Abstract
In the past decade, the development of highbush blueberry production in Poland has been followed by the occurrence of new pests in the plantations, including scales. Since both the assessment of the populations of natural enemies present in a territory and the knowledge of the scale species present in the crop are crucial for the correct application of IPM strategies, a study was carried out to address these aspects and evaluate the efficacy of several active substances in controlling Parthenolecanium spp. in several highbush blueberry plantations. Specimens of adult larvae collected on several plantations were phylogenetically closely linked to two species, P. corni and P. fletcheri. However, considering the ecology and behavior of these species, it was concluded that the pest population was more likely to belong to P. corni. Analyzing the scale parasitoids’ community present in the different locations, it emerged that it was quite diversified, including species affecting both the initial and adult biological phases of the scales, with differences also in the population size and diversity, including both general or specialized parasitoids and predators. The different active substances tested in the efficacy trials, which included both synthetic and bio-based compounds, were suitable for controlling the scale infestation. However, the different efficacy observed between them, depending on season and location, could be interpreted taking into consideration the initial level of infestation. It is concluded that applying an IPM strategy that combines agronomical practices with the application of insecticides with different mechanisms of action, attentive to the benefit of protecting natural enemies, can result in satisfactory control of P. corni in highbush blueberry plantations.
1. Introduction
Highbush blueberry production in Poland has been rapidly developed in the past two decades, allowing the country to become the second largest producer in the EU after Spain, with about 24% of the total EU harvest [1]. This production trend has been followed by the occurrence of “new” pests in the plantations across the country [2,3], including Parthenolecanium spp. (Hemiptera: Coccoidea) [4]. Among Parthenolecanium spp., several species have been recorded in Poland [5], such as the European fruit lecanium (P. corni [Bouchè]), a scale species that is damaging crops either directly or indirectly [6], normally observed on a wide range of fruit host plants (EPPO Global Database, https://gd.eppo.int/taxon/LECACO, accessed on 10 May 2023). The control of Parthenolecanium spp. is made difficult by their biological cycle and phenology, requiring that insecticide applications target crawler (mobile instar) emergence to achieve an effective reduction of the scale insect population [7].
Integrated Pest Management (IPM), which is compulsorily applied in the European Union since January 2014, can benefit from the populations of natural enemies present in a territory [8,9]. Important natural enemies of soft scales include predators, particularly members of the Anthribidae and Coccinellidae (Coleoptera) [10], as well as parasitoids, mainly belonging to the hymenopterous family Encyrtidae [11]. Outbreaks of scale insects have been explained by the reduction of natural enemies [12] and eventually as an effect of pesticide application [13].
Scale insects management is complicated by difficulties in their morphological identification [14]. This condition could result in less effective control by using methods or products not fully appropriate for the species present in the crop. However, the recent development of DNA barcoding techniques has also provided useful information for classifying scale insects [15,16]. Recently, specimens of a soft-scale insect were found on Vaccinium corymbosum cultivar ‘Bluecrop’ in Poland, which seemed to be close to Parthenolecanium corni (Bouché) based on their morphology but showed significant differences in their life cycle and in their settlement sites.
Therefore, a study was performed to (i) evaluate the efficacy in the control of Parthenolecanium spp. under field conditions in several locations and seasons with products having different mechanisms of action; (ii) assess the composition of natural enemies’ populations in these orchards; and (iii) determine whether specimens collected in these orchards should be considered congeneric with P. corni or whether they formed a separated (undescribed) species.
2. Materials and Methods
2.1. Taxonomic Identification of Scales Sampled from Blueberry Plantations
2.1.1. DNA Extraction, Amplification, and Sequencing
Young adult females of Parthenolecanium sp. were collected on Vaccinium corymbosum cv. Bluecrop in various locations (Table 1) and stored at −20 °C until analysis. The DNA extraction was performed using the CTAB method [17], with slight modifications. The DNA concentration was estimated on a 1.5% agarose gel and compared with GeneRuler 100 bp DNA Ladder Plus (Thermo Fisher Scientific, Waltham, MA, USA). Next, the DNA samples were diluted to give a concentration of 20 ng/µL and stored at −20 °C for downstream analyses.

Table 1.
Samples of Parthenolecanium species used in the molecular analyses.
To study the phylogenetic relationships among specimens, a fragment of DNA containing the mitochondrial cytochrome oxidase subunit I (COI) gene was amplified. The PCR amplifications were performed in a total volume of 25 μL containing: 20 ng/μL of template DNA, 2.5 μL 10 × Buffer Taq (750 mM Tris HCl pH 8.8; 200 mM (NH4)2SO4, 0.1% Tween 20) (Thermo Fisher Scientific Waltham, MA, USA), MgCl2 1500 μM, dNTP mix 800 μM, 0.2 μM of each primer, and 1.0 U Taq polymerase (Fermentas AB, Vilnius, Lithuania). The COI (mtDNA) was amplified with the primer pairs PcoF1 and HCO (Table 1). PCR conditions for COI were set as follows: an initial denaturation at 94 °C for 2 min, followed by 40 cycles of denaturation, annealing, and extension, and a final extension step at 72 °C for 10 min. For the COI amplification, the 35 cycles consisted of 30 s at 95 °C, 50 s at 50 °C, and 1 min at 72 °C. A negative control was used for PCR reactions.
PCR products with an addition of fluorescent dye were separated electrophoretically in a 1.5% agarose gel at 80 V for 1.5 h in 1 × TBE buffer containing 0.01% EtBr and visualized under UV light. After checking and determining the size of the resulting PCR product, the DNA was subjected to purification using an agarose gel of low melting point. The sequencing was conducted by Genomed S.A. (Warsaw, Poland) using the PCR primers together with a Big Dye® Terminator Cycle Sequencing Kit V. 3.1 of Applied Biosystems (Life Technologies, Warsaw, Poland) and separated using a capillary sequencer 3730XL DNA Analyzer.
2.1.2. DNA Sequence Alignment and Phylogenetic Analysis
DNA COI fragments of five Parthenolecanium species (P. corni, P. fletcheri, P. persicae, P. pomeranicum, and P. pruinosum) (Table 2) were included in the sequence alignment to compare genetic diversity with the specimens collected on V. corymbosum. Sequences were assembled and edited using BioEdit v. 7.2.5. Related DNA sequences of COI were compared using the BLAST function of GenBank. Multiple sequence alignments were performed with Clustal W using BioEdit v. 7.2.5 [18].

Table 2.
Primers and PCR protocols used.
2.2. Assessment of Parthenolecanium spp. Parasites
Parasitism of Parthenolecanium spp. Larvae was determined on sampled shoots (three shoots from four sites in each plantation) that showed the presence of scale females, which were collected during three observation periods (April, May, and June). The healthy females and those with clear parasite damage or parasites inside were separately counted.
Specimens of parasites/parasitoids were obtained by keeping the sampled shoots with the female scales in isolators until the hatched parasites were visible and systematically collected. The specimens were then sent to the Natural History Museum of London for identification.
2.3. Trials of Parthenolecanium sp. Control on Blueberry Plantations
Trials testing different products and strategies for the control of Parthenolecanium spp. Were conducted in 2017–2019 on several plantations of highbush blueberry cv. Bluecrop located in four locations in Mazovian Voievodship (Central Poland). The application of control products was carried out during the period of the overwintering larvae’s migration.
The experimental field design in each trial consisted of four replications arranged in randomized blocks. Each plot (replication) covered 52.55 m2 (three rows of 15 m length, for a total of 45 plants). Tested and reference products having different mechanisms of action (Table 3) were applied with a motorized knapsack sprayer (“Stihl SR 420“) with a spray volume of 750 L/ha.

Table 3.
List of active substances utilized in the field trials for the control of highbush blueberry scales.
The scale population density was estimated just before the treatment and approximately 2–3 weeks after spraying. The number of alive scale larvae was counted under a stereoscopic microscope on three stems (each 30 cm long) taken randomly from each plot/replication on each counting date.
2.4. Statistical Analysis
2.4.1. Phylogenetic Analysis
The phylogenetic analysis was performed with MEGA11 software [21], and the evolutionary history was inferred using the Maximum Likelihood method based on the Tamura-Nei model [22]. The bootstrap consensus tree was inferred from 1000 replicates [23]. Branches corresponding to partitions reproduced in less than 50% of bootstrap replicates collapsed. The initial tree(s) for the heuristic search were obtained automatically by applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura-Nei model and then selecting the topology with the superior log likelihood value. This analysis involved 16 nucleotide sequences. Codon positions included were 1st, 2nd, 3rd, and noncoding. All positions containing gaps and missing data were discarded. There were a total of 378 positions in the final dataset. The nucleotide sequences were compared with sequences collected in the NCBI GenBank databases using BLAST software [24] (http://www.ncbi.nlm.nih.gov/BLAST/, accessed on 15 January 2023).
The Bayesian inference analysis was carried out using the Markov Chain Monte Carlo (MCMC) algorithm with the mrbayes program ver. 3.2.2 [25], using Biolinux 8.0 as OS, and applying the default parameters.
2.4.2. Field Data Analysis
The data from field trials were analyzed by ANOVA performed on values transformed by log(x) + 1 in order to assure a normal distribution. The significance of differences between means was assessed with the Tukey multiple range test at p ≤ 0.05 using the package Statistica v.6.1. The value of actual mortality was calculated according to Abbott’s formula [26].
3. Results
3.1. Taxonomic Identification of Highbush Blueberry Scale Specimens
A phylogenetic tree was constructed based on the sequence of the COI gene fragments obtained from the Parthenolecanium sp. specimens collected in the study and the sequences of the genus Parthenolecanium deposited in GenBank (Figure 1). Two main clades can be distinguished in the trees obtained with both bootstrap and Bayesian analyses. The first clade is formed by the species P. prunoisum, while the second is formed by P. pomeraniucum, P. persicae, P. fletcheri, and P. corni, including the specimens from V. corymbosum as well. Two branches can be distinguished within the second clade, one of which contains P. pomeranicum. The specimens collected from blueberry plants were located on the second branch, positioned close to P. fletcheri and P. corni, indicating a close relationship between these taxa.

Figure 1.
Dendrograms showing the phylogenetic relationship of the adult females scale specimens collected on V. corymbosum with isolates of different Parthenolecanium species based on the analysis of molecular data by the maximum likelihood method (A) and Bayesian inference analysis (B). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test is shown below the branches.
3.2. Level of Parasitism of Parthenolecanium sp. under Field Conditions
The number of larvae found during the assessment was, on average, about 240 per shoot. The dynamic of the population and the percentage of parasitized larvae varied depending on the location (Figure 2). However, a clear difference in the dynamic was observed comparing the plantation in Piskórka with the other three: the increase (more than doubled) in the population size in Piskórka from April to May was paralleled by an increase of the parasitized larvae, while in the other two locations the population size was either steady (Rokotów) or decreased (Prazmów) in a similar pattern for healthy or parasitized larvae (Figure 2). The Piskórka plantation was also characterized by having almost 20% parasitized larvae during the whole season, ranging from 15% up to six times that of the other three plantations throughout the whole period of assessment (Figure 3).
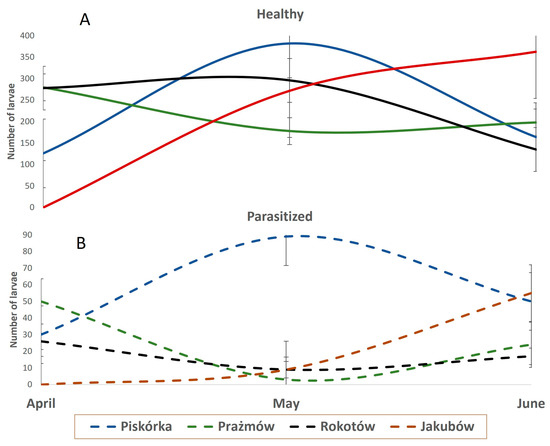
Figure 2.
Dynamic impact of natural parasites on the infestation of highbush blueberry plantations by Parthenolecanium spp.: number of healthy (A) and parasitized (B) larvae. Bars represent SD, n = 12.
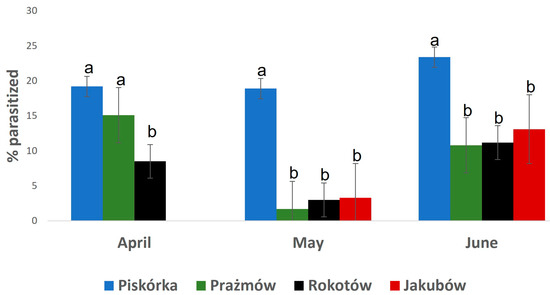
Figure 3.
Percent of parasitized Parthenolecanium larvae in the sampled population. Mean ± SD, n = 12. Different letters at each time point represent the statistical difference for p ≤ 0.05.
3.3. Identification of Parthenolecanium spp. Parasites
The number of parasitic species or genera found in the four plantations ranged from four to nine (Table 4). The plantation located in Prażmów was characterized by the highest biodiversity in this respect, also including a predator (Anthribidae spp.) not found in other locations. On the other hand, three species (Blastothrix brittanica, Coccophagus lycimnia, and Encyrtus infelix) were common to all sites, and two (Syrphophagus taeniatus and Metaphycus insidiosus) were found in three sites. One genus, Scelionidae spp., was found only in one location (Jakubów).

Table 4.
List of the parasites/parasitoids identified from larvae of Parthenolecanium spp. collected from different highbush blueberry plantations.
3.4. Control of Parthenolecanium sp. on Blueberry Plantations
The infestation by the scales varied depending on the season and the location, averaging from a few individuals to some hundreds (Table 5, Table 6 and Table 7). However, interestingly, the same location, Rokotów, recorded both the lowest (on average 2.5 individuals per shoot) and the highest (on average about 190 individuals per shoot) infestations in 2018 and 2019, respectively.

Table 5.
Effect of different products on the control of Parthenolecanium sp. on different blueberry plantations in 2017. Mean ± SD, n = 12. Different letters in columns represent statistical differences for p ≤ 0.05.

Table 6.
Effect of different products on the control of Parthenolecanium sp. on different blueberry plantations in 2018. Mean ± SD, n = 12. Different letters in columns represent statistical differences for p ≤ 0.05.

Table 7.
Effect of different products on the control of Parthenolecanium sp. on different blueberry plantations in 2019. Mean ± SD, n = 12. Different letters in columns represent statistical differences for p ≤ 0.05.
The different products applied, expressing different mechanisms of action, including some allowed in organic farming (i.e., camelina oil and spinosad), were in general effective in reducing the number of living larvae (Figure 4), but their efficacy was also influenced by the location, season, and initial level of infestation (Table 5, Table 6 and Table 7). For example, in the case of the synthetic active substances, during the three years of trials at the Piskórka plantation, only spirotetramat consistently reduced the number of living larvae significantly compared to the control, reaching an efficacy of 85–100%. Flonicamid or acetamiprid instead had a more variable effect, both when comparing the same location across the years (Piskórka) or confronting different locations (Piskórka and Rokotów) or the initial level of infestation (Table 5, Table 6 and Table 7), resulting in a lower efficacy than spirotetramet (Figure 4). The products allowed in organic farming (camelina oil and spinosad), as well as those based on silicon polymers, also had a variable effect, influenced by the same factors as the synthetic substances (Table 5, Table 6 and Table 7). However, in some cases, their efficacy was similar to the synthetic molecules, even for the protected crops, thus showing good control potential.
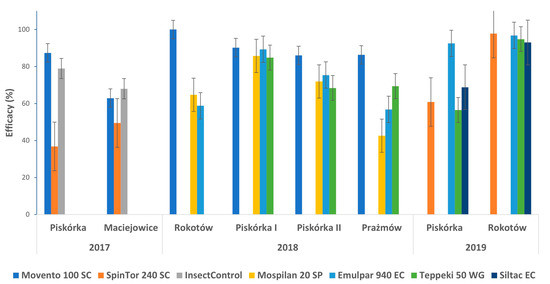
Figure 4.
Efficacy rate of the different active substances in controlling larvae of Parthenolecanium spp. on different blueberry plantations in 2017–2019. Mean ± SD, n = 12.
4. Discussion
4.1. Phylogenetic Identification of the Specimens
The specimens collected from the highbush blueberry orchards were grouped together, thus indicating a common genetic relationship, even though they were collected from orchards located in different regions. All specimens were phylogenetically closely linked to two other species, P. corni and P. fletcheri, which practically did not show genetic distance between them and the collected specimens. The dendrograms visually suggested a closer relationship between the specimens and P. fletcheri compared to the P. corni samples analyzed, but the genetic distance resulted in similar results. Both species have been recorded in Poland [5]. However, P. fletcheri, even though it was recorded for the first time in Poland in 1935, is still considered an alien species [27]. P. corni and P. fletcheri are morphologically very similar and share high variability in many morphological characters that are normally used in classification [28]. Moreover, considering that P. corni is a widespread species normally found on fruit trees, while P. fletcheri tends to be found mainly on a few forest tree species, it is more likely that the specimens analyzed belonged to the former species.
4.2. Ecology of Parthenolecanium sp. Parasites
Genera and species found to parasitize Parthenolecanium spp. in Polish highbush blueberry plantations are known natural enemies, either as parasitoids (Blastothrix, Coccophagus, Encyrtus, and Metaphycus) or predators (Anthribus) [29,30,31]. Coccophagus lycimnia and Blastothrix species are cosmopolitan, generalist parasitoid taxa that attack many scale species [30,32], so it was not surprising to find them in all the highbush blueberry plantations. Moreover, Blastothrix longipennis, Metaphycus insidiosus, and Coccophagus lycimnia were reported to be common and important parasitoids of P. corni in Poland [33]. Interestingly, the number of parasitoid genera found in association with Parthenolecanium spp. under Polish conditions was similar to that found in a fruit-producing region of Bulgaria [34]. Under those conditions, C. lycimnia and B. confusa were found to have the greatest importance in regulating the population density of the pest, as they have different targets: C. lycimnia parasitizing overwintering larvae and B. confusa parasitizing adult females.
Analyzing the parasitoid community attacking P. corni at different stages of its development, it emerged that it was quite diversified in its range, affecting both initial and adult biological phases, but also in its occurrence in different locations, as more species were present in Prażmów compared to Jakubów or Rokotów. Moreover, Prażmów was also the only location where a predator of the Anthribidae genera was observed. The presence of natural coniferous woods surrounding the plantation at Prażmów could suggest a possible explanation for this finding, as scales are frequently found on such and other forest plants under Polish conditions [35,36], thus giving the possibility of developing a complex population of natural enemies. This would also support the only finding in this location of Anthribidae spp., a predator species, as ladybirds can be an effective natural enemy of coniferous scales [37]. Anthribus nebulosus is an effective natural enemy of scale insects, including P. corni, as its larvae can act as parasitoids of adult scale individuals, while adults are able to act as predators in all stages of their hosts [9,38,39]. The efficacy of A. nebulosus in reducing the populations of P. corni was about 22% in Serbia [40].
The dynamic of the healthy or parasitized scale populations recorded in Jakubów, a location densely filled with fruit orchards, could be considered a classic example of the relation between pest and parasite populations [41], with the latter mainly formed by generalistic species, including scelionids. Most scelionids are solitary, and all are egg parasitoids that utilize the eggs of a wide variety of insects [42]. However, species of this family have been used successfully in classical biological control programs [43]. The recent release of Trissolcus japonicus to control Halyomorpha halys [44] is also an example of the potential biocontrol from using specific parasitoids of this family for Partenolecanium spp. control. Even though the level of parasitization was much higher in Piskórka, a site characterized by a natural environment rich in coniferous woods, compared to the other plantations in all three sampling periods, the parasitization rate found in the highbush blueberry plantations was in general low compared to other reports from other fruit growing areas [34].
4.3. Is It Possible to Develop a Strategy for the Integrated Control of Parthenolecanium sp. in Highbush Blueberry Orchards with Low Environmental Impact?
The different active substances tested in the trials proved suitable for controlling the scale infestation. However, the different efficacy observed between them should be interpreted taking into consideration the initial level of infestation. The high efficiency of Spinosad in the Rokotów 2019 trial was obtained when the untreated control had a high infestation level, almost unchanged from the assessment before the treatments. The same substance was less effective when the population in control plots was drastically reduced during the assessment period (Piskórka in 2019), likely as a result of the impact of natural enemies. In the latter case, only acetamiprid, a chloropyridinyl neonicotinoid, was highly effective. However, even though it was consistent in its efficacy, acetamipirid was sometimes less effective than the other synthetic substances (e.g., Rokotów and Prazmów in 2018). The use of acetamiprid for the control of the 1st instar crawler induced almost 97% mortality 21 days after the first treatment [45].
Spinosad, a bio-insecticide derived from the soil actinomycete Saccharopolyspora spinosa, is considered a valuable bioactive substance to control several pests [46]. It was found to be effective in controlling P. corni in grapes, but only at the beginning of the infestation [47]. Even though spinosad is classified as a substance with reduced environmental and toxicological risk [48,49], its toxicity on several hymenopteran parasitoids has been reviewed recently [50], and it was shown to cause 100% mortality on C. lycimnia, a specific parasitoid of P. corni, just 24 h after the treatment [51].
The silicon polymer showed intermediate efficacy in all trials. However, when the infestation in the control was high (Maciejowice in 2017 or Rokotów in 2019), the reduction of the number of larvae was significant. When applied to plants, the silicone polymer spreads on the treated surface, creating a three-dimensional grid structure with sticky properties that blocks the insect’s physical functions [52]. The efficacy of this kind of substance can thus depend on the application method and on the environmental conditions, as was also previously shown [4].
Camelina oil, characterized by a high content of poly-unsaturated and saturated fatty acids [53], was also more effective with a high infestation rate, including when the crop was grown under protected conditions. The mode of action for many oils is suffocation and water loss [54], and therefore they are unlikely to induce resistance in insect populations [55]. A canola oil treatment resulted in very high efficacy with concentrated sprays (90% with 20 L/ha and 99% with 30 L/ha), similar to that obtained with a tank of the same oil mix (1 L/ha) with chlorpyrifos-methyl [56]. Paraffin oil reduced the number of P. corni larvae by an average of about 80% in trials under different pedo-climatic conditions [57]. Other non-synthetic substances suitable for scale control under organic management of highbush blueberries were reported to have different efficacy. A mixture of vegetal amino acids and fatty acids or treatment with Quassia amara extract 50% and potassium soap showed insufficient efficacy [56]. On the other hand, a product based on polysaccharides and also containing chitosan showed a satisfactory level of efficacy [4]. The combined and alternated use of this kind of substance for the control of scales on highbush blueberries could thus represent a good strategy to reduce the environmental impact and improve the overall control efficacy.
The difficulty in controlling scales can be assessed considering the results from the treatments with synthetic active substances. The consistent and high efficacy shown by spirotetramat across locations and seasons, confirming previous results [4], can allow us to consider this product as a reference standard. However, spirotetramat efficacy changed significantly across seasons in a 2-year trial in Slovenia, from 98% in the first year to 70% in the second [58]. The efficacy of flonicamid, a selective systemic pesticide that interferes with the alimentary behavior of several insect species [59,60], was somehow inconsistent throughout the seasons and locations in the present study; its efficacy was significant only in two out of six trials where it was tested. However, as it was shown to have a small impact on parasitoids of other Coccidae species, causing the lowest reduction in their parasitism rate [61], its application and efficacy should be further verified.
Scale populations could remain below the economic threshold thanks to natural biological control, and it is believed that this has been the case for highbush blueberry plantations in Poland in the past, as no major need for their control was raised by producers. However, it could be argued that the need to assure protection against D. suzuki in recent years also in Poland, with increased use of pesticides, might have disrupted the natural control in highbush blueberry orchards, similarly to other cases [47,62], requiring the performance of some control measures against scales.
The presence of several genera and species of parasitoids in the highbush blueberry plantations concerned by the research allows us to envision their contribution to P. corni scale control even when pesticides are applied. Indeed, while insecticides are generally toxic to adult scelionids, it appears that preimaginal parasitoids within host eggs could escape high mortality in the field even when formulations based on insect growth regulators are applied [63]. Nevertheless, in addition to direct mortality, pesticides sublethal effects on beneficial insects’ physiology and behavior can also modify the population dynamics and biological control potential of scale parasitoids [11], thus requiring a careful selection of substances to be applied.
Control of scales is a major challenge because adult females and eggs are protected from pesticides, with only the first-instar nymphs being susceptible due to their mobility, making the timing of pesticide applications targeting them critical to achieving significant efficacy. A good integrated strategy to manage scales includes dormant pruning of old, weak canes and scale-infested wood, which prevents increasing their population density. Applying a complex strategy, combining the application of several synthetic insecticides with different MoAs and timings, allowed us to achieve an efficacy above 90% [64]. A good control was also possible by introducing a control based on non-synthetic substances (this report; [4]). The importance of P. corni control is also derived from its function as a vector of several viruses [65,66]. Recently, it was shown that larval stages and adult females of P. corni feeding on highbush blueberry plants infected by the blueberry red ringspot virus carried the virus, even though it was not proven they were able to transmit it [67].
5. Conclusions
The substances tested for the control of P. corni scales on highbush blueberry during several years and in different locations were suitable, but the level of efficacy depended on various factors and should be interpreted taking also into consideration the initial level of infestation. It is argued that the infestation level could have been affected by the population composition of natural enemies, which was diversified at the studied sites. Therefore, applying an IPM strategy that combines agronomical practices with the application of insecticides with different mechanisms of action, attentive to the benefit of protecting natural enemies, can result in satisfactory control of P. corni in highbush blueberry plantations. Even though the scales present in the highbush blueberry plantations presented some uncommon morphological characteristics, the comparison based on COI sequences of their DNA with those of other accessions from P. corni or P. fletcheri did not show significant differences.
Author Contributions
Conceptualization, M.T.; methodology, M.T. for field trials and A.F.-G. for phylogenetic analysis; laboratory analyses, B.S. for field samples, A.F.-G. for phylogenetic analysis; investigation, M.T. and E.M.; data curation, M.T.; writing—original draft preparation, E.M. and M.T.; writing—review and editing, all authors. funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Education and Sciences (grant number ZORpSz/3/2016–d. 2.2.2); “Monitoring inwazyjnych owadów i roztoczy zagrażających uprawom sadowniczym oraz opracowanie biologicznych podstaw ich zwalczania”.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
Christina Fisher and Max Barclay from the Natural History Museum of London is acknowledged for the identification of the scale parasites. Barbara Łagowska i Katarzyna Golan from Uniwersytet Przyrodniczy w Lublinie for the advices on phylogenetic analyses. The support from the farmers during the field trials is also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Blueberry Organization. Global State of the Blueberry Industry Report 2022. Available online: https://www.internationalblueberry.org/2022-report/ (accessed on 28 March 2023).
- Łabanowska, B.H.; Piotrowski, W. The spotted wing drosophila Drosophila suzukii (Matsumura, 1931)—Monitoring and first records in Poland. J. Hortic. Res. 2015, 23, 49–57. [Google Scholar] [CrossRef]
- Kalinowska, E.; Paduch-Cichal, E.; Chodorska, M.; Sala-Rejczak, K. First report of Blueberry red ringspot virus in highbush blueberry in Poland. J. Plant Pathol. 2011, 93, Supplement pp. S4.73. [Google Scholar]
- Tartanus, M.; Malusá, E.; Sas, D.; Łabanowska, B. Integrated control of Lecanium scale (Parthenolecanium spp.) on highbush blueberry in open field and protected crops. J. Plant Prot. Res. 2018, 58, 297–303. [Google Scholar] [CrossRef]
- Łagowska, B. Czerwce (Coccoidea), Zabielicowate (Ortheziidae), Czerwcowate (Margarodidae), Czerwce mączyste (Pseudococcidae), Pilśnikowate (Eriococcidae), Kermesowate (Kermesidae), Miłkowate (Cerococcidae), Misecznikowate (Coccidae), Gwiazdosze (Asterolecaniidae), Tarczniki (Diaspididae). In Fauna Polski—Charakterystyka i wykaz gatunków; Bogdanowicz, W., Chudzicka, E., Pilipiuk, I., Skibińska, E., Eds.; Muzeum and Instytut Zoologii PAN: Warszawa, Poland, 2004; pp. 266–269. [Google Scholar]
- Vranjic, J.A. Effects on host plant. In Soft Scale Insects: Their Biology, Natural Enemies and Control; Ben-Dov, Y., Hodgson, C.J., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1997; Volume 7A, pp. 323–336. [Google Scholar]
- Robayo Camacho, E.; Chong, J.-H. General biology and current management approaches of soft scale pests (Hemiptera: Coccidae). J. Integr. Pest. Manag. 2015, 6, 1–22. [Google Scholar]
- Stark, J.D.; Vargas, R.; Banks, J.E. Incorporating ecologically relevant measures of pesticide effect for estimating the compatibility of pesticides and biocontrol agents. J. Econ. Entomol. 2007, 100, 1027–1032. [Google Scholar] [CrossRef]
- Trdan, S.; Laznik, Ž.; Bohinc, T. Thirty years of research and professional work in the field of biological control (predators, parasitoids, entomopathogenic and parasitic nematodes) in Slovenia: A review. Appl. Sci. 2020, 10, 7468. [Google Scholar] [CrossRef]
- Ponsonby, D.J.; Copland, M.J.W. Coccinellidae and other Coleoptera. In Soft Scale Insects: Their Biology, Natural Enemies and Control; Ben-Dov, Y., Hodgson, C.J., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1997; Volume 7A, pp. 29–60. [Google Scholar]
- Kapranas, A.; Tena, A. Encyrtid Parasitoids of Soft Scale Insects: Biology, Behavior, and Their Use in Biological Control. Ann. Rev. Entomol. 2015, 60, 195–211. [Google Scholar] [CrossRef]
- Price, P.W.; Denno, R.F.; Eubanks, M.D.; Finke, D.L.; Kaplan, I. Insect Ecology: Behavior, Populations and Communities. Cambridge University Press: New York, NY, USA, 2011. [Google Scholar]
- Raupp, M.J.; Holmes, J.J.; Sadof, C.; Shrewsbury, P.; Davidson, J.A. Effects of cover sprays and residual pesticides on scale insects and natural enemies in urban forests. J. Arboric. 2001, 27, 203–214. [Google Scholar] [CrossRef]
- Gullan, P.J.; Cook, L.G. Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Zootaxa 2007, 425, 413–425. [Google Scholar] [CrossRef]
- Andersen, J.C.; Wu, J.; Gruwell, M.E.; Gwiazdowski, R.; Santana, S.H.; Feliciano, N.H.; Morse, G.E.; Normark, B.B. A phylogenetic analysis of armored scale insects (Hemiptera: Diaspididae), based upon nuclear, mitochondrial, and endosymbiont gene sequences. Mol. Phylogenet. Evol. 2010, 57, 992–1003. [Google Scholar] [CrossRef]
- Wang, X.-B.; Deng, J.; Zhang, J.-T.; Zhou, Q.-S.; Zhang, Y.-Z.; Wu, S.-A. DNA barcoding of common soft scales (Hemiptera: Coccoidea: Coccidae) in China. Bull. Entomol. Res. 2015, 105, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987, 19, 11–15. [Google Scholar]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Park, D.S.; Suh, S.J.; Oh, H.W.; Hebert, P.D.N. Recovery of the mitochondrial COI barcode region in diverse Hexapoda through tRNA-based primers. BMC Genom. 2010, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Ronquist, F.M.; Teslenko, P.; van der Mark, D.; Ayres, A.; Darling, S.; Höhna, B.; Larget, L.; Liu, M.; Suchard, A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Łagowska, B.; Golan, K.; Kot, I.; Kmieć, K.; Górska-Drabik, E.; Goliszek, K. Alien and invasive scale insect species in Poland and their threat to native plants. Bull. Insectology 2015, 68, 13–22. [Google Scholar]
- Stepaniuk, K.; Łagowska, B. Number and arrangement variation of submarginal tubercles in adult females Parthenolecanium corni group (Hemiptera, Coccidae) and its value as a taxonomic character. Pol. J. Entomol. 2006, 75, 293–301. [Google Scholar]
- Schultz, P.B. Natural enemies of oak lecanium (Homoptera: Coccidae) in eastern Virginia. Environ. Entomol. 1984, 13, 1515–1518. [Google Scholar] [CrossRef]
- Noyes, J.S. Universal Chalcidoidea Database; World Wide Web Electronic Publication, UK: 2019. Available online: http://www.nhm.ac.uk/chalcidoids (accessed on 10 March 2023).
- Meineke, E.K.; Dunn, R.R.; Frank, S.D. Early pest development and loss of biological control are associated with urban warming. Biol. Lett. 2014, 10, 20140586. [Google Scholar] [CrossRef] [PubMed]
- Robayo Camacho, E.; Chong, J.-H.; Braman, S.K.; Frank, S.D.; Schultz, P.B. Natural enemy communities and biological control of Parthenolecanium spp. (Hemiptera: Coccidae) in the Southeastern United States. J. Econ. Entomol. 2018, 111, 1558–1568. [Google Scholar] [CrossRef]
- Moglan, I. Complexes parasitaires de quelques espèces de coccides (Homoptera, Coccidae) en Roumanie. Entomol. Rom. 2007, 12, 267–275. [Google Scholar]
- Arnaoudov, V.; Olszak, R.; Kutinkova, H. Natural enemies of plum brown scale Parthenolecanium corni Bouché (Homoptera: Coccidae) in plum orchards in the region of Plovdiv. IOBC/Wprs Bull. 2006, 29, 105–109. [Google Scholar]
- Koteja, J. Notes on the Polish scale insect fauna (Homoptera, Coccoidea). IV. Polskie Pismo Entomol. 1972, 42, 565–571. [Google Scholar]
- Goliszek, K.; Łagowska, B.; Golan, K. Scale insects (Hemiptera, Sternorrhyncha, Coccoidea) on ornamental plants in the field in Poland. Acta Sci. Pol. Hortorum Cultus 2011, 10, 75–84. [Google Scholar]
- Graora, D.; Spasić, R.; Mihajlović, L. Bionomy of spruce bud scale, Physokermes piceae (Schrank) (Hemiptera: Coccidae) in the Belgrade area, Serbia. Arch. Biol. Sci. 2012, 64, 337–343. [Google Scholar] [CrossRef]
- Kosztarab, M.; Kozar, F. Introduction of Anthribus nebulosus (Coleoptera: Anthribidae) in Virginia for control of scale insects: A review. Virginia J. Sci. 1983, 34, 223–236. [Google Scholar]
- Kosztarab, M.; Kozár, F. Scale Insects of Central Europe; Series Entomologica; Akademiai Kiado: Budapest, Hungary, 1988; Volume 41, 456p. [Google Scholar]
- Dervisevic, M.; Graora, D. The life cycle and efficacy of Anthribus nebulosus Forster in reducing soft scale populations in Belgrade Fresenius Environ. Bull. 2019, 28, 1981–1985. [Google Scholar]
- Kidd, N.A.C.; Jervis, M.A. Population Dynamics. In Insects as Natural Enemies; Jervis, M.A., Ed.; Springer: Dordrecht, Germany, 2007. [Google Scholar] [CrossRef]
- Masner, L. Revisionary notes and keys to world genera of Scelionidae (Hymenoptera: Proctotrupoidea). Mem. Ent. Soc. Canada 1976, 108, 1–87. [Google Scholar] [CrossRef]
- Greathead, D.J. Opportunities for biological control of insect pests in tropical Africa. Rev. Zool. Afr. 1986, 100, 85–96. [Google Scholar]
- Falagiarda, M.; Carnio, V.; Chiesa, S.G.; Pignalosa, A.; Anfora, G.; Angeli, G.; Ioriatti, C.; Mazzoni, V.; Schmidt, S.; Zapponi, L. Factors influencing short-term parasitoid establishment and efficacy for the biological control of Halyomorpha halys with the samurai wasp Trissolcus japonicus. Pest Manag. Sci. 2023. [Google Scholar] [CrossRef]
- Seok-Min, L.; Bu-Keun, C.; Dong-Wan, K.; Kyung-Mi, P.; In-Young, H.; Jin-Hyeuk, K.; Heung-Su, L. Seasonal development and control of Parthenolecanium corni in blueberry Shrubs. Korean J. App. Entomol. 2021, 60, 403–415. [Google Scholar]
- Bacci, L.; Lupi, D.; Savoldelli, S.; Rossaro, B. A review of Spinosyns, a derivative of biological acting substances as a class of insecticides with a broad range of action against many insect pests. J. Entamol. Acarol. Res. 2016, 48, 40–52. [Google Scholar] [CrossRef]
- Duso, C.; Pozzebon, A.; Lorenzon, M.; Fornasiero, D.; Tirello, P.; Simoni, S.; Bagnoli, B. The Impact of Microbial and Botanical Insecticides on Grape Berry Moths and Their Effects on Secondary Pests and Beneficials. Agronomy 2022, 12, 217. [Google Scholar] [CrossRef]
- Williams, T.; Valle, J.; Viñuela, E. Is the naturally derived insecticide Spinosad® compatible with insect natural enemies? Biocontrol Sci. Technol. 2003, 13, 459–475. [Google Scholar] [CrossRef]
- Santana, V.; Santos, V.; Barbosa Pereira, B. Properties, toxicity and current applications of the biolarvicide spinosad. J. Toxicol. Environ. Health Part B 2020, 23, 13–26. [Google Scholar] [CrossRef]
- Biondi, A.; Mommaerts, V.; Smagghe, G.; Viñuela, E.; Zappalà, L.; Desneux, N. The non-target impact of spinosyns on beneficial arthropods. Pest Manag. Sci. 2012, 68, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Suma, P.; Zappalà, L.; Mazzeo, G.; Siscaro, G. Lethal and sub-lethal effects of insecticides on natural enemies of citrus scale pests. BioControl 2009, 54, 651–661. [Google Scholar] [CrossRef]
- Somasundaran, P.; Mehta, S.C.; Purohit, P. Silicone emulsions. Adv. Colloid Interface Sci. 2006, 128-130, 103–109. [Google Scholar] [CrossRef]
- Hrastar, R.; Petrisic, M.G.; Ogrinc, N.; Kosir, I.J. Fatty acid and stable carbon isotope characterization of Camelina sativa oil: Implications for authentication. J. Agric. Food Chem. 2009, 57, 579–585. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural products that had been used commercially as crop protection agents. Pest Manag. Sci, 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Skalský, M.; Niedobová, J.; Popelka, J. The efficacy of European fruit lecanium, Parthenolecanium corni (Bouché, 1844) control using natural products. Hortic. Sci. 2019, 46, 195–200. [Google Scholar] [CrossRef]
- Gantner, M.; Jaśkiewicz, B.; Golan, K. Occurrence of Parthenolecanium corni (Bouché) on 18 cultivars of hazelnut. Folia Hortic. 2004, 16, 95–100. [Google Scholar]
- Vončina, A.; Novljan, M. Effect of selected insecticides on European fruit lecanium population (Parthenolecanium corni Buche) in a northern highbush blueberry (Vaccinium corymbosum L.) orchard. In Proceedings of the 14. Slovensko Posvetovanje o Varstvu Rastlin z Mednarodno Udelezbo, Maribor, Slovenija, 5–6 March 2019; pp. 247–251. [Google Scholar]
- Taylor-Wells, J.; Gross, A.D.; Jiang, S.; Demares, F.; Clements, J.S.; Carlier, P.R.; Bloomquist, J.R. Toxicity, mode of action, and synergist potential of flonicamid against mosquitoes Pestic. Biochem. Physiol. 2018, 151, 3–9. [Google Scholar]
- Morita, M.; Ueda, T.; Yoneda, T.; Koyanagi, T.; Haga, T. Flonicamid, a novel insecticide with a rapid inhibitory effect on aphid feeding. Pest Manag. Sci. 2007, 63, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, P.; Shera, P.S. Lethal and sublethal effects of insecticides used in cotton crop on the mealybug endoparasitoid Aenasius arizonensis. Int. J. Pest Manag. 2020, 66, 13–22. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-effects of pesticides on non-target insects in agriculture: A mini-review. Sci. Nat. 2022, 109, 17. [Google Scholar] [CrossRef] [PubMed]
- Orr, D.B. Scelionid wasps as biological control agents: A review. Fla. Entomol. 1988, 71, 506–528. Available online: https://www.jstor.org/stable/3495011 (accessed on 10 March 2023). [CrossRef]
- Sial, A. Identification and Management of Scale Insects in Blueberries. 2022. Available online: https://site.extension.uga.edu/ipm/2022/08/05/identification-management-of-scale-insects-in-blueberries/ (accessed on 10 March 2023).
- Bahde, B.W.; Poojari, S.; Alabi, O.J.; Naidu, R.A.; Walsh, D.B. Pseudococcus maritimus (Hemiptera: Pseudococcidae) and Parthenolecanium corni (Hemiptera: Coccidae) are capable of transmitting Grapevine leafroll-associated virus between Vitis × labruscana and Vitis vinifera. Environ. Entomol. 2013, 42, 1292–1298. [Google Scholar] [CrossRef]
- Hommay, G.; Komar, V.; Lemaire, O.; Herrbach, E. Grapevine virus A transmission by larvae of Parthenolecanium corni. Eur. J. Plant Pathol. 2008, 121, 185–188. [Google Scholar] [CrossRef]
- Szyndel, M.S.; Paduch-Cichal, E. Detection of Blueberry red ringspot virus in different stages of Parthenolecanium corni in Poland. Ann. Wars. Univ. LifeSci.–SGGW Hortic. Landsc. Archit. 2020, 41, 77–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).