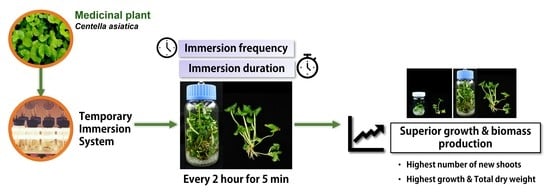
Optimal Growth and Biomass of Centella asiatica Using a Twin-Bottle Temporary Immersion Bioreactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Explant Preparation
2.2. Effect of Immersion Time on Growth and Biomass Production of Centella asiatica
2.3. Comparison of Different Culture Systems on Growth and Biomass Production of Centella asiatica
2.4. Experimental Design and Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belwal, T.; Andola, H.C.; Atanssova, M.S.; Joshi, B. Gotu Kola (Centella asiatica) in Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 265–275. [Google Scholar]
- Oyenihi, A.B.; Ahiante, B.O.; Oyenihi, O.R.; Masola, B. Centella asiatica: Its Potential for the Treatment of Diabetes in Diabetes, 2nd ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 213–222. [Google Scholar]
- Brinkhaus, B.; Lindner, M.; Schuppan, D.; Hahn, E.G. Chemical, pharmacological and clinical profile of the East Asian medical plant C. asiatica . Phytomedicine 2000, 7, 427–448. [Google Scholar] [CrossRef]
- Singh, R.H.; Singh, L. Studies on the anti-anxiety effects of the medhya rasayana drug Bacopa monniera (L.) Wettst. International J. Ayurveda Pharm. Res. 1980, 1, 33–148. [Google Scholar]
- Gohil, K.; Jagruti, A.P.; Anuradha, K.G. Pharmacological Review on Centella asiatica: A Potential Herbal Cure-all. Indian J. Pharm. Sci. 2010, 72, 546. [Google Scholar] [CrossRef] [PubMed]
- Bandara, S.M.; Lee, E.L.; Thomas, J.E. Goti kola (Centella aiatica L.): An Under-utilized Herb. Am. J. Plant Sci. 2011, 5, 20–29. [Google Scholar]
- Prakash, V.; Jaiswal, N.; Srivastava, M.A. review on medicinal properties of Centella asiatica . Asian J. Pharm. Clin. Res. 2017, 10, 69–74. [Google Scholar] [CrossRef]
- Sudhakaran, M.V. Botanical pharmacognosy of Centella asiatica (Linn.) urban. Pharmacogn. Mag. 2017, 9, 546–558. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A systematic review of the effect of Centella asiatica on wound healing. Int. J. Environ. Health Res. 2022, 19, 3266. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.H.; Wong, L.S.; Tan, A.L.; Yap, C.K. Effects of metal-contaminated soils on the accumulation of heavy metals in gotu kola (Centella asiatica) and the potential health risks: A study in Peninsular Malaysia. Environ. Monit. Assess. 2016, 188, 40. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Parveen, O.; Pandey, V.P.; Mathur, A.; Dwivedi, U.N. Heavy metal accumulation efficiency, growth and centelloside production in the medicinal herb Centella asiatica (L.) urban under different soil concentrations of cadmium and lead. Ind. Crops Prod. 2020, 157, 112948. [Google Scholar] [CrossRef]
- Haque, S.M.; Chakraborty, A.; Dey, D.; Mukherjee, S.; Nayak, S.; Ghosh, B. Improved micropropagation of Bacopa monnieri (L.) Wettst. (Plantaginaceae) and antimicrobial activity of in vitro and ex vitro raised plants against multidrug-resistant clinical isolates of urinary tract infecting (UTI) and respiratory tract infecting (RTI) bacteria. Clin. Phytosci. 2017, 3, 1–10. [Google Scholar]
- Nath, T.K.; Chandra, S.N.; Tiwari, V.; Deo, S.B. Micropropagation of Centella asiatica (L.), a valuable medicinal herb. Plant Cell Tissue Organ Cult. 2000, 63, 179–185. [Google Scholar] [CrossRef]
- Loc, N.H.; An, N.T.T. Asiaticoside production from centella (Centella asiatica L. Urban) cell culture. Biotechnol. Bioprocess Eng. 2010, 15, 1065–1070. [Google Scholar] [CrossRef]
- Naidu, T.B.; Rao, S.N.; Mani, N.S.; Mohan, Y.J.; Pola, S. Conservation of an endangered medicinal plant Centella asiatica through plant tissue culture. Drug Invent. Today 2010, 2, 17–21. [Google Scholar]
- Gallego, A.; Ramirez-Estrada, K.; Vidal-Limon, H.R.; Hidalgo, D.; Lalaleo, L.; Khan Kayani, W.; Palazon, J. Biotechnological production of centellosides in cell cultures of Centella asiatica (L) Urban. Eng. Life Sci. 2014, 14, 633–642. [Google Scholar] [CrossRef]
- Rahayu, S.; Roostika, I.; Bermawie, N. The effect of types and concentrations of auxins on callus induction of Centella asiatica . Nus. Biosci. 2016, 8, 283–287. [Google Scholar] [CrossRef]
- Watt, M.P. The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechnol. 2012, 11, 14025–14035. [Google Scholar]
- Valdiani, A.; Hansen, O.K.; Nielsen, U.B.; Johannsen, V.K.; Shariat, M.; Georgiev, M.I.; Abiri, R. Bioreactor-based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2019, 39, 20–34. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant Biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Berthouly, M.; Etienne, H. Temporary immersion system: A new concept for use liquid medium in mass propagation. In Liquid Culture Systems for In Vitro Plant Propagation; Eide, A.K.H., Preil, W., Eds.; Springer Science & Business Media: Dordrecht, The Netherlands, 2005; pp. 165–195. [Google Scholar]
- Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary immersion system for production of biomass and bioactive compounds from medicinal plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Kunakhonnuruk, B.; Inthima, P.; Kongbangkerd, A. Improving bacoside yield of Bacopa monnieri (L.) Wettst. in temporary immersion system by increasing immersion time and lowering the intervals. Ind. Crops Prod. 2023, 191, 115859. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Frometa, O.M.; Morgado, M.M.E.; Silva, J.A.T.; Morgado, D.T.P.; Gradaille, M.A.D. In vitro propagation of Gerbera jamesonii Bolus ex Hooker f. in a temporary immersion bioreactor. Plant Cell Tissue Organ Cult. 2017, 129, 543–551. [Google Scholar] [CrossRef]
- Zhang, B.; Song, L.; Bekele, L.D.; Shi, J.; Jia, Q.; Zhang, B. Optimizing factors affecting development and propagation of Bletilla striata in a temporary immersion bioreactor system. Sci. Hortic. 2018, 232, 121–126. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 437–497. [Google Scholar] [CrossRef]
- Paek, K.Y.; Chakrabarty, D.; Hahn, E.J. Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tissue Organ Cult. 2005, 81, 287–300. [Google Scholar] [CrossRef]
- Distabanjong, C.; Distabanjong, K.; Woo, J.G.; Jang, S.W. Production of phytoplasma-free plants in sugarcane (Saccharum spp.) using temporary immersion bioreactor. Acta Hortic. 2018, 1205, 727–734. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Inglese, P.; Barone, E.; Sottile, F. In vitro regeneration of Capparis spinosa L. by using a temporary immersion system. Plants 2019, 8, 177. [Google Scholar] [CrossRef]
- Carvalho, L.S.O.; Ozudogru, E.A.; Lambardi, M.; Paiva, L.V. Temporary immersion system for micropropagation of tree species: A bibliographic and systematic review. Not. Bot. Hortic. Agrobot. Cluj Napoca 2019, 47, 269–277. [Google Scholar] [CrossRef]
- Ibrahim, R. The potential of bioreactor technology for large-scale plant micropropagation. Acta Hortic. 2017, 1155, 573–584. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Wlodarska, P.; Zabiegala, B.; Bucinski, A.; Luczkiewicz, M. Bioreactor shoot cultures of Rhododendron tomentosum for a large-scale production of bioactive volatile compounds. Plant Cell Tissue Organ Cult. 2017, 131, 51–64. [Google Scholar] [CrossRef]
- Vijendra, P.D.; Jayanna, S.G.; Kumar, V.; Gajula, H.; Rajashekar, J.; Sannabommaji, T.; Anuradha, C.M. Rapid in vitro propagation of Lucas aspera Spreng. A potential multipurpose Indian medicinal herb. Ind. Crops Prod. 2017, 107, 281–287. [Google Scholar] [CrossRef]
- Bayraktar, M. Micropropagation of Stevia rebaudiana Bertoni using RITA bioreactor. HortScience 2019, 54, 725–731. [Google Scholar] [CrossRef]
- Martínez-Estrada, E.; Islas-Luna, B.; Pérez-Sato, J.A.; Bello-Bello, J.J. Temporary immersion improves in vitro multiplication and acclimatization of Anthurium andreanum Lind. Sci. Hortic. 2019, 249, 185–191. [Google Scholar] [CrossRef]
- Perez-Alonso, N.; Wilken, D.; Gerth, A.; Jahn, A.; Nitzsche, H.M.; Kerns, G. Cardiotonic glycosides from biomass of Digitalis purpurea L. cultured in temporary immersion systems. Plant Cell Tissue Organ Cult. 2009, 99, 151–156. [Google Scholar] [CrossRef]
- Roels, S.; Escalona, M.; Cejas, I.; Noceda, C.; Rodrıguez, R.; Canal, M.J.; Sandoval, J.; Debergh, P. Optimization of plantain (Musa AAB) micropropagation by temporary immersion system. Plant Cell Tissue Organ Cult. 2005, 82, 57–66. [Google Scholar] [CrossRef]
- Vendrame, W.A.; Xu, J.; Beleski, D.G. Micropropagation of Brassavola nodosa (L.) Lindl. using SETIS™ bioreactor. Plant Cell Tissue Organ Cult. 2023, 153, 67–76. [Google Scholar] [CrossRef]
- Ilczuk, A.; Winkelmann, T.; Richartz, S.; Witomska, M.; Serek, M. In vitro propagation of Hippeastrum × chmielii Chm.–influence of flurprimidol and the culture in solid or liquid medium and in temporary immersion systems. Plant Cell Tissue Organ Cult. 2005, 83, 339–346. [Google Scholar] [CrossRef]
- Vives, K.; Andújar, I.; Lorenzo, J.C.; Concepción, O.; Hernández, M.; Escalona, M. Comparison of different in vitro micropropagation methods of Stevia rebaudiana . Plant Cell Tissue Organ Cult. 2017, 131, 195–199. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Cruz-Cruz, C.A.; Perez-Guerra, J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). Vitr. Cell. Dev. Biol.-Plant 2019, 55, 313–320. [Google Scholar] [CrossRef]
- Shaik, S.; Dewir, Y.H.; Singh, N.; Nicholas, A. Micropropagation and bioreactor studies of the medicinally important plant Lessertia (Sutherlandia) frutescens L. S. Afr. J. Bot. 2010, 76, 180–186. [Google Scholar] [CrossRef]
- Escalona, M.; Aragon, C.; Capote, I.; Pina, D.; Cejas, I.; Rodríguez, R. Physiology of effects of temporary immersion bioreactor (TIB) on micropropagated plantlets. Acta Hortic. 2007, 748, 95–101. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, W.; Wang, Y.; Saxena, P.K.; Liu, C.Z. Improved mass multiplication of Rhodiola crenulate shoots using temporary immersion bioreactor with forced ventilation. Appl. Biochem. Biotechnol. 2012, 166, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Aragon, C.; Escalona, M.; Rodriguez, R.; Canal, M.; Capote, I.; Pina, D.; Gonzalez-Olmedo, J. Effect of sucrose, light, and carbon dioxide on plantain micropropagation in temporary immersion bioreactors. Vitr. Cell. Dev. Biol.-Plant 2010, 46, 89–94. [Google Scholar] [CrossRef]
- Jova, M.C.; Kosky, R.G.; Cuellar, E.E. Effect of liquid media culture systems on yam growth (Dioscorea alata L. ‘Pacala Duclos’). Biotechnol. Agron. Soc. Environ. 2011, 15, 515–521. [Google Scholar]
- Kunakhonnuruk, B.; Kongbangkerd, A.; Inthima, P. Improving large-scale biomass and plumbagin production of Drosera communis A. St.-Hil. by temporary immersion system. Ind. Crops Prod. 2019, 137, 197–202. [Google Scholar] [CrossRef]


| Factors | Survival | Number/Explant a | Leaf Length a | Clump (g/Clump) a | Total Dry Weight b | |||
|---|---|---|---|---|---|---|---|---|
| (%) | Shoots | Roots | Leaves | (cm) | Fresh Weight | Dry Weight | (g/Replication) | |
| Temporary immersion | ||||||||
| 3 times/day for 1 min | 100 ± 0.0 ns | 2.6 ± 0.0 abc | 3.7 ± 0.0 c | 6.2 ± 0.1 bc | 7.2 ± 0.1 cd | 2.05 ± 0.02 d | 0.18 ± 0.02 c | 2.75 ± 0.87 c |
| 5 min | 100 ± 0.0 | 2.2 ± 0.0 bc | 3.4 ± 0.1 c | 7.0 ± 0.1 b | 8.6 ± 0.2 bc | 3.22 ± 0.10 bc | 0.31 ± 0.02 b | 4.72 ± 1.03 b |
| 10 min | 100 ± 0.0 | 2.6 ± 0.1 abc | 3.8 ± 0.6 c | 6.6 ± 0.2 b | 7.1 ± 0.3 cd | 2.05 ± 0.15 d | 0.17 ± 0.01 c | 2.60 ± 0.70 c |
| 6 times/day for 1 min | 100 ± 0.0 | 1.5 ± 0.1 c | 4.6 ± 0.5 bc | 4.2 ± 0.1 c | 5.8 ± 0.1 d | 0.81 ± 0.09 e | 0.07 ± 0.00 d | 1.02 ± 0.14 d |
| 5 min | 100 ± 0.0 | 2.5 ± 0.1 abc | 10.9 ± 0.3 a | 8.4 ± 0.1 ab | 9.4 ± 0.2 ab | 4.20 ± 0.11 ab | 0.44 ± 0.01 a | 6.54 ± 0.43 a |
| 10 min | 100 ± 0.0 | 3.1 ± 0.2 ab | 3.7 ± 0.2 c | 8.1 ± 0.3 ab | 9.2 ± 0.1 ab | 3.12 ± 0.05 bcd | 0.32 ± 0.00 b | 4.74 ± 0.24 b |
| 12 times/day for 1 min | 100 ± 0.0 | 3.6 ± 0.3 a | 4.8 ± 0.4 bc | 8.4 ± 0.6 ab | 7.5 ± 0.2 c | 2.48 ± 0.29 cd | 0.23 ± 0.02 bc | 3.39 ± 1.29 bc |
| 5 min | 100 ± 0.0 | 3.6 ± 0.2 a | 8.3 ± 1.2 ab | 10.2 ± 0.2 a | 10.7 ± 0.2 a | 5.06 ± 0.13 a | 0.48 ± 0.01 a | 7.27 ± 0.60 a |
| 10 min | 100 ± 0.0 | 3.2 ± 0.1 ab | 4.6 ± 0.5 bc | 7.8 ± 0.6 b | 9.3 ± 0.4 ab | 4.60 ± 0.27 a | 0.43 ± 0.02 a | 6.44 ± 1.17 a |
| Culture systems | ||||||||
| Temporary immersion | 100 ± 0.0 ns | 3.6 ± 0.2 * | 8.3 ± 1.2 * | 10.2 ± 0.2 * | 10.7 ± 0.2 * | 5.06 ± 0.13 * | 0.48 ± 0.01 * | 7.27 ± 0.60 * |
| Semi-solid | 100 ± 0.0 | 1.3 ± 0.0 | 0.2 ± 0.0 | 3.6 ± 0.0 | 3.3 ± 0.0 | 0.46 ± 0.02 | 0.05 ± 0.00 | 0.68 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongsa, T.; Kongbangkerd, A.; Kunakhonnuruk, B. Optimal Growth and Biomass of Centella asiatica Using a Twin-Bottle Temporary Immersion Bioreactor. Horticulturae 2023, 9, 638. https://doi.org/10.3390/horticulturae9060638
Wongsa T, Kongbangkerd A, Kunakhonnuruk B. Optimal Growth and Biomass of Centella asiatica Using a Twin-Bottle Temporary Immersion Bioreactor. Horticulturae. 2023; 9(6):638. https://doi.org/10.3390/horticulturae9060638
Chicago/Turabian StyleWongsa, Thanakorn, Anupan Kongbangkerd, and Boworn Kunakhonnuruk. 2023. "Optimal Growth and Biomass of Centella asiatica Using a Twin-Bottle Temporary Immersion Bioreactor" Horticulturae 9, no. 6: 638. https://doi.org/10.3390/horticulturae9060638
APA StyleWongsa, T., Kongbangkerd, A., & Kunakhonnuruk, B. (2023). Optimal Growth and Biomass of Centella asiatica Using a Twin-Bottle Temporary Immersion Bioreactor. Horticulturae, 9(6), 638. https://doi.org/10.3390/horticulturae9060638