Abstract
When used for ornamental purposes, the herbaceous peony is usually propagated by root ramets, but the replanting of divided seedlings in the original planting area results in poor growth and development. No research has reported on a compound microbial agent used for herbaceous peony. The purpose of this experiment is to provide a reference for low–cost soil improvement in production, promote the growth and development of herbaceous peony, and solve the problem of planting back obstacles. In this study, 3–year–old seedlings of herbaceous peony ‘Zifengyu’ were selected and planted into an ordinary garden and replanted soils. Four root irrigation treatments were conducted using the compound microbial agent ‘Junzhongjun’ to determine the physicochemical properties of rhizosphere soil, root physiology, and growth and development of ‘Zifengyu’ under different soil conditions. The growth and development of the aboveground parts of herbaceous peony were promoted by the treatment of the compound microbial agent in the following order: garden soil > sieved–root soil > unsieved–root soil. Root vigour was enhanced in the following sequence: sieved–root soil > unsieved–root soil > garden soil. The organic matter and available potassium in the rhizosphere soil of herbaceous peony increased, and the promotional effect in the sieved–root soil was significantly better than that in the other two soils. The results show that the compound microbial agent is low in cost and has a stimulating effect on the growth and development of herbaceous peony. In the process of production, the residual broken roots in the soil can be sieved and combined with the application of the compound microbial agent to further alleviate the barriers of replanting. The concentration and frequency of agent application should be further optimized at a later stage.
1. Introduction
To maintain the good characteristics of a variety of herbaceous peony, production is performed by propagating with root ramets. However, the divided seedlings which are then planted back in situ will face changes in the soil microbial community, acid–base imbalance, the self–toxic substances produced by the secretion of the plant–root system, and other factors, and thus the growth becomes weaker each year, resulting in the occurrence of back to planting obstacles [1,2].
The study found that the overuse of chemical fertilisers in plant production can pollute the environment [3]. In recent years, there are many researches on the application of some organic fertilisers and soil improvers to promote plant growth and improve soil fertility [4,5].
The plant rhizosphere is a unique microbial environment formed by interactions between the root system and soil microorganisms. Active microbial species in the plant rhizosphere are closely associated with plant growth and development. These microorganisms play essential roles, such as regulating the decomposition of soil organic matter, promoting the cycling of nutrients such as C, N, and P, improving soil fertility, and promoting plant growth and development [6]. Compound microbial agents comprise one or more beneficial microorganisms and microbial carriers and contain a certain number of viable bacteria [7]. These agents can be used as indirect fertilisers to rapidly replenish beneficial bacteria in the soil, inhibit harmful bacteria, adjust soil pH, improve soil fertility, enhance plant resistance to diseases and insect pests, and promote plant growth [8]. In recent years, it has been found that a compound microbial agent contains a large number of beneficial microorganisms, which can accelerate the decomposition of soil organic matter, promote the transformation of fixed nutrients in the soil to effective nutrients, and improve enzyme activity. Some specific microorganisms can decompose toxic substances produced by the plant’s secondary metabolism and inhibit the production of harmful microorganisms, which in turn promotes the growth of cucumbers and tomatoes [9,10,11]. Li et al. found that the microorganisms contained in the compound microbial agent could effectively decrease the allelochemicals generated by continuous cropping of Rehmannia Glutinosa Libosch. [12]. After the application of microbial agents (the main components include Bacillus subtilis and Paenibacillus polymyxa), huai–chrysanthemum can prevent disease, increase production, improve quality, and increase fertiliser utilization rate [13].
At present, the researches on herbaceous peony mainly focus on cultivation techniques, regulation of the flowering period, production of fresh–cut flowers, adverse environmental stress, etc. The application of compound microbial agents to improve soil fertility and promote the growth and development of herbaceous peony has not been reported. In this study, we conducted root irrigation treatment of herbaceous peony planted under different soil conditions with a composite microbial fungicide, using the beneficial microbial flora and organic matter it contained to supplement the types and quantity of beneficial microorganisms in the rhizosphere soil of herbaceous peony. Subsequently, a comprehensive analysis of the changes in the growth and physiology of herbaceous peony, the rhizosphere soil microorganisms, and other indicators was performed to study the effect of the composite microbial fungicide on herbaceous peony growth and rhizosphere soil. The objective of this study was to provide a reference for applying the composite microbial fungicide in herbaceous peony production to improve soil fertility, promote plant growth and development, and address the problems of back planting.
2. Materials and Methods
2.1. Experimental Materials
In October 2020, healthy and pest–free herbaceous peony of the cultivar ‘Zifengyu’ (3 years old) were selected and planted in ordinary garden soil (Y) where no herbaceous peony had been planted previously and in back–planted soil wherein herbaceous peony had been planted previously (the soil with residual broken roots sieved was labelled S and the unsieved soil was labelled B). The experiment was conducted from April 2021 to September 2022 at the Herbaceous Peony Resource and Horticultural Experiment Center, Horticultural Experiment Station, Shandong Agricultural University. The site had favourable climatic conditions with an average annual temperature of approximately 13 °C and rainfall of approximately 697 mm.
The compound microbial agent ‘Junzhongjun’ (Weifang Kangendi Biotechnology Co., Ltd., Weifang, Shandong, China), a kind of microbial agent, which contains Bacillus subtilis, Lactobacillus, Saccharomyces, Azotobacteraceae, Bacillus miltiorrhizae, Bacillus gummi, and a variety of organic matter, was selected and used for herbaceous peony root irrigation at a concentration of 1 billion cfu/L.
2.2. Experimental Treatment and Sampling
The following treatment (W) and control groups (CK) were used: garden soil control group (Y–CK), garden soil treatment group (Y–W), sieved–root soil control group (S–CK), sieved–root soil treatment group (S–W), unsieved–root soil control group (B–CK), and unsieved–root soil treatment group (B–W).
Three replicate plots were established for each treatment: 20 herbaceous peony plants were planted in each plot, the plant spacing was 80 cm × 80 cm, and resin FRP (fiber reinforced polymer) isolation plates with a depth of 60 cm were buried between adjacent plots [14]. The compound microbial agent was diluted at a ratio of 1:600; 300 mL of the diluted agent was poured per plant in the treatment group each time and 300 mL of water was poured simultaneously in the control group, and four treatments were performed on 25th May (in the flowering period), 22nd July (in the fruiting period), and 27th October (in the dry leaf period) 2021 and 15th March (in the germination period) 2022 (Figure 1) [15]. Routine management and maintenance were performed. At the end of the high growth period (in the active microbial phase, 24 April 2022), three herbaceous peony plants were randomly selected from each plot, dug up, and brought back to the laboratory, and the close–knit soil around the roots of the herbaceous peony within 1–2 mm was collected as rhizosphere soil.
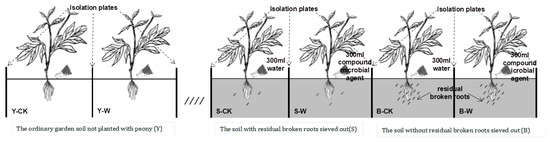
Figure 1.
Experimental material treatment. Notes: Y–CK, garden soil control group; Y–W, garden soil treatment group; S–CK, sieved–root soil control group; S–W, sieved–root soil treatment group; B–CK, unsieved–root soil control group; B–W, unsieved–root soil treatment group.
2.3. Test Methods
2.3.1. Determination of Plant Morphological Indicators
Three herbaceous peony plants were randomly selected for sampling in each plot, and plant height, stem thickness, leaf length, leaf width, leaf area, fresh weight, and dry weight were measured directly. The flowering rate and root–to–crown ratio were calculated as follows:
Flowering rate = number of flowers/number of stems (lateral buds were not counted);
Root–to–crown ratio = fresh weight of belowground parts/fresh weight of aboveground parts.
2.3.2. Determination of Root Physiological Indicators
Superoxide dismutase (SOD) activity was determined via photochemical reduction using the nitrogen blue tetrazolium method, peroxidase (POD) activity was determined using the Guaiacol method, and catalase (CAT) activity and malondialdehyde (MDA) concentration were determined using the method described by Cang and Zhao [16]. Soluble sugar concentration was determined using the anthrone colorimetric method, soluble protein concentration was determined using the Komas Brilliant Blue method, proline concentration was determined using the sulfosalicylic acid method, and root vigour was determined using the triphenyltetrazolium chloride method [17]. Paeoniflorin concentration was determined using the method described by Li et al. [18]. Spermidine concentration was determined using the method of Liu et al. [19]. Indoleacetic acid and abscisic acid concentrations were determined using the method described by Gong et al. [20].
2.3.3. Determination of Rhizosphere Soil Indicators
The concentrations of soil ammonium nitrogen and nitrate nitrogen were determined using an AA3 flow injection analyser [21]. Soil organic matter, fast–acting phosphorus, and fast–acting potassium concentrations were determined as described by Bao [22]. Soil enzyme activities were determined as described by Geng and Wang [23]. Reagents were prepared and utilised as described by Ma et al. [24]. The soil microbial population was determined as described by Cheng and Xue [25].
2.3.4. Statistical Analysis
The experimental data were processed and plotted using Microsoft Excel (Microsoft, Redmond, WA, USA), and the data were tested for significance and errors using SPSS Statistics version 23.0 (IBM Corporation, Armonk, NY, USA).
The growth rate was calculated as follows: Growth rate (%) = (W (treatment group)—CK (control group))/CK (control group).
3. Results and Analysis
3.1. Changes in Morphological Indices of Herbaceous Peony under Different Soil Conditions after Treatment with Compound Microbial Agent
The stem thickness and flowering rate of herbaceous peony increased following treatment with the compound microbial agent. The growth rates of stem thickness were: sieved–root soil (21.89%) > garden soil (20.83%) > unsieved–root soil (13.42%), and the flowering rates were: garden soil (36.07%) > sieved–root soil (9.74%) > unsieved–root soil (9.56%) (Figure 2, Table 1). The plant height of herbaceous peony planted in both garden and sieved–root soils increased in both garden (14.78%) and sieved–root soils (6.11%), while that of herbaceous peony planted in unsieved–root soil decreased by 7.33%. The leaf width of herbaceous peony planted in sieved–root soil increased by 2.90%, whereas that of herbaceous peony planted in garden and unsieved–root soils decreased in the unsieved–root (−0.98%) and garden soils (−4.60%). Leaf length and leaf area decreased with growth rate in the following order: leaf length: unsieved–root soil (−3.69%) > sieved–root soil (−8.74%) > garden soil (−10.79%); leaf area: sieved–root soil (−4.95%) > unsieved–root soil (−7.48%) > garden soil (−22.27%).

Figure 2.
The flowering period of herbaceous peony ‘Zifengyu’ under different soil conditions after treatment with the compound microbial agent. CK, control group; W, treatment group; Y, the ordinary garden soil not planted with herbaceous peony previously; S, the soil with sieved residual broken roots; B, the soil with unsieved residual broken roots.

Table 1.
Changes in the aboveground growth index of herbaceous peony after treatment with the compound microbial agent under different soil conditions.
The fresh weight of herbaceous peony planted in both garden and sieved–root soils increased after treatment with the compound microbial agent. The promotion effect of the agent on herbaceous peony in sieved–root soil was significantly higher than that in garden soil, and the fresh weight of herbaceous peony planted in unsieved–root soil decreased. The aboveground, belowground, and total dry weights of peony planted in sieved–root soil increased by 83.70%, 34.72%, and 51.20%, respectively, compared with the CK, whereas those in both garden and unsieved–root soils decreased, with the most significant decrease being observed in herbaceous peony planted in unsieved–root soil (Appendix A, Table A1).
3.2. Changes in the Herbaceous Peony Root System after Treatment with the Compound Microbial Agent under Different Soil Conditions
3.2.1. Changes in Antioxidant Enzyme Activities and MDA in the Herbaceous Peony Root System
Herbaceous peony planted in garden soil and unseived–root soil treated with a compound microbial agent showed enhanced SOD activity in the root system (garden soil (20.32%) > unsieved–root soil (6.92%)); in contrast, it was reduced by 36.40% in sieved–root soil (Figure 3(3–1)). POD activity decreased by 14.81% in the roots of herbaceous peony planted in unsieved–root soil, showed no significant change in sieved–root soil, and increased by 200% in garden soil after treatment with the compound microbial agent (Figure 3(3–2)). As shown in Figure 3(3–3), CAT activity increased in the roots of herbaceous peony planted in unsieved–root and sieved–root soil (76.56% and 4626.67%, respectively) and decreased in garden soil by 2.45%. As shown in Figure 3(3–4), the MDA concentration of the roots of herbaceous peony planted in unsieved–root and garden soils increased after treatment with the compound microbial agent and was higher in the roots of plants planted in unsieved–root soil (466.26%) than those of plants planted in garden soil (31.87%), whereas it decreased by 86.85% in sieved–root soil.
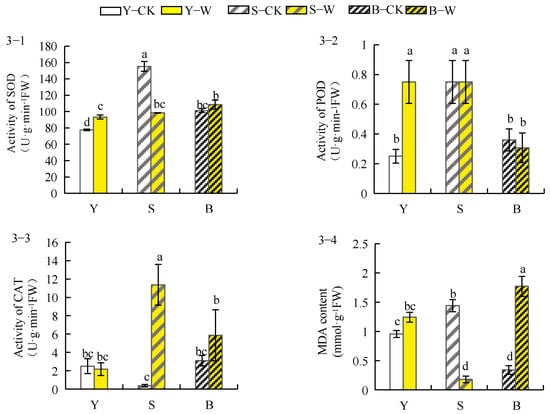
Figure 3.
Effect of compound microbial agent treatment on antioxidant enzyme activity and MDA of the herbaceous peony root system under different soil conditions. Notes: 3–1, activity of SOD; 3–2, activity of POD; 3–3, activity of CAT; 3–4, MDA content; Y–CK, garden soil control group; Y–W, garden soil treatment group; S–CK, sieved–root soil control group; S–W, sieved–root soil treatment group; B–CK, unsieved–root soil control group; B–W, unsieved–root soil treatment group. The same lowercase letter mark in the graph indicates that the difference did not reach the significant level (p > 0.05); different lowercase letters indicate significant differences (p < 0.05).
3.2.2. Changes in Osmoregulatory Substances and Root Vigour in the Herbaceous Peony Root System
The soluble sugar concentration in herbaceous peony roots treated with the compound microbial agent under different soil conditions decreased in all groups (Figure 4(4–1)) in the following order: garden soil (−4.11%) > unsieved–root soil (−11.42%) > sieved–root soil (−26.45%). The soluble protein concentration also decreased to varying degrees (Figure 4(4–2)) in the following order: sieved–root soil (−0.63%) > garden soil (−31.22%) > unsieved–root soil (−35.46%). As shown in Figure 4(4–3), the proline concentration in the roots of herbaceous peony planted in both unsieved–root and garden soils increased to different extents, with garden soil (63.11%) > unsieved–root soil (22%), whereas that in roots of plants planted in the sieved–root soil decreased by 37.1%. As shown in Figure 4(4–4), the root vigour of herbaceous peony was significantly enhanced in different soil conditions in the following order: sieved–root soil (176.18%) > unsieved–root soil (118.92%) > garden soil (56.44%).
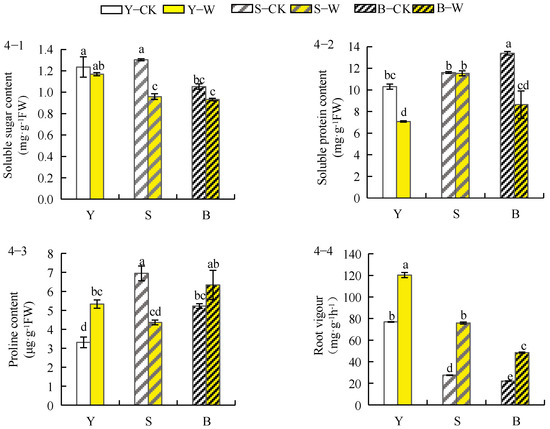
Figure 4.
Effect of compound microbial agent treatment on osmoregulatory substances and root vigor of herbaceous peony root system under different soil conditions. Notes: 4–1, soluble sugar content; 4–2, soluble protein content; 4–3, proline content; 4–4, root vigour; Y–CK, garden soil control group; Y–W, garden soil treatment group; S–CK, sieved–root soil control group; S–W, sieved–root soil treatment group; B–CK, unsieved–root soil control group; B–W, unsieved–root soil treatment group. The same lowercase letter mark in the graph indicates that the difference did not reach the significant level (p > 0.05); different lowercase letters indicate significant differences (p < 0.05).
3.2.3. Changes in Secondary Metabolites in the Herbaceous Peony Root System
The concentration of paeoniflorin in the roots of herbaceous peony planted in garden and unsieved–root soils increased by 25.94% and 38.74%, respectively, after treatment with the compound microbial agent, whereas that in roots of plants planted in the sieved–root soil decreased by 38.42% (Figure 5(5–1)). The effect of compound microbial agent treatment on the concentration of spermidine in herbaceous peony roots under different soil conditions varied considerably (Figure 5(5–2)). The root spermidine concentration of herbaceous peony planted in garden soil decreased by 32.92% after treatment with the compound microbial agent. In contrast, the root spermidine concentration of herbaceous peony in unsieved–root soil increased significantly by 40.27%, without significant change in the root spermidine concentration of herbaceous peony in sieved–root soil after treatment. As shown in Figure 5(5–3), the IAA concentration in herbaceous peony roots planted in garden and unsieved–root soils increased by 39.50% and 23.18%, respectively, after treatment with the compound microbial agent, whereas that in roots of plants planted in sieved–root soil decreased by 40.25%. The ABA concentration of herbaceous peony roots planted in both garden and unsieved–root soils decreased by 31.62% and 3.28%, respectively, whereas that in roots of plants planted in sieved–root soil increased by 4.86% (Figure 5(5–4)).
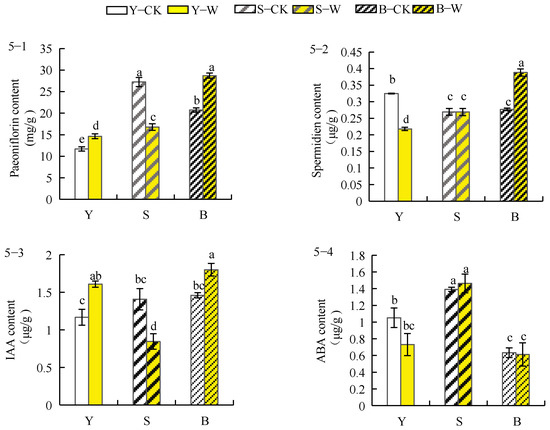
Figure 5.
Effect of compound microbial agent treatment on secondary metabolites within the root system of herbaceous peony under different soil conditions. Notes: 5–1, paeoniflorin content; 5–2, spermidine content; 5–3, IAA content; 5–4, ABA content; Y–CK, garden soil control group; Y–W, garden soil treatment group; S–CK, sieved–root soil control group; S–W, sieved–root soil treatment group; B–CK, unsieved–root soil control group; B–W, unsieved–root soil treatment group. The same lowercase letter mark in the graph indicates that the difference did not reach the significant level (p > 0.05); different lowercase letters indicate significant differences (p < 0.05).
3.3. Changes in Rhizosphere Soil of Herbaceous Peony under Different Soil Conditions after Treatment with the Compound Microbial Agent
3.3.1. Changes in Soil Organic Matter and Soil Nutrients between Herbaceous peony Roots
Organic matter content in the rhizosphere soil of herbaceous peony increased under different soil conditions (Figure 6(6–1)) in the following order: sieved–root soil (89.92%) > unsieved–root soil (40.12%) > garden soil (13.29%). As shown in Figure 6(6–2), the ammonium nitrogen concentration of herbaceous peony rhizosphere soil in unsieved–root and garden soils decreased by 9.77% and 36.29%, respectively. The ammonium nitrogen concentration of herbaceous peony rhizosphere soil in the sieved–root soil increased by 8.5%. Nitrate–nitrogen concentration in the herbaceous peony rhizosphere soil decreased in unsieved–root and garden soils by 17.41% and 46.34%, respectively, whereas that in the herbaceous peony rhizosphere soil in sieved–root soil increased by 8.7% (Figure 6(6–3)). The concentration of available phosphorus in the herbaceous peony rhizosphere soil in unsieved–root and garden soils decreased by 7.75% and 13.61%, respectively. The concentration of available phosphorus in the sieved–root soil increased by 4.16% (Figure 6(6–4)). As shown in Figure 6(6–5), the concentration of available potassium in herbaceous peony rhizosphere soil increased in the three soil conditions after treatment with the compound microbial agent by 29%, 22.54%, and 11.6% in the sieved–root, unsieved–root, and garden soils, respectively.
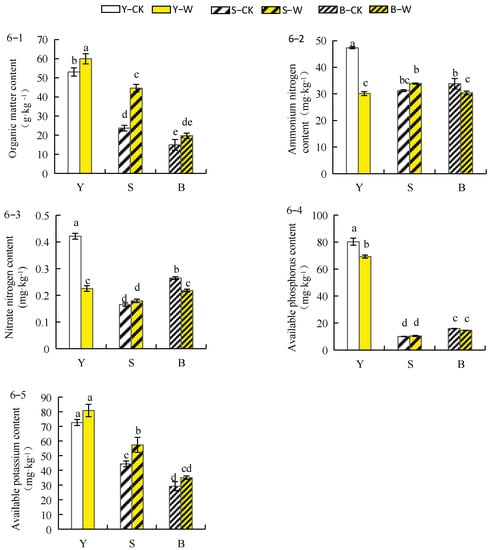
Figure 6.
Effect of compound microbial agent treatment on organic matter and soil nutrients of herbaceous peony rhizosphere soil under different soil conditions. Notes: 6–1, organic matter content; 6–2, ammonium nitrogen content; 6–3, nitrate nitrogen content; 6–4, available phosphorus content; 6–5, available potassium content; Y–CK, garden soil control group; Y–W, garden soil treatment group; S–CK, sieved–root soil control group; S–W, sieved–root soil treatment group; B–CK, unsieved–root soil control group; B–W, unsieved–root soil treatment group. The same lowercase letter mark in the graph indicates that the difference did not reach the significant level (p > 0.05); different lowercase letters indicate significant differences (p < 0.05).
3.3.2. Changes in Soil Enzyme Activity between Herbaceous Peony Roots
As shown in Figure 7(7–1), sucrase activity in the rhizosphere soil of herbaceous peony planted in sieved–root and garden soils treated with the compound microbial agent decreased by 6.76% and 22.17%, respectively, and sucrase activity in the rhizosphere soil of unsieved–root soil increased by 32%. The phosphatase activity of the rhizosphere soil increased by 20.42% and 13.46% in herbaceous peony planted in garden and unsieved–root soils treated with the compound microbial agent, respectively, whereas the phosphatase activity in the rhizosphere soil of sieved–root soil decreased by 4.09% (Figure 7(7–2)). Figure 7(7–3) shows the different effects of treatment with the compound microbial agent on dehydrogenase activity in the herbaceous peony rhizosphere soil under different soil conditions. The dehydrogenase activity in the rhizosphere soil of herbaceous peony planted in sieved–root soil was enhanced by 115.5% after treatment with the compound microbial agent, and the dehydrogenase activity in the rhizosphere soil of garden and unsieved–root soils was reduced by 31.12% and 8.33%, respectively. The urease activity in the rhizosphere soil of herbaceous peony planted in unsieved–root soil was significantly improved by 6.05% compared with that in CK after treatment with the compound microbial agent, whereas the urease activity in the rhizosphere soil of garden and sieved–root soils was reduced by 6.56% and 2.01%, respectively (Figure 7(7–4)). The hydrogen peroxidase activity in the rhizosphere soil of herbaceous peony was reduced to different degrees after treatment with the compound microbial agent (Figure 7(7–5)) in the following order: garden soil (−3.66%) > unsieved–root soil (−4.61%) > sieved–root soil (−5.76%).
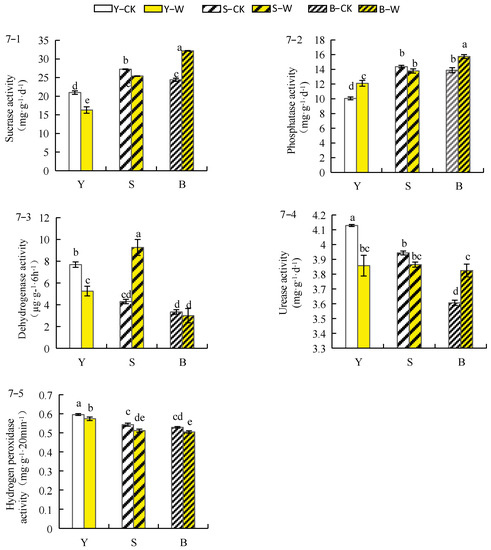
Figure 7.
Effect of compound microbial agent treatment on the enzyme activity of herbaceous peony rhizosphere soil under different soil conditions. Notes: 7–1, sucrase activity; 7–2, phosphatase activity; 7–3, dehydrogenase activity; 7–4, urease activity; 7–5, hydrogen peroxidase activity; Y–CK, garden soil control group; Y–W, garden soil treatment group; S–CK, sieved–root soil control group; S–W, sieved–root soil treatment group; B–CK, unsieved–root soil control group; B–W, unsieved–root soil treatment group. The same lowercase letter mark in the graph indicates that the difference did not reach the significant level (p > 0.05); different lowercase letters indicate significant differences (p < 0.05).
3.3.3. Changes in Soil Microbial Abundance between Herbaceous Peony Roots
As shown in Figure 8(8–1), the number of bacteria in the rhizosphere soil of herbaceous peony planted in both garden and unsieved–root soils treated with compound microbial agent decreased by 87.05% and 67.82%, respectively, while the number of bacteria in the rhizosphere soil of sieved–root soil increased by 5.56%. The number of fungi in the rhizosphere soil of herbaceous peony planted in garden and unsieved–root soils treated with the compound microbial agent decreased compared with CK by 4.83% and 2.96%, respectively, while the number of fungi in the rhizosphere soil of sieved–root soil increased by 10.24% (Figure 8(8–2)). As shown in Figure 8(8–3), after treatment with the compound microbial agent, the number of actinomycetes in the rhizosphere soil of herbaceous peony planted under different soil conditions decreased in all groups in the following order: garden soil (−6.76%) > sieved–root soil (−26.97%) > unsieved–root soil (−76.47%).
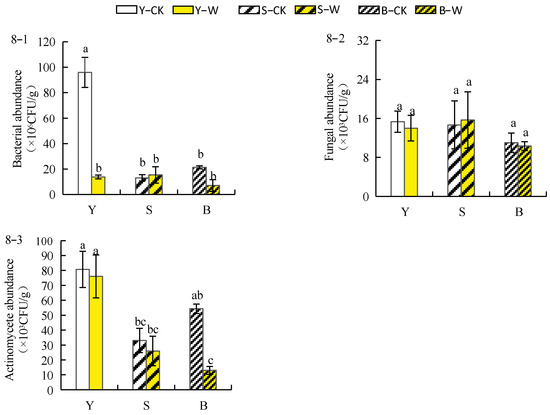
Figure 8.
Effect of compound microbial agent treatments on the abundance of soil microorganisms between the roots of herbaceous peony under different soil conditions. Notes: 8–1, bacterial abundance; 8–2, fungal abundance; 8–3, actinomycete abundance; Y–CK, garden soil control group; Y–W, garden soil treatment group; S–CK, sieved–root soil control group; S–W, sieved–root soil treatment group; B–CK, unsieved–root soil control group; B–W, unsieved–root soil treatment group. The same lowercase letter mark in the graph indicates that the difference did not reach the significant level (p > 0.05); different lowercase letters indicate significant differences (p < 0.05).
4. Discussion
4.1. Effect of Compound Microbial Agent Treatment on the Growth and Development of Herbaceous Peony under Different Soil Conditions
Studies have shown that beneficial microorganisms in compound microbial agents promote plant growth by secreting plant growth hormones, dissolving phosphorus, fixing nitrogen, and promoting plant nutrient functions. The application of a compound microbial agent significantly promotes the elongation of roots, stems, and leaves of roasted tobacco, which increases the dry weight and the root–to–crown ratio of the roots, stems, and leaves [26]. In cucumbers, microbial gent application reportedly increased net photosynthetic intensity, female flowering node, and seating rates and promoted growth and development [27]. In the present study, we found that a compound microbial agent application increased plant height, stem thickness, flowering rate, plant water content, and root–to–crown ratio. Additionally, consistent with the results reported by Wang et al. [27], the growth of herbaceous peony planted in garden soil increased after treatment with a compound microbial fungicide, and plant height, stem thickness, flowering rate, plant water content, and dry matter content of herbaceous peony planted in sieved–root soil increased. However, the root–to–crown ratio decreased, indicating that the compound microbial agent promoted the growth of the root system in sieved–root soil and significantly increased the growth of the aboveground parts of herbaceous peony. The overall growth of herbaceous peony planted in unsieved–root soil treated with the compound microbial agent was promoted to a lesser extent. In contrast, the root–to–crown ratio increased, presumably because the many chemosensitive substances secreted by the broken roots of herbaceous peony in the soil seriously impacted plant growth. The exogenous application of the compound microbial agent slightly improved and promoted the aboveground parts, owing to the treatment time and agent concentration.
4.2. Effect of Compound Microbial Agent Treatment on the Herbaceous Peony Root System under Different Soil Conditions
SOD, POD, and CAT are essential enzymes for plant disease resistance and constitute the antioxidant system, which can scavenge excessive free radicals, protect proteins and the cell membrane from reactive oxygen species, and help maintain the stability of the plant cell membrane structure [28]. When plants are exposed to adverse stress, their ability to scavenge excess free radicals decreases, the balance of free radical dynamics is disrupted, and the degree of cell membrane lipid peroxidation and subsequently MDA concentration increase [29]. Studies have shown that compound microbial agents can significantly increase SOD, POD, and CAT activities in cotton leaves and roots, promote the decomposition of peroxides, ensure normal redox potential in vivo, reduce oxidative membrane damage, lower MDA concentration, and improve plant stress resistance [30]. However, in the present study, SOD and POD activities in herbaceous peony roots planted in garden soil were found to be significantly increased. Furthermore, MDA concentration slightly increased after treatment with the compound microbial agent. The CAT activity in the sieved–root soil significantly increased and the MDA concentration decreased. The antioxidant enzyme activities in the unsieved–root soil changed slightly, the SOD and CAT activities increased slightly, and the MDA concentration increased significantly. It is hypothesised that the positive enzymatic activity in the herbaceous peony root system under different soil conditions is low, owing to the influence of the treatment exposition and concentration of the agents and soil conditions, among other factors. The dynamic balance of free radicals in herbaceous peony is disrupted by many chemosensitive substances in the unsieved–root soil, and the MDA concentration substantially increased, and alleviating the degree of membrane lipid peroxidation in the cells via the application of compound microbial bacterial agents is difficult [31].
Soluble sugars and proteins, as well as proline, are essential osmoregulatory substances in plants. Under environmental stress, plants maintain cellular osmotic pressure and reduce oxidative damage by accumulating osmoregulatory substances [32]. The root system is a critical absorption and metabolic organ of plants, and its growth and development directly affect the growth of aboveground stems and leaves as well as crop yield. Root vigour is an important index of the functional absorption of the root system [33]. The concentrations of soluble sugars and proteins as well as that of proline in ginseng leaves and leaves of white spurge seedlings increased after compound microbial agent application [34,35]. In the present study, after applying the compound microbial agent, the proline concentration in herbaceous peony roots planted in garden and unsieved–root soils increased, while that in sieved–root soil decreased. The soluble sugar and soluble protein concentrations in herbaceous peony roots under different soil conditions decreased to varying degrees, in contrast to the results of previous studies, suggesting that the osmoregulatory substances in the herbaceous peony root system were not promoted due to the short exposure to the compound microbial agent or the excessive accumulation of organic matter in the soil after application, which affects the osmotic balance. Li et al. [36] found that applying three different microbial agents to cucumbers in facility cultivation promoted plant growth and enhanced the root vigour of cucumbers to various degrees. In the present study, the root vigour of herbaceous peony was improved after treatment with the compound microbial agent under different soil conditions. The best effect was observed in sieved–root soils, consistent with the results of previous studies.
Secondary metabolites are produced during plant environmental adaptation and are important medicinal compounds. Under environmental stress, plants inhibit the growth of other plants by releasing secondary metabolites into their external environment to improve their competitiveness [37]. Paeoniflorin is the main monoterpene glycoside isolated from herbaceous peony root systems. It has medicinal value and is a secondary metabolite produced during adversity. Polyamines play a dominant role in plant bud differentiation and vary depending on the plant species. Spermidine is an endogenous polyamine that plays a dominant role in regulating herbaceous peony bulb development.
Plant hormones regulate plant growth, development, and environmental adaptation. Indoleacetic acid promotes the formation of lateral and adventitious roots in plants and maintains apical dominance. Gibberellin has regulatory effects on plant seed germination, stem elongation, leaf spreading, photosynthesis, and plant flowering and significantly promotes plant seed germination and seedling growth under adverse conditions [38]. In the present study, we found that the concentration of paeoniflorin and indoleacetic acid secreted in herbaceous peony roots planted in garden soil increased and the concentration of spermidine and abscisic acid decreased after treatment with the compound microbial agent. The concentrations of paeoniflorin, spermidine, and indoleacetic acid increased and that of abscisic acid decreased in the unsieved–root soil. In contrast, in the sieved–root soil, the concentrations of paeoniflorin and indoleacetic acid decreased and that of abscisic acid increased. No increase in secondary metabolites in the herbaceous peony root system was observed after the compound microbial agent was applied.
4.3. Effect of Compound Microbial Agent Treatment on Rhizosphere Soil under Different Soil Conditions
Soil organic matter is an important indicator of soil fertility and an essential soil component. Maintaining or increasing soil organic matter concentration promotes the formation of agglomerates and maintains their stability, providing energy for the activities of soil microorganisms [39]. Nitrogen is one of the primary nutrients required for plant growth. Ammonium nitrogen and nitrate nitrogen in soil are the two forms of nitrogen that plants can directly absorb and use and are important indicators of soil nitrogen supply capacity. Fast–acting phosphorus in the soil is more easily absorbed and utilised by plants and is an essential indicator for evaluating the level of soil phosphorus supply [40]. Studies have shown that compound microbial agents contain a large amount of nutrients and organic matter, which can improve the soil nutrient and organic matter concentration when applied to the soil. Moreover, they can accelerate the reproduction and metabolism of rhizosphere soil microorganisms under conditions of sufficient nitrogen and carbon supply, increase the concentration of microbial secretions and physiologically active substances, activate insoluble and immobile nutrient elements in the soil, and improve the effectiveness of soil nutrients [41,42]. In this study, the ammonium nitrogen, nitrate nitrogen, and available phosphorus concentrations in the rhizosphere of sieved–root soil increased slightly, and the available potassium and organic matter concentrations significantly increased after treatment with the compound microbial agent, which was consistent with above findings. The organic matter and available potassium concentrations in the rhizosphere soil of garden and unsieved–root soils increased slightly, whereas the ammonium nitrogen, nitrate nitrogen, and available phosphorus concentrations decreased, in contrast to the results of previous studies. Because herbaceous peony needs to continuously absorb water and nutrients from the soil during growth, the compound microbial agent treatment may have promoted root growth, and the ability to absorb nutrients from the soil increased or the soil nutrient concentration decreased.
Soil enzymes are proteins produced by decomposing plant and animal residues, secretion of plant roots, and metabolism of soil microorganisms and are essential in many critical soil biochemical processes. Soil sucrase is associated with the conversion of carbon and is an essential hydrolytic enzyme that characterises the biological activity of soil. Urease activity reflects the ability of soil organic nitrogen to be converted into active nitrogen and the availability of inorganic nitrogen. Phosphatase participates in the soil phosphorus cycle and is related to the effective phosphorus concentration in the soil. Catalase can break down hydrogen peroxide in the soil, reducing its toxic effects on plants and characterising soil biochemical activity [43]. Application of the compound microbial agent increases soil phosphatase, sucrase, and urease activities [42,44]. Soil urease, dehydrogenase, neutral phosphatase, and sucrase activities reportedly increased after the application of compound microbial agents to cotton [30]. In the present study, phosphatase activity was significantly enhanced while other enzyme activities were reduced in the rhizosphere of garden soil after applying the compound microbial agent. Furthermore, dehydrogenase activity was increased in the sieved–root soil, and sucrase, phosphatase, and urease activities were enhanced and dehydrogenase and catalase activities were reduced in the unsieved–root soil. This finding differs from that of a previous study. Studies have shown that after treating roasted tobacco with different concentrations of compound microbial agents, enzyme activity tends to increase and then decrease with increasing concentration [26]. It is possible that the activities of the various enzymes investigated in this experiment were affected differently by the type of the compound microbial agent, soil conditions, and application concentration.
Soil microorganisms play an irreplaceable role in the ecological environment, and the higher the microbial concentration of the soil, the more functional the microbial community and the more adequate the soil fertility [45]. When applied to soil, studies have shown that compound microbial agents create beneficial microflora around crop roots, improve soil microbial activity, and increase the soil microbial population [46,47]. After facility–cultivated tomatoes and Pinyi sweet tea seedlings were treated with different compound microbial agents, the number of beneficial bacteria and actinomycetes in the rhizosphere soil increased significantly, the number of harmful fungi decreased, and the soil microbial population structure was optimised [48]. In the present study, the number of bacteria, fungi, and actinomycetes in the rhizosphere soil of herbaceous peony planted in garden and unsieved–root soils decreased after application of the compound microbial agent. In the rhizosphere soil of sieved–root soil, the number of bacteria and fungi increased and the number of actinomycetes decreased, in contrast to previous findings. It is presumed that the structure of beneficial and harmful microbial communities in the rhizosphere soil of herbaceous peony differs slightly from that of other plants. Therefore, the changes in specific beneficial and detrimental microbial communities in the rhizosphere soil of herbaceous peony after the application of the compound microbial agent need to be further studied.
5. Conclusions
In this study, we found that application of a compound microbial agent altered the microbial community structure in the soil between herbaceous peony roots and promoted the growth and development of herbaceous peony. The organic matter and available potassium concentrations in the rhizosphere soil of herbaceous peony under different soil conditions increased after treatment with the compound microbial agent. Promotion of organic matter and nutrients was best in the sieved–root soil. The microbial community in the soil changed, and the number of bacteria, fungi, and actinomycetes decreased in the garden and unsieved–root soils. The number of bacteria and fungi increased, and the number of actinomycetes decreased in the sieved–root soil. The growth and development of the aboveground part of herbaceous peony were promoted by the treatment of compound microbial agent in the following order: garden soil > sieved–root soil > unsieved–root soil. Root vigour was enhanced in the sequence: sieved–root soil > unsieved–root soil > garden soil. It is concluded that in the production of herbaceous peony, on the basis of sieving the residual broken roots in the soil, combined with the application of the compound microbial agent can further alleviate the barriers of replanting. For herbaceous peony planted in soil without sieved residual broken roots, it is necessary to further increase the concentration and frequency of the compound microbial agent based on them set in this experiment. Further research on the concentration and frequency of application of the compound microbial agent and the beneficial and detrimental microbial communities in the rhizosphere soil is required to provide a reference for improving the soil, promoting the growth and development of herbaceous peony, reducing production costs, and addressing the problems of replanting.
Author Contributions
L.Y. co–designed and undertook most of the study, analysed the data, and wrote the paper. L.S. and C.Z. provided comments on the first draft of this paper. X.Y., D.Z., A.X., Y.S., F.L. and L.D. participated in tests and data analysis. X.S. co–designed the study, revised drafts of the manuscript, and provided resources. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Shandong Province Improved Seed Project (No.2021S230304–02583).
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Changes in root–to–crown ratio of herbaceous peony after treatment with the compound microbial agent under different soil conditions.
Table A1.
Changes in root–to–crown ratio of herbaceous peony after treatment with the compound microbial agent under different soil conditions.
| Soil Conditions | Fresh Weight Growth Rate (%) | Dry Weight Growth Rate (%) | Root-Shoot Ratio Growth Rate (%) | ||||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Total | Shoot | Root | Total | ||
| Y | 10.12 ± 0.04 b | 11.92 ± 0.06 b | 11.09 ± 0.05 b | −3.17 ± 0.07 b | −16.39 ± 0.04 b | −11.29 ± 0.05 b | 1.50 ± 0.02 b |
| S | 66.05 ± 0.07 a | 40.21 ± 0.05 a | 51.57 ± 0.06 a | 83.70 ± 0.22 a | 34.72 ± 0.19 a | 51.20 ± 0.20 a | −15.51 ± 0.01 c |
| B | −49.67 ± 0.01 c | −34.07 ± 0.03 c | −41.23 ± 0.01 c | −42.66 ± 0.03 c | −30.24 ± 0.01 c | −34.87 ± 0.01 c | 31.13 ± 0.03 a |
Notes: Y, the ordinary garden soil not planted with herbaceous peony previously; S, the soil with sieved residual broken roots; B, the soil with unsieved residual broken roots. The same lowercase letter mark in the graph indicates that the difference did not reach the significant level (p > 0.05); different lowercase letters indicate significant differences (p < 0.05).
References
- Liu, Z.M.; Wang, H.Y.; Li, Y.; Li, X.; Shi, Y.J.; Yang, L.J.; Yang, X.; Zheng, C.S.; Sun, X. Effects of exogenous albiflorin on the growth of herbaceous peony (Paeonia lactiflora) and rhizosphere soil. Plant Physiol. J. 2022, 58, 873–888. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Xu, J.G.; Bao, M.Y.; Liu, M.M.; Xie, A.Q.; Zhang, D.L.; Sun, X. Effect of waterlogging stress on root morphology and polyamine content of herbaceous herbaceous peony (Paeonia lactiflora). Plant Physiol. J. 2020, 56, 1445–1457. [Google Scholar] [CrossRef]
- Filipović, V.; Ugrenović, V.; Popović, V.; Dimitrijević, S.; Popović, S.; Aćimović, M.; Dragumilo, A.; Pezo, L. Productivity and flower quality of different pot marigold (Calendula officinalis L.) varieties on the compost produced from medicinal plant waste. Ind. Crop Prod. 2023, 192, 116093. [Google Scholar] [CrossRef]
- Bielski, S.; Szwejkowska, B. Effect of fertilization on the development and yields of pot marigold (Calendula officinalis L.). Herba Pol. 2013, 59, 5–12. [Google Scholar] [CrossRef]
- Dimitrijevic, S.; Pavlovic, M.; Maksimovic, S.; Ristic, M.; Filipovic, V.; Antonovic, D.; Dimitrijevic-Brankovic, S. Plant growth-promoting bacteria elevate the nutritional and functional properties of black cumin and flaxseed fixed oil. J. Sci. Food. Agr. 2018, 98, 1584–1590. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Savarese, C.; Cozzolino, V.; Verrillo, M.; Vinci, G.; De Martino, A.; Scopa, A.; Piccolo, A. Combination of humic biostimulants with a microbial inoculum improves lettuce productivity, nutrient uptake, and primary and secondary metabolism. Plant Soil 2022, 481, 285–314. [Google Scholar] [CrossRef]
- Balestrini, R.; Salvioli, A.; Dal Molin, A.; Novero, M.; Gabelli, G.; Paparelli, E.; Marroni, F.; Bonfante, P. Impact of an arbuscular mycorrhizal fungus versus a mixed microbial inoculum on the transcriptome reprogramming of grapevine roots. Mycorrhiza 2017, 27, 417–430. [Google Scholar] [CrossRef]
- Xie, D.F.; Wang, G.Q.; Xie, R.; Zhu, Z.J.; Xue, S.H. Effects of Microbial Fertilizers on Growth and Defense-related Enzymes of Continuously Cropped Cucumbers. Fujian J. Agric. Sci. 2018, 33, 696–701. [Google Scholar] [CrossRef]
- Yue, M.C.; Wang, Z.G.; Chen, Q.S.; Xu, M.X.; Li, W.M.; Wu, X.D.; Liu, Q.Y.; Chen, L.L.; Wang, D.S.; Jiao, J.G. Effects of Reduction of Chemical Fertilizer Combined with Application of Microbial Agents on Growth and Soil Fertility of Cherry Tomato. Soils 2020, 52, 68–73. [Google Scholar] [CrossRef]
- Hassine, M.; Aydi-Ben-Abdallah, R.; Jabnoun-Khireddine, H.; Daami-Remadi, M. Soil-borne and compost-borne Penicillium sp. and Gliocladium spp. as potential microbial biocontrol agents for the suppression of anthracnose-induced decay on tomato fruits. Egypt. J. Biol. Pest. Co. 2022, 32, 12. [Google Scholar] [CrossRef]
- Li, Z.G.; Wang, X.M.; Liu, T.Y.; Zhang, X.G.; Jie, X.L.; Zhao, Y.J. Restor ation of continuous Cropping Obstacles of Rehmannia Glutinosa Libosch by Applying Compound Bacterial Manure. Hunan Agric. Sci. 2008, 5, 62–65. [Google Scholar] [CrossRef]
- Wang, A.M.; Yang, S.L.; Ding, A.P.; Guo, Y.H.; Yu, X.X. Study on the fattening effect of “the microbial agent” on Huaichrysanthemum. Henan Agric. 2022, 24, 44–46. [Google Scholar] [CrossRef]
- Lv, M.W.; Xu, J.G.; Du, J.; Gao, C.R.; Lu, J.; Zhang, Q.X.; Wang, T.L.; Sun, X. Effects of exogenous gibberellin A3 and paclobutrazol on development of herbaceous peony (Paeonia lactiflora) bulbils. Plant Physiol. 2018, 54, 790–802. [Google Scholar] [CrossRef]
- Zhang, D.L.; Sun, L.M.; Xie, A.Q.; Li, X.; Li, Y.; Liu, Z.M.; Sun, X. Effects of residual broken roots on the growth and rhizosphere soil of herbaceous peony. Eur. J. Hortic. Sci. 2022, 87, 10. [Google Scholar] [CrossRef] [PubMed]
- Cang, J.; Zhao, H.J. Experimental Course of Plant PhySiology; Higher Education Press: Beijing, China, 2013; pp. 68–85. [Google Scholar]
- Yu, Q.Y. Plant Physiology Laboratory Tutorial; Beijing University of Technology Press: Beijing, China, 2014. [Google Scholar]
- Li, F.Y.; Zang, X.D.; Cao, Y. Determination of paeoniflorin of cultivated herbaceous peony root in Mudanjiang by HPLC. North. Hortic. 2017, 20, 149–153. [Google Scholar] [CrossRef]
- Liu, J.; Ji, X.J.; Liu, Y.L. A high-performance liquid chromatographic method for the determination of polyamines in plant tissues. Plant Physiol. Lett. 2002, 38, 596–598. [Google Scholar] [CrossRef]
- Gong, X.C.; Song, C.F.; Wang, M.H.; Zheng, F.C.; Miao, W.G.; Wang, J.S. Determination of growth factors intobacco and cotton by high performance liquid chromatography. Jiangsu J. Agric. Sci. 2012, 28, 225–227. [Google Scholar] [CrossRef]
- Xu, S.Z.; Liu, Y.S.; Xia, X.X.; Wang, Y.P.; Chen, X.S.; Shen, X.; Yin, C.M.; Mao, Z.Q. Dazomet fumigation plus short-term crop rotation of onion significantly alleviates crop succession disorder in apple. Acta Hortic. Sin. 2018, 45, 11–20. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Geng, Y.Q.; Wang, D.M. Research progress of soil hydrolytic enzyme activity determination methods. Chin. J. Eco-Agric 2012, 20, 387–394. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Duan, Y.N.; Shen, X.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. Effects of mixed planting of different crops with young replanted apple trees on replanting plants and soil environment. Sci. Agric. Sin. 2018, 51, 3815–3822. [Google Scholar] [CrossRef]
- Cheng, L.J.; Xue, H.Q. Experimental Techniques in Microbiology; World Book Publishing Co.: Xi’an, China, 2000. [Google Scholar]
- Zhu, J.F.; Wang, X.D.; Guo, C.B.; Li, Y.J.; Jing, X.F.; Liu, L.; Cui, Z.Z. Influence of microbial bacterial application on soil key enzyme activity and root growth of roasted tobacco. Acta Agric. Jiangxi 2015, 9, 31–35. [Google Scholar] [CrossRef]
- Wang, M.Y.; Li, G.Z.; Yang, X.F.; Zhang, H.; Xin, B. Preliminary study on the effect of microbial fertilizer on fertility, yield and quality of cucumber in protected areas. China Soils Fert. 2003, 3, 38–41. [Google Scholar] [CrossRef]
- Annamaria, R.; Antonella, C.; Barbara, B.; Gian Franco, S. Iron deficiency differently affects peroxidase isoforms in sunflower. J. Exp. Bot. 2001, 52, 25–35. [Google Scholar] [CrossRef]
- Zhang, H.N.; Lu, X.H.; Jin, Z.N.; Li, Y.; Wang, R.F.; Li, Z.X.; Liu, L.K. Effects of rare earth tailing sand drought on physiological characteristics of four plant species under high temperature conditions. Acta Ecol. Sin. 2019, 39, 2426–2434. [Google Scholar] [CrossRef]
- Song, Y.L.; Yu, J.; Chen, S.G.; Xiao, C.Z.; Li, Y.H.; Su, X.R.; Ding, F.J. Effects of compound microbial agents on physiological characteristics and inter-root soil microbial and chemical properties of cotton. Soils 2019, 51, 477–487. [Google Scholar] [CrossRef]
- Yuan, L.; Kheemu, Y.L.; Zhang, L.Q. Effects of NaCl stress on reactive oxygen metabolism and cell membrane stability in pistachio seedlings. J. Plant Ecol. 2005, 6, 119–125. [Google Scholar] [CrossRef]
- Yan, M.; Wang, Y.; Bao, J.K.; Wang, C.C.; Lu, D.Y.; Wu, C.Y. Effects of mixed salinity stress on osmoregulatory substances and antioxidant enzyme activities in jujube. Shandong Agric. Sci. 2022, 54, 37–43. [Google Scholar] [CrossRef]
- Xiong, M.B.; Luo, M.S.; Tian, Y.B.; Song, G.Y.; Cao, S.Y. Dynamics of soil nutrition and wheat root activities and their relationships during wheat growth. Soil Fertil. 2005, 3, 8–11. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Yang, S.; Zhao, S.; Zheng, W.B.; Zhou, X.; Li, M. Study on the effect of biofertilizer on osmoregulatory substances in ginseng leaves. J. Ginseng Res. 2020, 3, 50–52. [Google Scholar] [CrossRef]
- Tian, F.; Chen, X.; Wang, P.C.; Zhong, L.; Ou, E.L. Effect of microbial fungicides on drought-resistant enzyme system and physiological and biochemical indexes of white spurge seedlings. J. Plant Physiol. 2022, 58, 435–446. [Google Scholar] [CrossRef]
- Li, Y.Q.; Xin, S.J.; Ao, Y.S. Effect of microbial fertilizer on the growth, yield and quality of greenhouse cucumber. Chin. Agric. Sci. Bull. 2012, 28, 259–263. [Google Scholar] [CrossRef]
- Huang, L.Q.; Guo, L.P. Accumulation of secondary metabolites under environmental stress and the formation of daoji medicinal herbs. Chin. J. China Mater. Med. 2007, 4, 277–280. [Google Scholar]
- Wei, Z.M.; Hu, X.J.; Mo, H. Effect of exogenous gibberellin on seed germination and seedling growth of Marigold under salt stress. North. Hortic. 2022, 16, 69–75. [Google Scholar]
- Wang, Q.K.; Wang, S.L.; Gao, H.; Liu, Y.; Yu, X.J. Effects of land use practices on soil organic matter. J. Ecol. 2005, 4, 360–363. [Google Scholar] [CrossRef]
- Matse, D.T.; Huang, C.H.; Huang, Y.M.; Yen, M.Y. Effects of coinoculation of Rhizobium with plant growth promoting rhizobacteria on the nitrogen fixation and nutrient uptake of Trifolium repens in low phosphorus soil. J. Plant Nutr. 2020, 43, 739–752. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front. Microbiol. 2018, 9, 1–25. [Google Scholar] [CrossRef]
- Deng, L.; Wang, T.; Luo, W.; He, L.Y.; Liang, Z.S. Effects of a compound microbial agent and plants on soil properties, enzyme activities, and bacterial composition of Pisha sandstone. Environ. Sci. Pollut. Res. 2021, 28, 53353–53364. [Google Scholar] [CrossRef]
- Gao, J.X.; Gao, Y.; Wu, X.M.; Niu, Y.Q.; Pei, H.X.; Xie, H. Response of microbial community and soil ions to microbicides in pepper continuous cropping soil. Southwest. China. J. Agric. Sci. 2020, 33, 1659–1664. [Google Scholar] [CrossRef]
- Guo, D.; Ren, C.Y.; Ali, A.; Li, R.H.; Du, J.; Liu, X.Y.; Guan, W.D.; Zhang, Z.Q. Streptomyces pactum combined with manure compost alters soil fertility and enzymatic activities, enhancing phytoextraction of potentially toxic metals (PTMs) in a smelter-contaminated soil. Ecotoxicol. Environ. Saf. 2019, 181, 312–320. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Zhang, L.R.; Chen, H.; Kang, P.Z.; Shen, R.Q. Effects of different microbial agents on tomato yield and soil microbial population. Hubei Agric. Sci. 2013, 52, 5452–5454,5518. [Google Scholar] [CrossRef]
- Lei, X.D.; Li, J.W.; Xu, X.L.; Zhang, H.L.; Cao, L.K. Influence of microbicides on growth characteristics and soil microbial diversity of spinach. Chin. J. Eco-Agric. 2012, 20, 488–494. [Google Scholar] [CrossRef]
- Liu, L.Y.; Liu, K.X.; Chi, X.L.; Zhang, X.; Xu, C.; Zhu, H.; Jin, X.; Liu, W.W.; Sun, Z.T.; Mao, Z.Q. Effects of Bacillus subtilis SNB-86 fertilizer on the growth and soil environment of continuous Pingyi sweet tea seedlings. Acta Hortic. Sin. 2018, 45, 2008–2018. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).