Light Intensity during Green-Leaf Butterhead Lettuce Propagation Influences Yield and Carotenoids at Harvest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Production
2.2. Carotenoid and Chlorophyll Extraction
2.3. HPLC Analysis
2.4. Data Collection and Statistical Analyses
3. Results
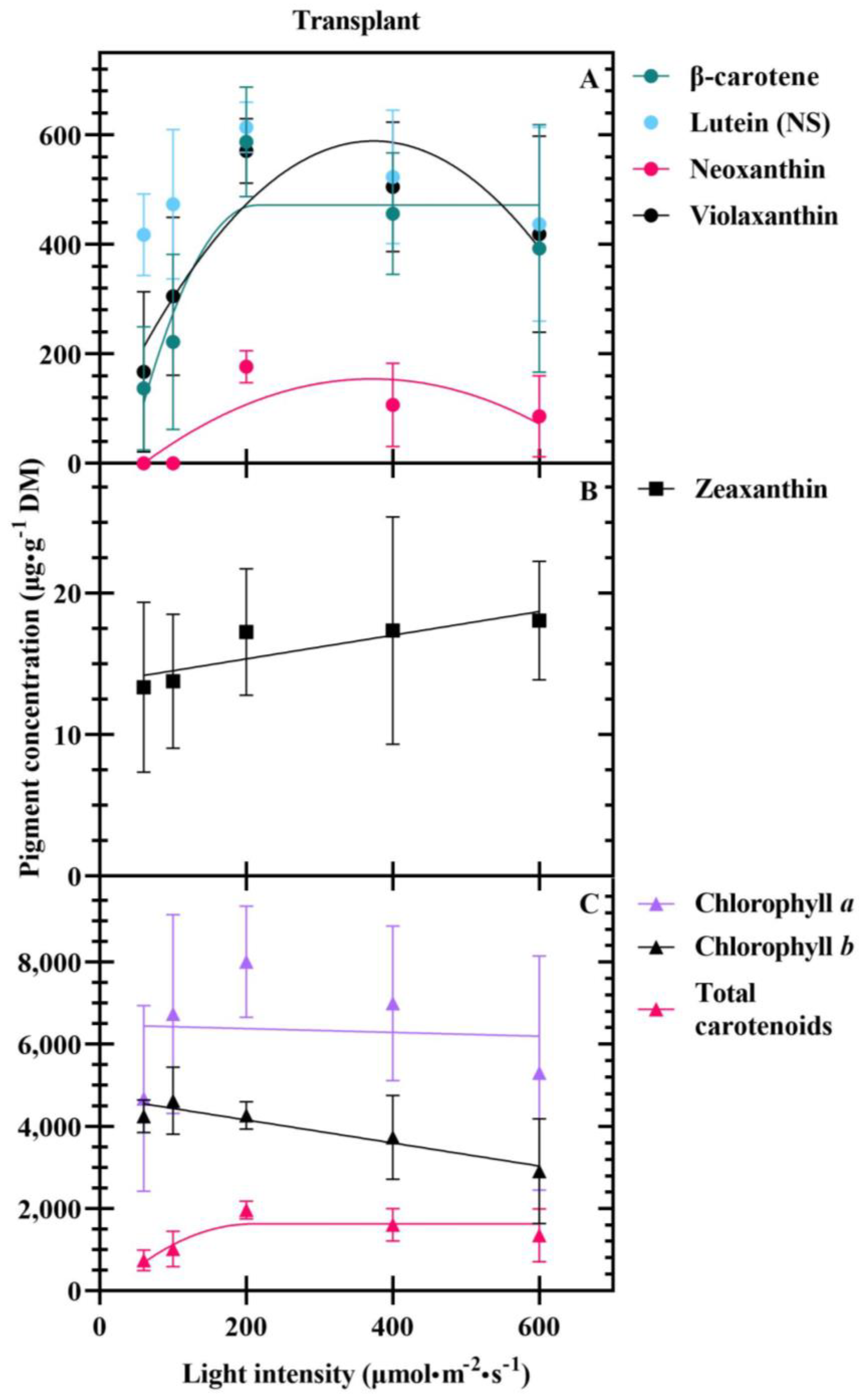
3.1. Seedlings
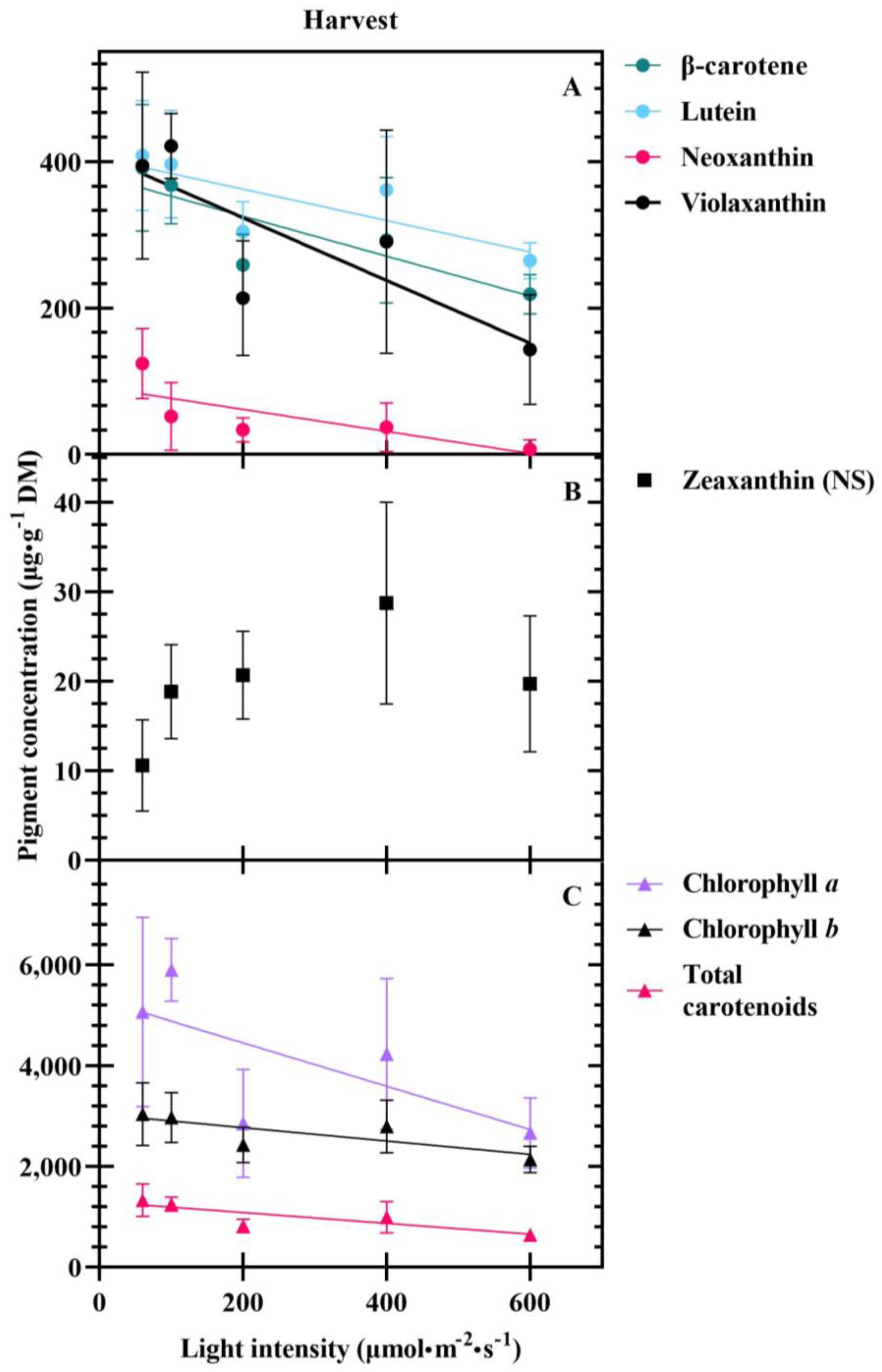
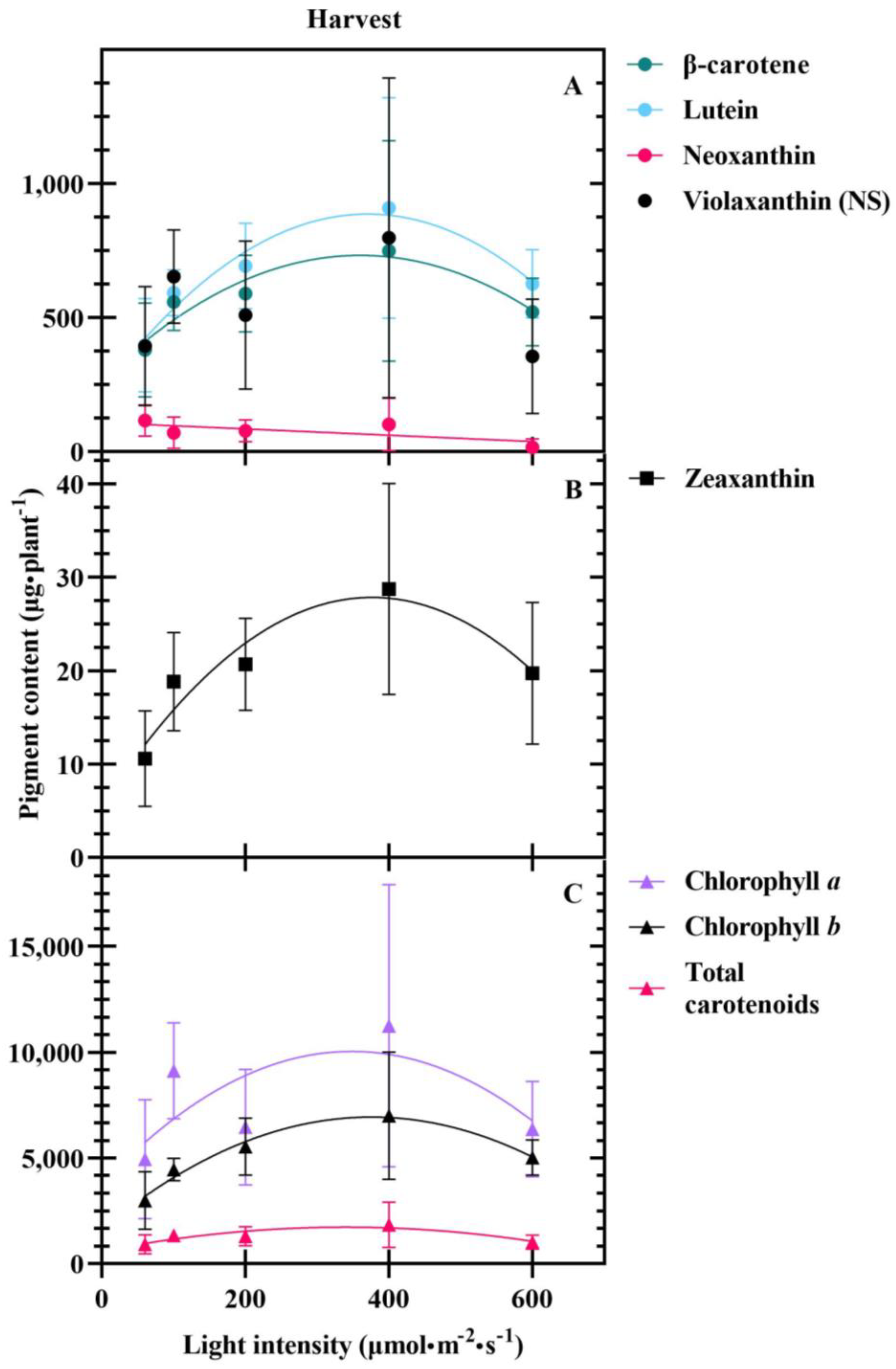
3.2. Harvest
4. Discussion
4.1. Yield at Transplant and Harvest Increases with DLI to A Point
4.2. DLI Alters Plant Morphology
4.3. Carotenoids Are Differentially Impacted at Transplant and Harvest
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Agriculture—National Agriculture Statistics Service (USDA–NASS). Vegetables 2019 Summary. 2020. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/vegean20.pdf (accessed on 29 April 2021).
- Eaves, J.; Eaves, S. Comparing the profitability of a greenhouse to a vertical farm in Quebec. Can. J. Agric. Econ. /Rev. Can. D’Agroeconomie 2018, 66, 43–54. [Google Scholar] [CrossRef]
- Hoops, G.P.; Olshansky, H.P.; Tavares, J.; Rosen, R. Copenhagen’s Case for Urban Farming: A Feasibility Study. Bachelor’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2018. [Google Scholar]
- Zeidler, C.; Schubert, D.; Vrakking, V. Feasibility Study: Vertical Farm EDEN; DLR Institute of Space Systems, German Aerospece Center: Bremen, Germany, 2013. [Google Scholar]
- Sago, Y. Effects of light intensity and growth rate on tipburn development and leaf calcium concentration in butterhead lettuce. HortScience 2016, 51, 1087–1091. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, X.; Teng, Y.; Gu, Y.; Chen, L.; Kong, X.; Han, Y. Asia Pacific Academy of Science Pte. Ltd. Available online: https://aber.apacsci.com/index.php/ama/article/view/2109 (accessed on 31 October 2022).
- Brechner, M.; Both, A.J.; Staff, C.E.A. Hydroponic Lettuce Handbook; Cornell Controlled Environment Agriculture: Ithica NY, USA, 1996; pp. 504–509. [Google Scholar]
- Lopez, R.; Runkle, E.S. Light Management in Controlled Environments; Meister Media Worldwide: Willoughby, OH, USA, 2017. [Google Scholar]
- Walters, K.J.; Lopez, R.G. Basil seedling production environment influences subsequent yield and flavor compound concentration during greenhouse production. PLoS ONE 2022, 17, e0273562. [Google Scholar] [CrossRef]
- Wiseman, E.M.; Bar-El Dadon, S.; Reifen, R. The vicious cycle of vitamin a deficiency: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3703–3714. [Google Scholar] [CrossRef] [PubMed]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid–derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef]
- Frank, H.A.; Brudvig, G.W. Redox functions of carotenoids in photosynthesis. Biochemistry 2004, 43, 8607–8615. [Google Scholar] [CrossRef]
- Amengual, J.; Gouranton, E.; van Helden, Y.G.J.; Hessel, S.; Ribot, J.; Kramer, E.; Kiec-Wilk, B.; Razny, U.; Lietz, G.; Wyss, A.; et al. Beta–carotene reduces body adiposity of mice via bcmo1. PLoS ONE 2011, 6, e20644. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, J.H.; Kim, H. Β–carotene and lutein inhibit hydrogen peroxide–induced activation of nf–κb and il–8 expression in gastric epithelial ags cells. J. Nutr. Sci. Vitaminol. 2011, 57, 216–223. [Google Scholar] [CrossRef]
- Yao, N.; Yan, S.; Guo, Y.; Wang, H.; Li, X.; Wang, L.; Hu, W.; Li, B.; Cui, W. The association between carotenoids and subjects with overweight or obesity: A systematic review and meta–analysis. Food Funct. 2021, 12, 4768–4782. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, Q.; Yan, F.-F.; Wan, J.-F.; Lin, C.-S. Lutein protects against ischemia/reperfusion injury in rat skeletal muscle by modulating oxidative stress and inflammation. Immunopharmacol. Immunotoxicol. 2015, 37, 329–334. [Google Scholar] [CrossRef]
- Widomska, J. Why has nature chosen lutein and zeaxanthin to protect the retina? J. Clin. Exp. Ophthalmol. 2014, 5, 326. [Google Scholar] [CrossRef]
- Johnson, E.J.; Neuringer, M.; Russell, R.M.; Snodderly, D.M. Nutritional manipulation of primate retinas, iii: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xathophyll-free monkeys. Investigative Ophtamol. Visual Sci. 2005, 46, 692. [Google Scholar] [CrossRef]
- Tu, W.; Wu, L.; Zhang, C.; Sun, R.; Wang, L.; Yang, W.; Yang, C.; Liu, C. Neoxanthin affects the stability of the C 2 S 2 M 2 -type photosystem II supercomplexes and the kinetics of state transition in Arabidopsis. Plant J. 2020, 104, 1724–1735. [Google Scholar] [CrossRef]
- Pizarro, L.; Stange, C. Light–dependent regulation of carotenoid biosynthesis in plants. Cienc. E Investig. Agrar. 2009, 36, 143–162. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Pantanizopoulos, N.I.; Sams, C.E.; Kopsell, D.E. Shoot tissue pigment levels increase in ‘Florida Broadleaf’ mustard (Brassica juncea L.) microgreens following high light treatment. Sci. Hortic. 2012, 140, 96–99. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J. Amer. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Weiguo, F.; Pingping, L.; Yanyou, W.; Jianjian, T. Effects of different light intensities on anti–oxidative enzyme activity, quality and biomass in lettuce. Hortic. Sci. 2012, 39, 129–134. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light–emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Pramuk, L.A.; Runkle, E.S. Photosynthetic daily light integral during the seedling stage influences subsequent growth and flowering of Celosia, Impatiens, Salvia, Tagetes, and Viola. HortScience 2005, 40, 1099C–1099. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X.L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.-L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Voß, B.; Maass, D.; Wüst, F.; Schaub, P.; Beyer, P.; Welsch, R. Carotenogenesis is regulated by 5′utr–mediated translation of phytoene synthase splice variants. Plant Physiol. 2016, 172, 2314–2326. [Google Scholar] [CrossRef]
- Simkin, A.J.; Zhu, C.; Kuntz, M.; Sandmann, G. Light–dark regulation of carotenoid biosynthesis in pepper (Capsicum annuum) leaves. J. Plant Physiol. 2003, 160, 439–443. [Google Scholar] [CrossRef]



| Light Intensity (µmol·m−2·s−1) | DLI (mol·m−2·d−1) | |
|---|---|---|
| Target | Actual | |
| 60 | 62 ± 3 | 5.4 |
| 100 | 103 ± 4 | 8.9 |
| 200 | 196 ± 6 | 16.9 |
| 400 | 415 ± 7 | 35.9 |
| 600 | 610 ± 13 | 52.7 |
| Rep. | Transplant Date | DLI (mol·m−2·d−1) | Air Temperature (°C) |
|---|---|---|---|
| 1 | 19 December 2020 | 15.5 ± 1.2 | 21.8 ± 1.2 |
| 2 | 17 January 2021 | 18.3 ± 2.7 | 23.5 ± 0.8 |
| 3 | 16 February 2021 | 16.0 ± 1.6 | 23.1 ± 1.3 |
| Parameter | a | b | c | R2 | |
|---|---|---|---|---|---|
| Fresh mass (g) | 0.17 z | 1.32 × 10−3 | −2.55 × 10−6 | 0.752 | |
| (0.02) y | (6.64 × 10−5) | (3.75 × 10−7) | |||
| Dry mass (g) | 2.34 × 10−3 | 8.77 × 10−5 | −8.91 × 10−8 | 0.816 | |
| (1.08 × 10−3) | (3.94 × 10−6) | (2.23 × 10−8) | |||
| Dry mass conc. (g·kg−1) | 29.05 | 0.08 | −7.96 × 10−5 | 0.675 | |
| (1.40) | (0.01) | (2.87 × 10−5) | |||
| Stem diameter (mm) | 0.65 | 1.58 × 10−3 | (−2.11 × 10−6) | 0.607 | |
| (0.04) | (1.43 × 10−4) | (8.04 × 10−7) | |||
| Leaf number | 1.93 | 2.65 × 10−3 | −3.57 × 10−6 | 0.646 | |
| (0.05) | (1.80 × 10−4) | (1.02 × 10−6) | |||
| Height (cm) | 7.90 | −0.03 | 287.93 | 0.641 | |
| (0.34) | (0.01) | (30.42) | |||
| β–carotene conc. (µg·g−1 DM) | −226.24 | 6.52 | 214.10 | 0.464 | |
| (−228.45) | (4.02) | (68.75) | |||
| Neoxanthin conc. (µg·g−1 DM) | 52.33 | 0.32 | −1.59 × 10−3 | 0.425 | |
| (16.48) | (0.06) | (3.38 × 10−4) | |||
| Violaxanthin conc. (µg·g−1 DM) | 337.71 | 0.78 | −3.83 × 10−3 | 0.458 | |
| (37.65) | (−0.14) | (7.73 × 10−4) | |||
| Zeaxanthin conc. (µg·g−1 DM) | 13.68 | 0.01 | 0.088 | ||
| (1.39) | (0.00) | ||||
| Chlorophyll b conc. (µg·g−1 DM) − | 4724.16 | −2.81 | 0.322 | ||
| (210.51) | (0.62) | ||||
| Total carotenoid conc. (µg·g−1 DM) − | −230.07 | 17.74 | 209.52 | 0.424 | |
| (677.77) | (12.05) | (72.49) | |||
| Lutein content (µg·plant−1) − | −7.94 | 0.15 | 369.09 | 0.709 | |
| (3.29) | (0.04) | (63.22) | |||
| Chlorophyll a content (µg·plant−1) − | −108.26 | 2.05 | 350.21 | 0.643 | |
| (−51.03) | (0.62) | (67.93) | |||
| Chlorophyll b content (µg·plant−1) − | −46.22 | 1.00 | 365.37 | 0.646 | |
| (25.04) | (0.30) | (71.78) | |||
| Total carotenoid content (µg·plant−1) − | −20.48 | 0.36 | 355.90 | 0.699 | |
| (7.78) | (0.10) | (61.18) |
| Parameter | a | b | c | R2 | |
|---|---|---|---|---|---|
| Fresh mass (g) | −5.49 z | 1.14 | 294.89 | 0.859 | |
| (7.26) y | (0.10) | (16.07) | |||
| Dry mass (g) | 0.01 | 0.03 | 300.92 | 0.438 | |
| (0.52) | (0.01) | (45.85) | |||
| Leaf number | 15.37 | 0.13 | 326.91 | 0.720 | |
| (1.32) | (0.02) | (27.89) | |||
| Height (cm) | 13.14 | 4.65 × 10−3 | −1.63 × 10−5 | 0.166 | |
| (0.24) | (8.60 × 10−4) | (4.83 × 10−6) | |||
| Β-carotene conc. (µg·g−1 DM) | 380.36 | −0.27 | 0.401 | ||
| (17.50) | (0.05) | ||||
| Lutein conc. (µg·g−1 DM) − | 405.37 | −0.22 | 0.309 | ||
| (16.93) | (0.05) | ||||
| Neoxanthin conc. (µg·g−1 DM) | 91.84 | −0.15 | 0.356 | ||
| (10.58) | (0.03) | ||||
| Violaxanthin conc. (µg·g−1 DM) | 409.19 | −0.43 | 0.376 | ||
| (28.98) | (0.09) | ||||
| Chlorophyll a conc. (µg·g−1 DM) | 5315.26 | −4.35 | 0.265 | ||
| (377.41) | (1.12) | ||||
| Chlorophyll b conc. (µg·g−1 DM) | 3037.61 | −1.35 | 0.240 | ||
| (124.94) | (0.37) | ||||
| Total carotenoid conc. (µg·g−1 DM) | 1299.29 | −1.08 | 0.431 | ||
| (64.70) | (0.19) | ||||
| Β-carotene content (µg·plant−1) | 527.74 | 0.64 | −3.54 × 10−3 | 0.217 | |
| (54.85) | (0.21) | (1.16 × 10−3) | |||
| Lutein content (µg·plant−1)− | 573.16 | 0.97 | −4.78 × 10−3 | 0.365 | |
| (54.81) | (0.21) | (1.16 × 10−3) | |||
| Neoxanthin content (µg·plant−1) | 107.90 | −0.12 | 0.135 | ||
| (15.88) | (0.048) | ||||
| Zeaxanthin content (µg·plant−1) | 16.92 | 0.03 | −1.56 × 10−4 | 0.371 | |
| (1.84) | (0.01) | (3.88 × 10−5) | |||
| Chlorophyll a content (µg·plant−1) | 7491.55 | 7.92 | −0.05 | 0.129 | |
| (1007.04) | (3.86) | (0.02) | |||
| Chlorophyll b content (µg·plant−1) | 4347.67 | 8.00 | −0.04 | 0.410 | |
| (404.70) | (1.55) | (0.01) | |||
| Total carotenoid content (µg·plant−1) | 1302.39 | 1.37 | −0.01 | 0.199 | |
| (146.35) | (0.56) | (3.09 × 10−3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Givens, S.R.; Del Moro, D.S.; Parker, S.E.; Renny, A.G.; Sams, C.E.; Walters, K.J. Light Intensity during Green-Leaf Butterhead Lettuce Propagation Influences Yield and Carotenoids at Harvest. Horticulturae 2023, 9, 223. https://doi.org/10.3390/horticulturae9020223
Givens SR, Del Moro DS, Parker SE, Renny AG, Sams CE, Walters KJ. Light Intensity during Green-Leaf Butterhead Lettuce Propagation Influences Yield and Carotenoids at Harvest. Horticulturae. 2023; 9(2):223. https://doi.org/10.3390/horticulturae9020223
Chicago/Turabian StyleGivens, Spencer R., Dustin S. Del Moro, Sarah E. Parker, Alexander G. Renny, Carl E. Sams, and Kellie J. Walters. 2023. "Light Intensity during Green-Leaf Butterhead Lettuce Propagation Influences Yield and Carotenoids at Harvest" Horticulturae 9, no. 2: 223. https://doi.org/10.3390/horticulturae9020223
APA StyleGivens, S. R., Del Moro, D. S., Parker, S. E., Renny, A. G., Sams, C. E., & Walters, K. J. (2023). Light Intensity during Green-Leaf Butterhead Lettuce Propagation Influences Yield and Carotenoids at Harvest. Horticulturae, 9(2), 223. https://doi.org/10.3390/horticulturae9020223







