Abstract
Nitrogen (N) is an essential nutrient that plants require and is, most of the time, limited in different terrestrial ecosystems. Forming symbioses with plants, arbuscular mycorrhizal (AM) fungi improve mineral element uptake and the net primary production of plants. Recent reports have suggested that AM fungi mediate N uptake in plants. However, there are fewer studies on the influence of AM fungi on the response of Lycium barbarum, a medicinal plant in northwest China, under different N-addition conditions. In this study, the effect of Rhizophagus irregularis, N forms (NO3− and NH4+), and N levels (1.5, 7.5, 15, 30 mM) on the performance of L. barbarum was evaluated through a pot experiment. The application of R. irregularis significantly improved L. barbarum biomass, net photosynthetic rate, and root tissue viability under adequate NO3− and NH4+ supplies, and mycorrhizal plants showed better performance under NO3− supply. AM colonization enhanced N acquisition under adequate NO3− supply and strongly induced the expression of LbAMT3-1 in L. barbarum roots. Based on these results, we propose that NO3−-dominated N supply favors mycorrhizal symbiosis to a greater extent than NH4+; this study provides a basis for maintaining beneficial AM symbiosis during nitrogen fertilizer use in arable land.
1. Introduction
Arbuscular mycorrhizal (AM) fungi, from the Glomeromycotina, participate and play key roles in plant productivity. AM fungi provide limiting mineral nutrients to exchange photosynthetic products (lipid and hexose) with its host plants, especially in nutrient-limited ecosystems [1]. During AM symbiosis development, the intraradical fungal hyphae invade root cells and develop highly branched tree-like structures, known as arbuscules, for the purpose of nutrient exchange. Meanwhile, extraradical hyphae explore regions of the soil that plant roots cannot reach and extract mineral nutrients and water from these regions [2]. After the establishment of a symbiotic system, plants can obtain nutrients through their own root hairs and root epidermis or through arbuscules’ interfacial apoplasts, where the exchange between AM fungi and the plant takes place [3].
Nitrogen (N) is the essential mineral that constitutes chlorophyll and proteins; in plants, it affects stem and leaf growth and fruit development. However, the N availability in soil is frequently lower than the demand of local plants. Ammonium (NH4+) and nitrate (NO3−) are the dominant forms of nitrogen absorbed by plants from the soil [4]. N limitation is the major reason for reduced net primary production in most terrestrial ecosystems [5]. In comparison with plants, microorganisms have advantages in terms of N uptake due to their unique characteristics, including fast growth rate and large surface area to volume ratio [6]. An increasing number of reports have demonstrated that AM fungi mediate plant N uptake [3,7,8]. AM fungi supplying N to the plant increases the competitive advantage of the host plant. AM fungi can take up inorganic forms (NH4+ [9] and NO3− [10]) or organic forms (amino acids [11]) of N from the soil through their extraradical hyphae. As a result, the N in the cytoplasm of extraradical hyphae is assimilated into arginine and transferred from the extraradical hyphae to the intraradical hyphae in combination with polyphosphate [12]. In arbuscules, NH4+ is released from arginine, but the mechanism through which N is released from arbuscules into the interfacial apoplast between the arbuscule and the plant root cell remains unclear. Two AM fungal aquaporins that are located in the intraradical hyphae and arbuscules were identified in a previous study and were proposed as candidates for NH4+ transport into interfacial apoplasts [13]. Additionally, two genes with substantial homology to ammonia transport outward protein3 (Ato3) in Saccharomyces cerevisiae were identified in Rhizophagus Irregularis; these genes were speculated to be candidate genes for NH4+ transport into interfacial apoplasts [14].
The import of NH4+ across the periarbuscular membrane into the root cortical cells is dependent on plant NH4+ transporters (AMTs). AMTs induced by AM fungi have been identified in several plants, including ZmAMT3;1 in maize [15], AMT3;1 and AMT4 in Poaceae species [16], AMT2;3 in Medicago truncatula [17], and LjAMT2;2 in Lotus japonicus [18]. These studies have suggested the existence of a mycorrhizal NH4+ uptake pathway. In addition to NH4+, Wang et al. [3] recently identified OsNPF4.5, a transporter from the nitrate transporter 1/peptide transporter family (NPF), in Oryza sativa. OsNPF4.5 is capable of transferring NO3− across the periarbuscular membrane under NO3−-supply conditions. Wang et al. [3] proposed one mycorrhizal NO3− uptake pathway that is conserved in gramineous species.
Lycium barbarum L. (wolfberry), originally cultivated in east Asia, is a plant species with high economic value in northwest China; it has been used as a traditional medical and food supplement in China for more than 2000 years [19]. Symbioses that have formed between L. barbarum and AM fungi are quite common [20]. Different N fertilizers alter the performance of the growth, and betaine and polysaccharide concentrations of L. barbarum [21]. However, the influence of AM fungi on the performance of L. barbarum with different N (forms and concentrations) fertilizers is not fully understood. The response of a recently isolated NH4+ transporter (LbAMT3-1) [15,22], which may regulate NH4+ transport from AM fungi to L. barbarum, is also unclear. Therefore, a pot experiment was conducted in this study with the following objectives: (1) to evaluate the effect of AM fungal colonization on the growth performance (biomass accumulation, N accumulation, photosynthesis, and root viability) of L. barbarum and (2) to compare the response of LbAMT3-1 expression to different N supplies.
2. Materials and Methods
2.1. Biological Materials and Growth Substrate
Seeds of L. barbarum L. (Ningqi 1) were kindly provided by Dr. Wang Yajun (Ningxia Academy of Agriculture and Forestry Sciences). Surface disinfection of L. barbarum seeds was achieved by soaking the seeds in 5% sodium hypochlorite for 5 min. Disinfected seeds were then rinsed 3 times with distilled water and then placed on moist filter paper for germination (3 days) at 28 °C. Germinated seeds were grown in a seedling tray filled with sterilized vermiculate until 4–5 leaves emerged (about 5 weeks) in a greenhouse (20–30 °C and a photoperiod of 12–14 h). During growth, the seedlings were watered daily and fertilized weekly with 5 mL of full-strength Hoagland’s solution [23]. The seedlings that showed similar growth and height were selected and used for the pot experiment.
Rhizophagus irregularis Blaszk., Wubet, Renker & Buscot (BGC BJ109) was provided by the Plant Nutrition and Resources Institutes of Beijing Academy of Agriculture and Forestry. Rhizophagus irregularis was the dominant AM fungal species in the rhizosphere of Goji in Ningxia province in China, so this AM fungal strain was used in a previous study [22]. Plantago asiatica L., which was cultivated using autoclaved sands, was used as the host plant for R. irregularis propagation for 12 months. The inoculum consisted of spores (20 per gram of sand), sand, and mycelia.
The growth substrate was a mixture of autoclaved (121 °C for 3 h) sand and vermiculite (1:1, v:v).
2.2. Experimental Design and Growth Condition
For the pot experiment, four seedlings as one independent biological replicate were transplanted in one plastic pot (10 × 8 ×10 cm in length, width, and height, respectively) filled with 500 g of growth substrate (sand: vermiculite, 1:1). There were 4 biological replicates (16 seedlings in total) for each treatment. The experiment consisted of a randomized complete block design with two AM situations (inoculated and noninoculated with R. irregularis), two N forms (NO3− or NH4+), and four N levels (1.5, 7.5, 15, or 30 mM), with 4 replicates per treatment. There were 16 treatments with 4 biological replicates for each treatment (64 pots in total). For inoculated treatments, 20 g of inoculum of R. irregularis was thoroughly mixed with growth substrate. The noninoculated treatments were achieved by inoculation with autoclaved inoculum.
After transplantation, the seedlings were grown in a greenhouse with 16 h/8 h (light/dark) photoperiod, at 24–28 °C and 40–65% relative humidity. Seedlings in each pot were watered daily. The N treatment was achieved by weekly fertilization with 50 mL of modified Hoagland’s solution [24] containing either NO3− (CaNO3) or NH4+((NH4)2SO4) (1.5, 7.5, 15, or 30 mM) and 0.2 mM phosphate. The N treatment lasted for 6 weeks.
2.3. Plant Sampling, Biomass, and AM Fungal Colonization Measurements
Six weeks after seedling transplantation, the seedlings from each treatment were harvested. Roots from each biological replicate (one pot) were separately collected, washed with tap water, dried with paper towel, cut into 1 cm fragments, and divided into 4 portions. One portion of the roots was stained with trypan blue [25] and used for colonization measurement based on the grading assessment method [26]. One portion was used for the root viability assessment. One portion of the roots was frozen with liquid nitrogen and stored in a refrigerator at −80 °C. The shoots and the last portion of the roots were dried at 65 °C until they reached a constant weight.
2.4. Net Photosynthesis Rate and Root Viability Measurements
The net photosynthesis rate (Pn) was measured one day before harvest with a Li−6400 potable open flow gas-exchange system (Li-Cor Inc. Lincoln, NE, United States). The measurement conditions were as follows: photosynthetically active irradiation, 500 mmol/m2 s; temperature, 25 °C; and CO2 concentration, 400 mmol/mol [27].
Fresh roots (100 mg) from each biological replicate in different treatments were used to assess the root tissue viability, which is shown as the reduction amount of triphenyltetrazolium chloride (TTC) per unit weight and reaction time [28].
2.5. Determination of N Concentration
Dried plant materials (100 mg) were digested with 98% H2SO4 and 30% H2O2, and the N concentration was analyzed using a Flowsys continuous flow analyzer (SYSTEA S.p.A., Anagni, Italy).
2.6. Analysis of LbAMT3-1 and Promoter Sequences
The physical and chemical properties, transmembrane structure, and subcellular localization of LbAMT3-1 were predicted using ProtParam (https://web.expasy.org/protparam/, accessed on 15 October 2021), Deep TMHMM version 1.0.0 (https://dtu.biolib.com/DeepTMHMM, accessed on 15 October 2021), and DeepLoc-1.0 (https://services.healthtech.dtu.dk/service.php?DeepLoc−1.0, accessed on 15 October 2021). The conserved amino acid sites were analyzed through a comparison with CaMep2 (Candida albicans N240A), EcAmtB (Escherichia coli), and LbAMT3-1. The tertiary structure of LbAMT3−1 was modeled and visualized using SWISS-MODEL (https://swissmodel.expasy.org/interactive, accessed on 15 October 2021) based on the crystal structure of the ammonium transporter CaMep2 from C. albicans N240A.
Through the BLAST comparison of the amino acid sequence of LbAMT3-1, the genes with high homology to the protein sequence of LbAMT3-1 were screened out, and the Poisson correction model was used in MEGA-X software (Pennsylvania State University, USA, accessed on 15 October 2021) to construct a neighbor-joining phylogenetic tree for genetic distance calculation (bootstrap test value 0–1000), and MEME Version 5.5.2 (https://meme-suite.org/meme/tools/meme, accessed on 15 October 2021) was used to analyze the conserved domains of LbAMT3-1 and its cognate genes.
Genomic DNA was extracted using a Plant Genomic DNA Extraction Kit (R2485, Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s instructions. Based on the coding sequence of LbAMT3-1 (GenBank accession number: OK335754), the thermal asymmetric interlaced PCR [29] was applied to amplify the promoter sequence upstream ATG of LbAMT3-1 (Table S1). Through Plant CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 17 October 2021) and PLACE 26.0 (https://www.dna.affrc.go.jp/PLACE/?action=newplace, accessed on 17 October 2021)), the cis-acting elements of the promoter sequence of LbAMT3-1 were predicted and analyzed.
2.7. Quantitative Real-Time PCR Analysis
The frozen roots that were stored at −80 °C were homogenized and ground into powder with a mortar and pestle and liquid nitrogen and used for total RNA extraction, which was carried out using an E.Z.N.ATM Plant RNA kit (Omega Bio-Tek, Norcross, GA, USA). First-strand cDNA synthesis was obtained from 1 μg of total RNA using HiScript® II Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China) following the supplier’s instructions [27].
Real-time quantitative PCR (qPCR) was performed on a CF96X Real-Time PCR System (Bio-Rad, Hercules, CA, USA) to analyze the transcript accumulation of LbAMT3−1. The reaction volume was 10 μL, which contained 0.5 μL of each gene-specific primer (10 μM), 1 μL of cDNA, 3 μL of RNase-free H2O, and 5 μL of ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) [22].
2.8. Statistical Analyses
Data were analyzed using SPSS (Version 26, IBM, Armonk, NY, USA). The data were tested for normality and homogeneity of variances. Analyses of variance (ANOVA) were applied, and Tukey’s honestly significant difference (HSD) tests at p < 0.05 were used when a significant impact by a factor was detected.
3. Results
3.1. Biomass Accumulation and AM Colonization
Six weeks after N treatment, the biomass accumulation of L. barbarum seedlings was recorded (Table 1). All inoculated L. barbarum plants showed a statistically significant increase in the root and shoot biomass compared with those of noninoculated plants, except for those supplied with 1.5 mM NO3− or NH4+. The inoculated plants showed no significant differences in biomass from the noninoculated control plants under 1.5 mM NH4+ supply but showed decreased root and shoot biomass under 1.5 mM NO3− supply. The improvement in the plant biomass accumulation by AM fungi was more prominent with NO3− supply than with NH4+ supply. Although noninoculated plants did not show any promotion of biomass accumulation with the increase in N levels, plants supplied with NO3− grew better than those supplied with NH4+.

Table 1.
Plant growth of L. barbarum under different N supplies.
The colonization intensity and arbuscular abundance were more than 50% and 30%, respectively, under all treatments after inoculation with R. irregularis, while no AM structure was observed in the noninoculated control plants. As the N level increased, colonization intensity and arbuscular abundance substantially increased. Lycium barbarum plants supplied with NO3− showed a significantly higher arbuscular abundance than the plants that received NH4+ (Table 2).

Table 2.
AM fungi colonization of L. barbarum under different N supplies.
3.2. N Concentration and Content
For N uptake, mycorrhizal L. barbarum plants fertilized with NO3− (except for those fertilized with 1.5 mM NO3−) showed a statistically significant increase in N concentrations in both the shoots and roots compared with those of their noninoculated counterparts. When L. barbarum plants were fertilized with 1.5 mM NO3−, the inoculation of AM fungus did not affect the N concentrations. In contrast with the NO3− supply, mycorrhizal L. barbarum plants supplied with NH4+ did not significantly differ in their N concentration in the roots from noninoculated plants and showed a decrease in the N concentration in their shoots under 15 mM and 30 mM NH4+ supplies (Figure 1). Fertilization with NO3− increased the N concentration in L. barbarum shoots but not in the roots compared with NH4+ supply at 7.5 mM, 15 mM, and 30 mM.
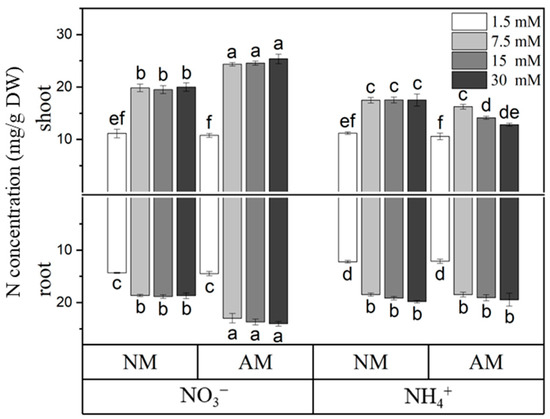
Figure 1.
N concentration in inoculated (AM) and noninoculated (NM) L. barbarum under different N supplies. DW, dry weight; NO3−, plants fertilized with CaNO3; NH4+, plants fertilized with (NH4)2SO4. Data are shown as means ± SD (n = 4). Values with the same letter did not significantly differ at p < 0.05 per Tukey’s HSD test.
3.3. Net Photosynthetic Rate
Pn showed significant differences among the different treatments (Figure 2a). Compared with NH4+ supply, plants with NO3− supply had a higher Pn. Low NO3− and excess NH4+ supplies decreased the photosynthetic rate. AM fungal colonization significantly increased the Pn under adequate N supply, except for in the 1.5 mM N supply treatment.
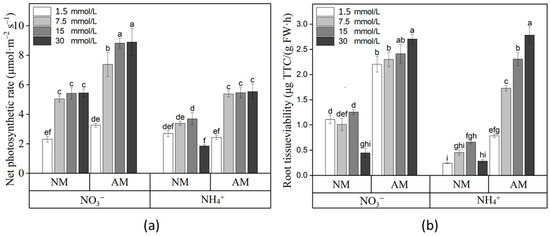
Figure 2.
Net photosynthetic rate (a) and root tissue viability (b) of L. barbarum under different N supplies and AM fungi treatments. AM, inoculated with R. irregularis; NM, noninoculated; NO3−, plants fertilized with CaNO3; NH4+, plants fertilized with (NH4)2SO4. Data are shown as means ± SD (n = 4). Values with the same letter did not significantly differ at p < 0.05 per Tukey’s HSD test.
3.4. Root Tissue Viability
Root tissue viability was notably affected by the N form, N level, and AM fungal treatment according to the variance analyses. For the noninoculated plants, the root tissue viability of the plants supplied with NO3− showed obvious increases in these variables compared with those plants supplied with NH4+, and 30 mM N supply significantly decreased the root tissue viability. For inoculated plants, AM fungal colonization significantly increased the plant root tissue viability under different N supplies, and the root tissue viability increased along with increasing N levels (Figure 2b).
3.5. Analysis of LbAMT3-1 and Promoter Sequences
LbAMT3-1 is constituted of 490 amino acids, has a molecular weight of 53,572.42, and has a theoretical isoelectric point of 5.75. It is a stable protein. A comparison with the reported amino acid sequences of the E. coli and C. albicans ammonium transporters showed important amino acid functional sites are conserved in LbAMT3-1, for instance, extracellular ammonium-binding sites, phenylalanine gates in hydrophobic channels, and double-histidine structures (Figure S1).
The results of Deep TMHMM transmembrane structure prediction showed that LbAMT3-1 is a membrane protein with 11 transmembrane helices (Figure S2), which conforms to the structural characteristics of a transporter. The results of DeepLoc prediction further indicated that LbAMT3-1 is a transporter located in the cell membrane. Using the ammonium transporter CaMep2 of C. albicans N240A as a template, the tertiary structure model of LbAMT3-1 was constructed. The results showed that LbAMT3-1 exists in a typical trimer assembly form, and there is a central pore surrounded by 11 transmembrane helices in each unit (Figure S3).
Phylogenetic analysis based on protein sequence alignment showed that LbAMT3-1 had a stronger genetic relationship with the SlAMT3-1 of tomato and is in the same branch as two other species of Solanaceae—capsicum and tobacco. LbAMT3-1 contains roughly the same motif as other AMTs, but LbAMT3-1, SlAMT3-1, CaAMT3-1, and NtAMT3-1 in Solanaceae all lack a motif with the amino acid sequence PALGQGFLIGQAGLPATVHYYHBGSVEE (Figure S4).
The promoter sequence of LbAMT3-1 contains multiple mycorrhizal or nodule-associated cis-regulatory elements, such as nodulation and AM-symbiosis-associated cis-acting elements OSE1ROOTNODULE (AAAGAT) and NODCON2GM/OSE2ROOTNODULE (CTCTT) and the cis-acting elements MYCS (TCTTGTTW) and P1BS (GNATATNC), which are located in the promoters of the phosphate transporter genes that are specifically induced by AM fungi (Figure S5).
3.6. Relative Expression of LbAMT3-1
In accordance with the colonization results, the expression of LbAMT3-1 was scarcely detected in noninoculated plant roots (Figure 3). LbAMT3-1 expression levels were higher with NO3− supply than with NH4+ supply under all N levels. Except for the 1.5 mM NO3− supply, the expression of LbAMT3-1 was maximized at the other NO3− levels.
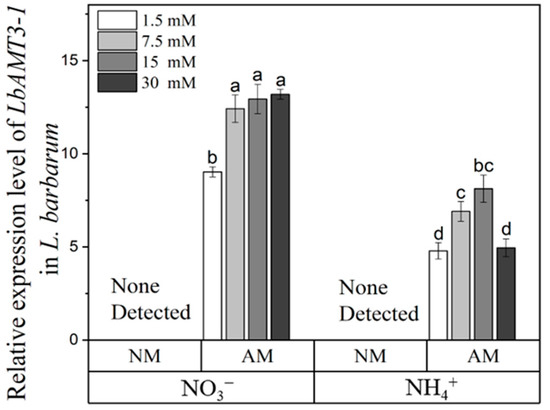
Figure 3.
Transcript levels of LbAMT3-1 in inoculated (AM) and noninoculated (NM) L. barbarum roots under different N supplies. NO3−, plants fertilized with CaNO3; NH4+, plants fertilized with (NH4)2SO4. Data are shown as means ± SD (n = 4). The actin gene of L. barbarum was used as an internal control gene. Values with the same letter did not significantly differ at p < 0.05 per Tukey’s HSD test.
4. Discussion
Recent studies have shown that N is one of nutrient elements that AM fungi transfer to plants, in addition to phosphorus, which is used for exchange with hosts’ photosynthates [15,17,19,30]. In this study, the response of AM symbiosis with L. barbarum to different N (forms and concentrations) supplies was assessed. The lowered colonization of R. irregularis caused by the low N (1.5 mM) supply could be attributed to the strong N competition between the host plants and AM fungi. There was a report that the extraradical hyphae of AM fungi constituted a large N sink and contained a N concentration at least four times higher than that of their plant partners. In N-limited conditions, due to inability of AM fungi in the mutual relationship with plants to keep their N reserves to themselves rather than to deliver N to plants, host plants may, in return, limit the develop of AM fungi inside the roots [31]. Similar results were observed in a previous study, where the AM colonization of Andropogon gerardii Vitman increased along with an increasing N level from 0 to 1.5 mM in nitrate form [32]. Similarly, Wu et al. [30] found that the AM arbuscular, hyphal, and total colonization rates of Populus × canadensis ‘Neva’ roots substantially increased with an increasing N level (from 0 to 15 mM NH4NO3). Our results indicated that an adequate N supply is crucial for the maintenance of a beneficial symbiotic relationship between host plants and AM fungi. In addition, the plant tissue N:P ratio threshold needs to be taken into consideration for maximizing the benefit of AM symbiosis through fertilizer application [32].
AM fungi have a rapid NH4+ assimilation capacity [33,34]. Although AM fungi prefer the direct uptake of NH4+, owing to the lower energy consumption [35,36], our results showed that when NH4+ was the only N source, the arbuscular abundance was significantly lower than when NO3− was the only N source. Meanwhile, the arbuscular abundance and colonization intensity significantly decreased under excessive NH4+ supply. Similar results have been reported: NH4+ supply decreases the AM fungal colonization [37,38]. Given the toxicity to plants, inhibition of root growth, and high C costs of uptake, NH4+ has an adverse impact on AM colonization.
The form of N supply can influence a plant’s biomass, photosynthetic rate, and total N concentration. In contrast to NH4+, a higher photosynthetic rate and a higher biomass accumulation were recorded in L. barbarum exposed to NO3−, although this was independent of AM colonization. Previous studies indicated that NH4+ hinders primary root growth by reducing the length of the meristem and the elongation zone and decreasing the elemental expansion rate in the root apex of Arabidopsis thaliana [39]. Thus, the toxicity of NH4+ causes the inhibition of plant growth and the Pn. When seedlings were fertilized with the same N form, the biomass accumulation did not differ among the different N levels in the noninoculated treatment. The slow-growing nature of L. barbarum and the low amount of total applied N (less than 252 mg/kg) in the growth substrate might have weakened the effect of the promotion of increasing N concentration on biomass accumulation [19,21]. AM colonization promoted plant growth and Pn under adequate (higher than 1.5 mM) N supply. Due to the ammonium toxicity to plant roots and the negative effects on arbuscular rates, the growth and Pn of the mycorrhizal plants supplied with NH4+ were weakened compared with those of plants supplied with NO3−.
For N uptake, our findings highlighted that AM fungal colonization could promote N uptake under NO3− supply but not under NH4+ supply. It is accepted that N acquired by extraradical hyphae is assimilated into arginine and binds to polyphosphate, is transferred from extraradical to intraradical hyphae and arbuscules, and is then released into the interfacial apoplast in NH4+ form [34,40]. Due to the limited ability of plants to assimilate NH4+, when NH4+ is the sole N source, NH4+ transferred via the mycorrhizal pathway may aggravate the toxicity of NH4+ to the root system. This also explained the decreases in the N concentration in the shoots when the concentration of NH4+ supply was. For NO3− supply, we speculated that NH4+ released into the interfacial apoplasts via the arbuscules can replenish the N to plants with less pressure caused by NH4+ assimilation. Meanwhile, the direct transfer of NO3− via the mycorrhizal pathway may also fulfill the demand of plants for N [3].
Plant root growth and development are strongly regulated by AM fungal symbiosis [41]. Colonization by AM fungi is instrumental in improving root-absorbing activity, root tissue viability, and root morphology [30,42]. For instance, Funneliformis mosseae colonization significantly increased the root tissue viability of Robinia pseudoacacia L. [43]. Decreases in root tissue viability were observed with excess N supply (30 mM), which was attributed to the stress response of plants to excess N supply [44]. Our results agree with those of previous studies in that AM fungal colonization increased root tissue viability and alleviated the inhibition of root tissue viability under excessive N supply. Increased root tissue viability indicated greater element uptake capacity and growth stimulation [45,46], which is consistent with the higher biomass accumulation observed in the inoculated plants.
NH4+ has been proposed in many studies to be an important candidate for N transfer to plants by AM fungi [12,34,47]. This prompted us to speculate that AM-fungi-induced L. barbarum ammonia transporter LbAMT3-1 might be important for N uptake at the symbiotic interface. Important functional features such as the extracellular ammonium binding site, the Phe gate, and the twin-His motif within the hydrophobic channel are very similar to those of CaMep2 and AtAMT1-1, which possess ammonium transport capacity [48,49]. The AMT3 cluster and the AMT3-1 orthologs may correspond to monocot–dicot AM-inducible AMTs [16]. OsAMT3-1, SbAMT3-1, and GmAMT3-1 were proven to be specifically induced by AM fungi; whether other AMTs in close proximity to LbAMT3-1 are induced by AM fungi requires further investigation. There are two conserved motifs (MYCS and P1BS) in the promoter region of LbAMT3-1. The deletion or mutation of any one of them led to a significant decrease or even complete deletion of the promoter activity of the phosphate transporter gene induced by AM fungi in eggplant and tobacco [50]. Although the MYCS and P1BS motifs were found to be cis-acting elements in the promoter region of the phosphate transporter, their presence suggested that they may serve as activating elements conserved in the promoter regions of all genes that respond to AM fungal induction. In addition to the MYCS and P1BS motifs, nodulation and AM-symbiosis-associated cis-acting elements (OSE1ROOTNODULE and NODCON2GM/OSE2ROOTNODULE) have also been identified, suggesting that there may be diverse regulatory pathways for LbAMT3-1 [51,52].
The expression of LbAMT3-1, which resembles the expression of ZmAMT3;1 [15], was only detected in AM-fungi-inoculated treatments, as in our previous study [22]. When the N supply was in the form of NO3−, the expression pattern of LbAMT3-1 was similar to those of the biomass and N concentration. As the transport of NH4+ from AM fungi toward plant roots was suggested to accompany phosphate [53], the highly induced expression of LbAMT3-1 may represent the promotion of both the N and phosphate uptake of L. barbarum. Another possible explanation of the increased N concentration may be the direct transport of NO3− through the mycorrhizal pathway with a nitrate transporter [3]. When the N supply was in the form of NH4+, the toxicity of NH4+ limited the arbuscular rates and the expression of LbAMT3-1. Although the transport of N through the mycorrhizal pathway may be hindered, the direct uptake of NH4+ by plant roots may fulfill the N demand of plants [15].
5. Conclusions
In summary, this study is the first to attempt to illustrate the preference of the symbiosis of AM fungi and L. barbarum for N (various forms and concentrations). The inoculation of R. irregularis formed colonization of the roots of L. barbarum and promoted plant biomass accumulation when adequate N (higher than 1.5 mM) was supplied. Compared with NH4+, the performance of L. barbarum with R. irregularis under NO3− fertilization was much higher in terms of growth, photosynthesis, and root tissue viability. An increased concentration of NH4+ mitigated the improvement in the N transport of R. irregularis as the expression of LbAMT3-1 was reduced under a high level of NH4+. However, a high level of NO3− did not affect the expression of LbAMT3-1. Further study with isotope-labeled N and an LbAMT3-1 mutant may provide more information about the transport of N through the mycorrhizal pathway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9060637/s1, Table S1: Primers used for amplification of promoter sequence of LbAMT3-1; Figure S1: Sequence conservation in ammonium transporters; Figure S2: Sequence conservation in ammonium transporters: Figure S3: Structure modeling of LbAMT3-1 based on ammonium transporter crystal structure of the N240A mutant of Candida albicans Mep2; Figure S4: Phylogenetic tree of ammonium transporter (AMT) and conservative motif of AMTs; Figure S5: Analysis of cis-acting elements in promoter sequence of LbAMT3-1.
Author Contributions
Conceptualization, M.G. and H.Z.; Investigation, M.G., Q.Z. and K.C.; Writing—Original Draft Preparation, M.G. and H.Z.; Writing—Review and Editing, M.G. and H.Z.; Supervision, M.G. and H.Z.; Project Administration, M.G. and H.Z.; Funding Acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (42277027, 31700530, 31870093, and 31800096).
Data Availability Statement
The data related to the present study are available upon request from the interested party.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, C.; Parniske, M. Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu. Rev. Cell Dev. Biol. 2013, 29, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Chen, A.Q.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; et al. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Fan, X.R.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef]
- Kobae, Y.; Tamura, Y.; Takai, S.; Banba, M.; Hata, S. Localized expression of arbuscular mycorrhiza-inducible ammonium transporters in soybean. Plant Cell Physiol. 2010, 51, 1411–1415. [Google Scholar] [CrossRef]
- He, X.H.; Critchley, C.; Bledsoe, C. Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs). Crit. Rev. Plant Sci. 2003, 22, 531–567. [Google Scholar] [CrossRef]
- Frey, B.; Schüepp, H. Acquisition of nitrogen by external hyphae of arbuscular mycorrhizal fungi associated with Zea mays L. New Phytol. 1993, 124, 221–230. [Google Scholar] [CrossRef]
- Tobar, R.; Azcón, R.; Barea, J.M. Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytol. 1994, 126, 119–122. [Google Scholar] [CrossRef]
- Cliquet, J.B.; Murray, P.J.; Boucaud, J. Effect of the arbuscular mycorrhizal fungus Glomus fasciculatum on the uptake of amino nitrogen by Lolium perenne. New Phytol. 1997, 137, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pfeffer, P.E.; Douds, D.D.; Piotrowski, E.; Lammers, P.J.; Shachar-Hill, Y. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 2005, 168, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, Y.J.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.; Doidy, J.; Zimmermann, S.D.; Wipf, D.; Courty, P.E. Take a trip through the plant and fungal transportome of mycorrhiza. Trends Plant Sci. 2016, 21, 937–950. [Google Scholar] [CrossRef]
- Hui, J.; An, X.; Li, Z.; Neuhäuser, B.; Ludewig, U.; Wu, X.; Schulze, W.; Chen, F.; Feng, G.; Lanbers, H.; et al. The mycorrhiza-specific ammonium transporter ZmAMT3;1 mediates mycorrhiza-dependent nitrogen uptake in maize roots. Plant Cell 2022, 34, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Koegel, S.; Mieulet, D.; Baday, S.; Chatagnier, O.; Lehmann, M.F.; Wiemken, A.; Boller, T.; Wipf, D.; Bernèche, S.; Guiderdoni, E.; et al. Phylogenetic, structural, and functional characterization of AMT3;1, an ammonium transporter induced by mycorrhization among model grasses. Mycorrhiza 2017, 27, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Breuillin-Sessoms, F.; Floss, D.S.; Gomez, S.K.; Pumplin, N.; Ding, Y.; Levesque-Tremblay, V.; Noar, R.D.; Daniels, D.A.; Bravo, A.; Eaglesham, J.B.; et al. Suppression of Arbuscule Degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2-3. Plant Cell 2015, 27, 1352–1366. [Google Scholar] [CrossRef]
- Guether, M.; Neuhauser, B.; Balestrini, R.; Dynowski, M.; Ludewig, U.; Bonfante, P. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 2009, 150, 73–83. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Hart, M.; Chen, H.; Tang, M. Arbuscular mycorrhizal symbiosis regulates hormone and osmotic equilibrium of Lycium barbarum L. under salt stress. Mycosphere 2016, 7, 828–843. [Google Scholar] [CrossRef]
- Chen, K.; Huang, G.; Li, Y.; Zhang, X.; Lei, Y.; Li, Y.; Xiong, J.; Sun, Y. Illumina Miseq sequencing reveals correlations among fruit ingredients, environmental factors, and AMF communities in three Lycium barbarum producing regions of China. Microbiol. Spect. 2022, 10, e02293-21. [Google Scholar] [CrossRef]
- Chung, R.S.; Chen, C.C.; Ng, L.T. Nitrogen fertilization affects the growth performance, betaine and polysaccharide concentrations of Lycium barbarum. Ind. Crops Prod. 2010, 3, 650–655. [Google Scholar] [CrossRef]
- Cheng, K.; Wei, M.; Jin, X.; Tang, M.; Zhang, H. LbAMT3-1, an ammonium transporter induced by arbuscular mycorrhizal in Lycium barbarum, confers tobacco with higher mycorrhizal levels and nutrient uptake. Plant Cell Rep. 2022, 41, 1477–1480. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 48, 356. [Google Scholar]
- Gamborg, O.L.; Wetter, L.R. Plant Tissue Culture Methods; National Research Council of Canada, Prairie Regional Laboratory: Saskatoon, SK, Canada, 1975. [Google Scholar]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzip, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une significantion fonctionnelle. In Physiological and Genetics Aspectes of Mycorrhizae; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Han, X.; Du, X.; Wu, Y.; Wei, M.; Gu, Y.; Aba, X.; Tang, M.; Zhang, H. Foliar-applied potassium improved mycorrhizal Goji (Lycium barbarum L.) growth of the potassium free-compartment in a compartmented culture system. Sci. Hortic. 2022, 293, 110681. [Google Scholar] [CrossRef]
- Ruf, M.; Brunner, I. Vitality of tree fine roots: Reevaluation of the tetrazolium test. Tree Physiol. 2003, 23, 257–263. [Google Scholar] [CrossRef]
- Liu, Y.G.; Chen, Y. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 2007, 43, 649–656. [Google Scholar] [CrossRef]
- Wu, F.; Fang, F.R.; Wu, N.; Li, L.; Tang, M. Nitrate transporter gene expression and kinetics of nitrate uptake by Populus × canadensis ‘Neva’ in relation to arbuscular mycorrhizal fungi and nitrogen availability. Front. Microbiol. 2020, 11, 176. [Google Scholar] [CrossRef]
- Chen, A.; Gu, M.; Wang, S.; Chen, J.; Xu, G. Transport properties and regulatory roles of nitrogen in arbuscular mycorrhizal symbiosis. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2018; Volume 74, pp. 80–88. [Google Scholar]
- Johnson, N.C.; Wilson, G.W.T.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal phenotypes and the law of the minimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef]
- Breuninger, M.; Trujillo, C.G.; Serrano, E.; Fischer, R.; Requena, N. Different nitrogen sources modulate activity but not expression of glutamine synthetase in arbuscular mycorrhizal fungi. Fungal Genet. Biol. 2004, 41, 542–552. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.R.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bucking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Courty, P.E.; Doubková, P.; Calabrese, S.; Niemann, H.; Lehmann, M.F.; Vosátka, M.; Selosse, M.A. Species-dependent partitioning of C and N stable isotopes between arbuscular mycorrhizal fungi and their C3 and C4 hosts. Soil Biol. Biochem. 2015, 82, 52–61. [Google Scholar] [CrossRef]
- Pérez-Tienda, J.; Valderas, A.; Camañes, G.; García-Agustín, P.; Ferrol, N. Kinetics of NH4+ uptake by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mycorrhiza 2012, 22, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.E.; Pérez-Fernández, M.A.; Valentine, A.J. Arbuscular mycorrhizae affect the N and C economy of nodulated Phaseolus vulgaris (L.) during NH4+ nutrition. Soil Biol. Biochem. 2009, 41, 2115–2121. [Google Scholar] [CrossRef]
- Valentine, A.J.; Osborne, B.A.; Mitchell, D.T. Form of inorganic nitrogen influences mycorrhizal colonisation and photosynthesis of cucumber. Sci. Hortic. 2002, 92, 229–239. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, N.; Gao, K.; Chen, F.; Yuan, L.; Mi, G. Ammonium inhibits primary root growth by reducing the length of meristem and elongation zone and decreasing elemental expansion rate in the root apex in Arabidopsis thaliana. PLoS ONE 2013, 8, e61031. [Google Scholar] [CrossRef]
- Belmondo, S.; Fiorilli, V.; Pérez-Tienda, J.; Ferrol, N.; Marmeisse, R.; Lanfranco, L. A dipeptide transporter from the arbuscular mycorrhizal fungus Rhizophagus irregularis is upregulated in the intraradical phase. Front. Plant Sci. 2014, 5, 436. [Google Scholar] [CrossRef]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Berta, G.; Trotta, A.; Fusconi, A.; Hooker, J.E.; Munro, M.; Atkinson, D.; Giovannetti, M.; Morini, S.; Foutuna, P.; Tisserant, B.; et al. Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Physiol. 1995, 15, 281–293. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Zhang, H.; Song, Y.; Chen, H.; Tang, M. Funneliformis mosseae enhances root development and Pb phytostabilization in Robinia pseudoacacia in Pb-contaminated soil. Front. Microbiol. 2019, 10, 2591. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.; Li, H.; Shi, W.; Polle, A.; Lu, M.; Sun, X.; Luo, Z.B. Global poplar root and leaf transcriptomes reveal links between growth and stress responses under nitrogen starvation and excess. Tree Physiol. 2015, 35, 1283–1302. [Google Scholar] [CrossRef] [PubMed]
- Langer, I.; Syafruddin, S.; Steinkellner, S.; Puschenreiter, M.; Wenzel, W.W. Plant growth and root morphology of Phaseolus vulgaris L. grown in a split-root system is affected by heterogeneity of crude oil pollution and mycorrhizal colonization. Plant Soil 2010, 332, 339–355. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Kobayashi, K.; Zhu, J.; Huang, J.; Yang, H.; Wang, Y.; Dong, G.; Liu, G.; Han, Y.; et al. Seasonal changes in the effects of free-air CO2 enrichment (FACE) on growth, morphology and physiology of rice root at three levels of nitrogen fertilization. Glob. Chang. Biol. 2008, 14, 1844–1853. [Google Scholar] [CrossRef]
- Chalot, M.; Blaudez, D.; Brun, A. Ammonia: A candidate for nitrogen transfer at the mycorrhizal interface. Trends Plant Sci. 2006, 11, 263–266. [Google Scholar] [CrossRef]
- van den Berg, B.; Chembath, A.; Jefferies, D.; Basle, A.; Khalid, S.; Rutherford, J.C. Structural basis for Mep2 ammonium transceptor activation by phosphorylation. Nat. Commun. 2016, 7, 11337. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Ludewig, U. Role of AMT1;1 in NH4+ acquisition in Arabidopsis thaliana. Plant Biol. 2006, 8, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Gu, M.; Sun, S.; Zhu, L.; Hong, S.; Xu, G. Identification of two conserved cis-acting elements, MYCS and P1BS, involved in the regulation of mycorrhiza-activated phosphate transporters in eudicot species. New Phytol. 2011, 189, 1157–1169. [Google Scholar] [CrossRef]
- Fehlberg, V.; Vieweg, M.F.; Dohmann, E.M.; Hohnjec, N.; Puhler, A.; Perlick, M.; Kuster, H. The promoter of the leghaemoglobin gene VfLb29: Functional analysis and identification of modules necessary for its activation in the infected cells of root nodules and in the arbuscule-containing cells of mycorrhizal roots. J. Exp. Bot. 2005, 56, 799–806. [Google Scholar] [CrossRef]
- Stougaard, J.; Jørgensen, J.E.; Christensen, T.; Kühle, A.; Marcker, K.A. Interdependence and nodule specificity of cis-acting regulatory elements in the soybean leghemoglobin lbc3 and N23 gene promoters. Mol. Gen. Genet. 1990, 220, 353–360. [Google Scholar] [CrossRef]
- Tian, C.; Kasiborski, B.; Koul, R.; Lammers, P.J.; Bücking, H.; Shachar-Hill, Y. Regulation of the nitrogen transfer pathway in the arbuscular mycorrhizal symbiosis: Gene characterization and the coordination of expression with nitrogen flux. Plant Physiol. 2010, 153, 1175–1187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).