M. Substrate Composition Affects Growth and Physiological Parameters of Blueberry. Sci. Hortic. 2023, 308, 111528. [Google Scholar] [CrossRef]
- Razavi, F.; Hajilou, J. Enhancement of Postharvest Nutritional Quality and Antioxidant Capacity of Peach Fruits by Preharvest Oxalic Acid Treatment. Sci. Hortic. 2016, 200, 95–101. [Google Scholar] [CrossRef]
- Guillén, F.; Zapata, P.J.; Martínez-Romero, D.; Castillo, S.; Valverde, J.M.; Valero, D.; Díaz-Mula, H.M.; Serrano, M. Postharvest Treatments with Oxalic Acid on Quality of the Early-Season Sweet Cherry Cultivar ‘Early Lory’. Acta Hortic. 2014, 1079, 173–178. [Google Scholar] [CrossRef]
- Zheng, X.; Jing, G.; Liu, Y.; Jiang, T.; Jiang, Y.; Li, J. Expression of Expansin Gene, MiExpA1, and Activity of Galactosidase and Polygalacturonase in Mango Fruit as Affected by Oxalic Acid during Storage at Room Temperature. Food Chem. 2012, 132, 849. [Google Scholar] [CrossRef]
- Gomes, E.P.; Vanz Borges, C.; Monteiro, G.C.; Filiol Belin, M.A.; Minatel, I.O.; Pimentel Junior, A.; Tecchio, M.A.; Lima, G.P.P. Preharvest Salicylic Acid Treatments Improve Phenolic Compounds and Biogenic Amines in ‘Niagara Rosada’ Table Grape. Postharvest Biol. Technol. 2021, 176, 111505. [Google Scholar] [CrossRef]
- Kayashima, T. Oxalic Acid Is Available as a Natural Antioxidant in Some Systems. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2002, 1573, 1–3. [Google Scholar] [CrossRef]
- Soleimani Aghdam, M.; Asghari, M.; Babalar, M.; Askari Sarcheshmeh, M.A. 8—Impact of Salicylic Acid on Postharvest Physiology of Fruits and Vegetables. In Eco-Friendly Technology for Postharvest Produce Quality; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 243–268. ISBN 978-0-12-804313-4. [Google Scholar]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef]
- José Aliaño González, M.; Carrera, C.; Barbero, G.F.; Palma, M. A Comparison Study between Ultrasound–Assisted and Enzyme–Assisted Extraction of Anthocyanins from Blackcurrant (Ribes nigrum L.). Food Chem. X 2022, 13, 100192. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Preharvest Salicylic Acid Treatments to Improve Quality and Postharvest Life of Table Grapes (Vitis vinifera L.) Cv. Flame Seedless. J. Food Sci. Technol. 2015, 52, 3607–3616. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Guillén, F.; Valero, D.; Serrano, M. The Effects of Salicylic Acid and Its Derivatives on Increasing Pomegranate Fruit Quality and Bioactive Compounds at Harvest and During Storage. Front. Plant Sci. 2020, 11, 668. [Google Scholar] [CrossRef]
- Grobelna; Kalisz; Kieliszek The Effect of the Addition of Blue Honeysuckle Berry Juice to Apple Juice on the Selected Quality Characteristics, Anthocyanin Stability, and Antioxidant Properties. Biomolecules 2019, 9, 744. [CrossRef]



| Value | Air Temperature (°C) | Soil Temperature (°C) | PPFD * |
|---|---|---|---|
| (μmol m−2 s−1) | |||
| Maximum | 32.0 | 30.6 | 2407 |
| Minimum | 16.1 | 19.5 | 439 |
| Average | 19.7 | 26.5 | 1495 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retamal-Salgado, J.; Adaos, G.; Cedeño-García, G.; Ospino-Olivella, S.C.; Vergara-Retamales, R.; Lopéz, M.D.; Olivares, R.; Hirzel, J.; Olivares-Soto, H.; Betancur, M. Preharvest Applications of Oxalic Acid and Salicylic Acid Increase Fruit Firmness and Polyphenolic Content in Blueberry (Vaccinium corymbosum L.). Horticulturae 2023, 9, 639. https://doi.org/10.3390/horticulturae9060639
Retamal-Salgado J, Adaos G, Cedeño-García G, Ospino-Olivella SC, Vergara-Retamales R, Lopéz MD, Olivares R, Hirzel J, Olivares-Soto H, Betancur M. Preharvest Applications of Oxalic Acid and Salicylic Acid Increase Fruit Firmness and Polyphenolic Content in Blueberry (Vaccinium corymbosum L.). Horticulturae. 2023; 9(6):639. https://doi.org/10.3390/horticulturae9060639
Chicago/Turabian StyleRetamal-Salgado, Jorge, Geber Adaos, George Cedeño-García, Sebastian Camilo Ospino-Olivella, Rosa Vergara-Retamales, María Dolores Lopéz, Raúl Olivares, Juan Hirzel, Héctor Olivares-Soto, and Matías Betancur. 2023. "Preharvest Applications of Oxalic Acid and Salicylic Acid Increase Fruit Firmness and Polyphenolic Content in Blueberry (Vaccinium corymbosum L.)" Horticulturae 9, no. 6: 639. https://doi.org/10.3390/horticulturae9060639
APA StyleRetamal-Salgado, J., Adaos, G., Cedeño-García, G., Ospino-Olivella, S. C., Vergara-Retamales, R., Lopéz, M. D., Olivares, R., Hirzel, J., Olivares-Soto, H., & Betancur, M. (2023). Preharvest Applications of Oxalic Acid and Salicylic Acid Increase Fruit Firmness and Polyphenolic Content in Blueberry (Vaccinium corymbosum L.). Horticulturae, 9(6), 639. https://doi.org/10.3390/horticulturae9060639








