Bioactive Compounds and Antioxidant Capacity of Several Blackberry (Rubus spp.) Fruits Cultivars Grown in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Determination of Fruit Weight, Dimensions, Moisture and Firmness of Blackberries
2.4. Determination of Total Soluble Solids Content (TSS), pH and Titratable Acidity of Blackberries
2.5. The Bioactive Compounds Determination
2.5.1. Preparation of Blackberry Extracts
2.5.2. Determination of Total Phenols (TPh), Flavonoids (TFlav) and Total Monomeric Anthocyanins (MAP) Content
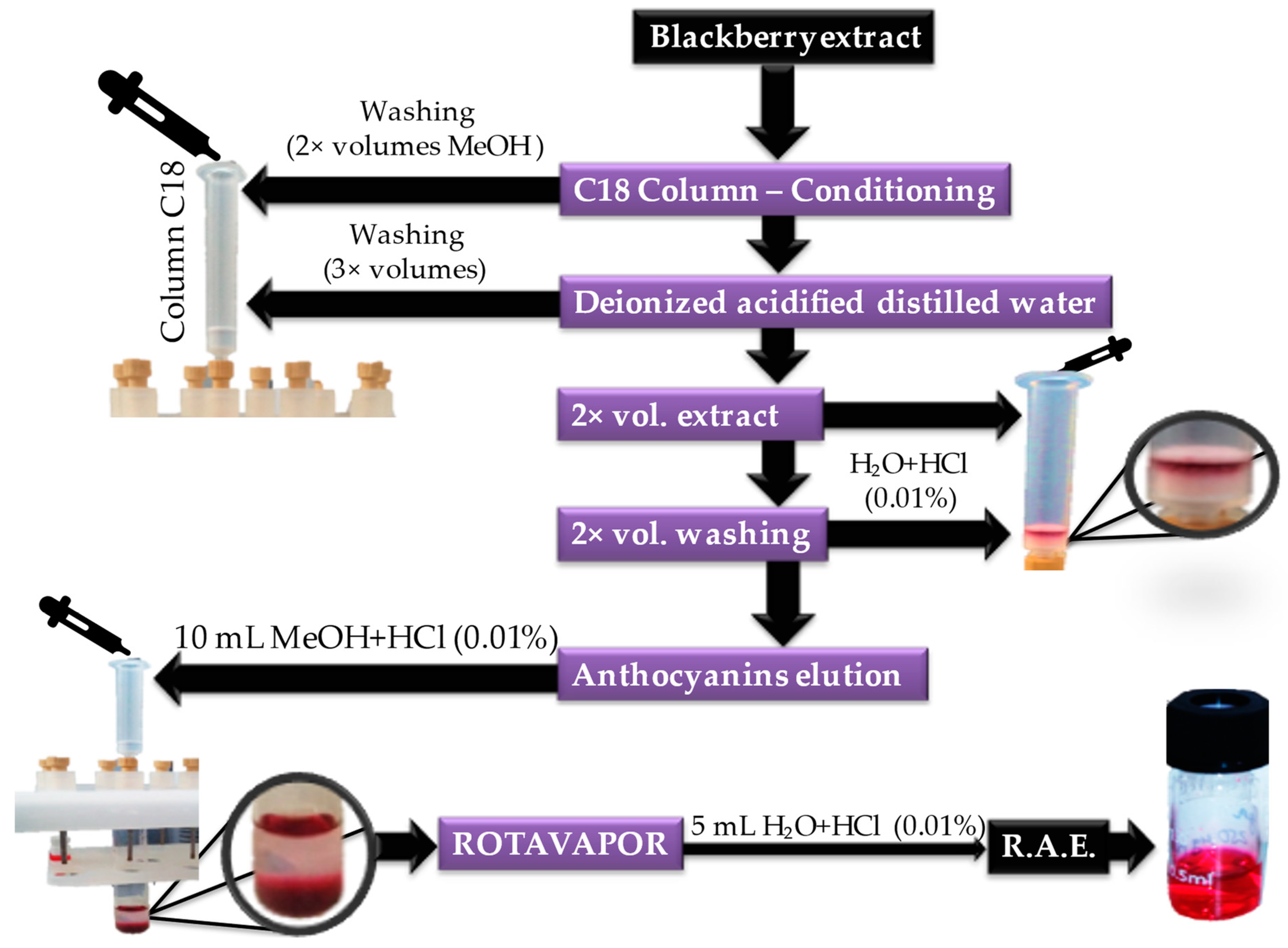
2.6. Solid Phase Extraction and HPLC-PDA Analysis of Anthocyanins
2.7. Determination of Antioxidant Capacity of Blackberries
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameters of Blackberries
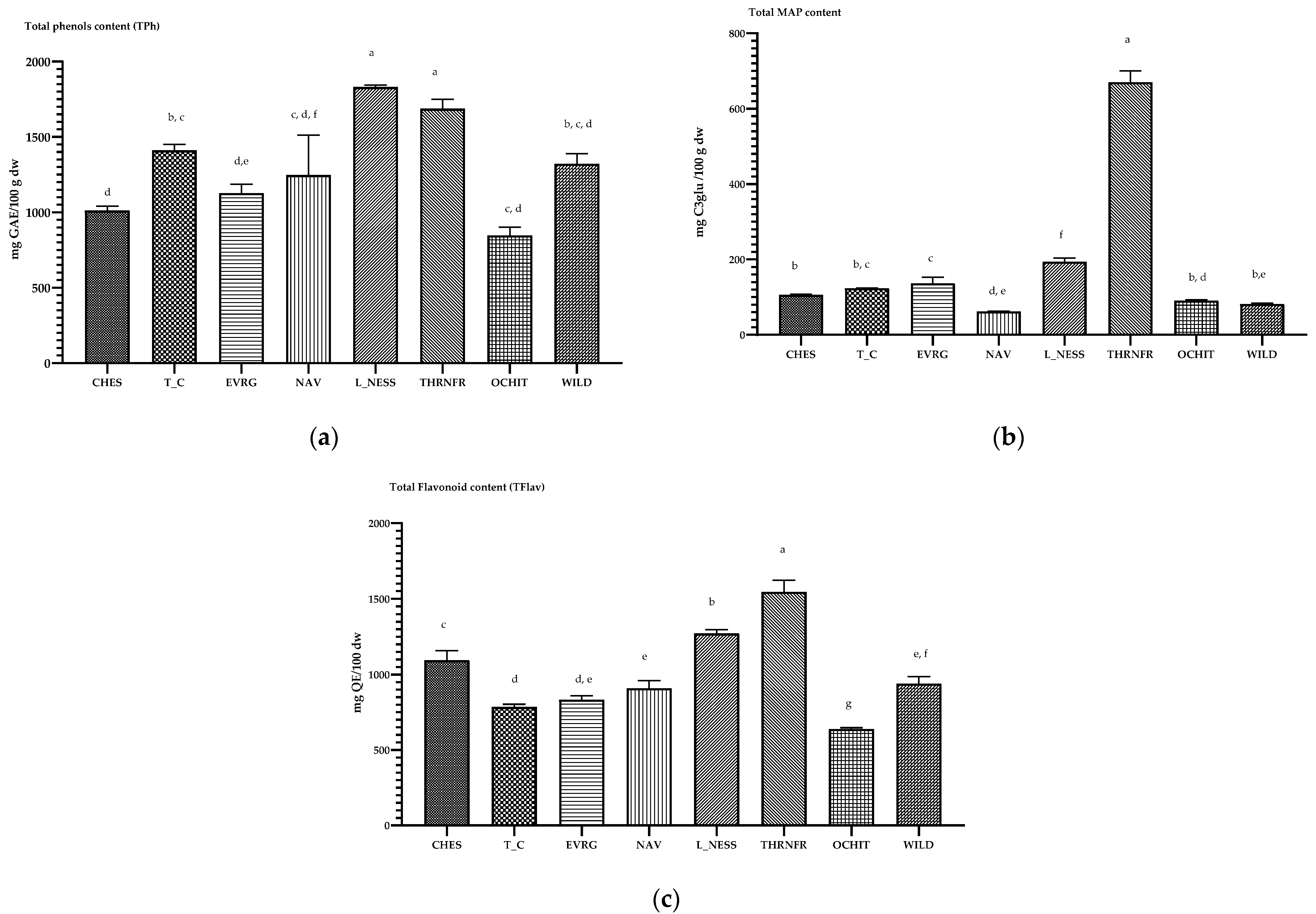
3.2. The Content of Total Phenols (TPh), Total Flavonoids (TFlav) in Blackberry Fruits and Monomeric Anthocyanins (MAP)
3.3. Anthocyanins Identified from Blackberry Fruits by HPLC-PDA
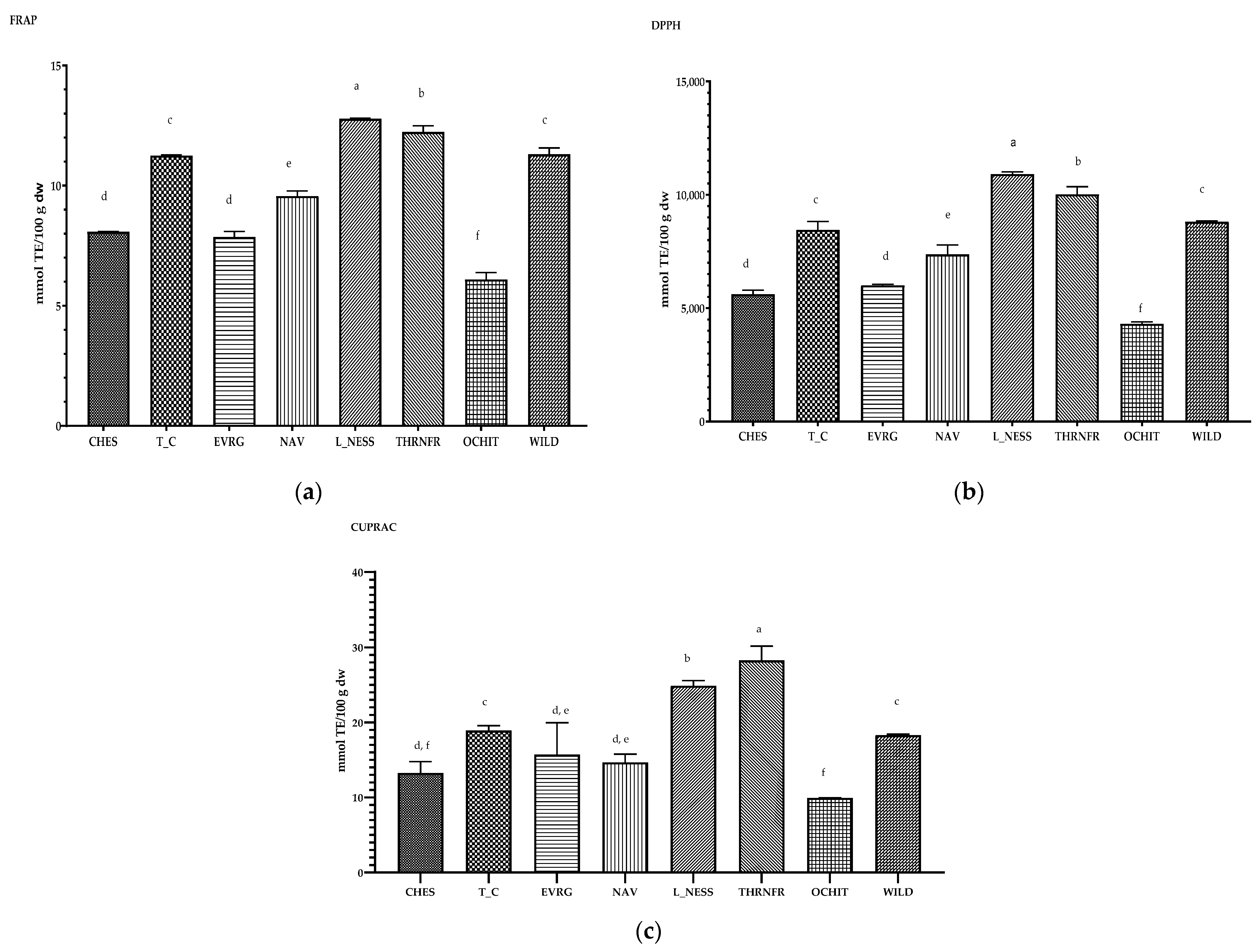
3.4. Antioxidant Capacity of Blackberry Fruits
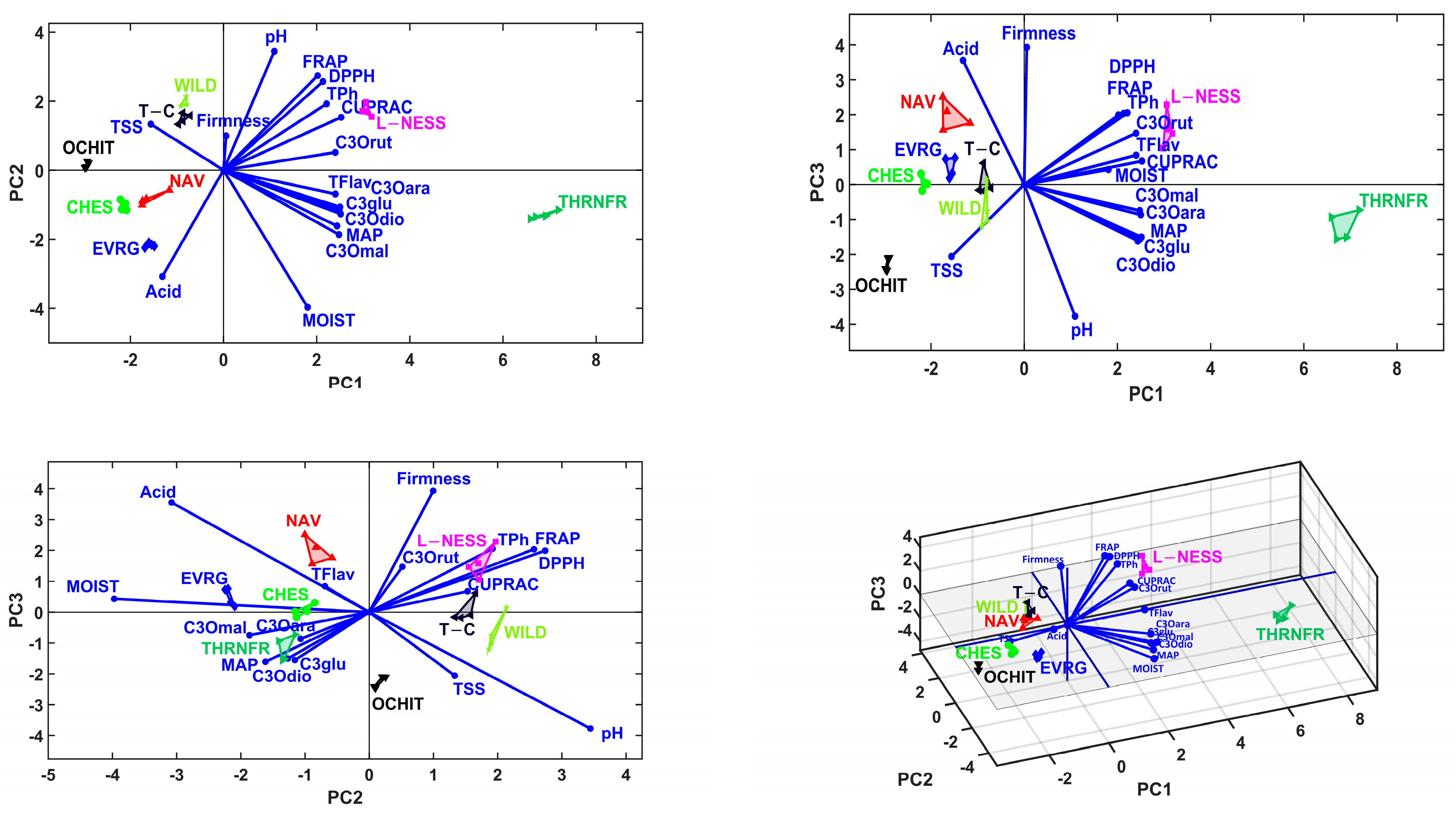
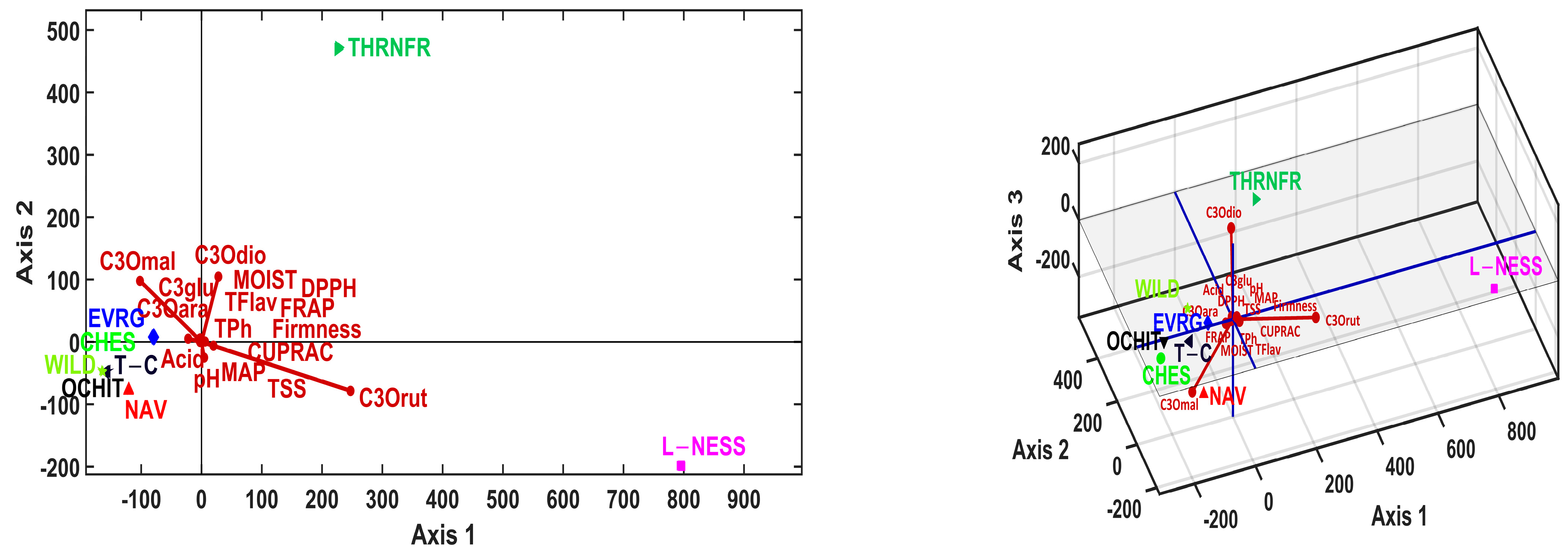
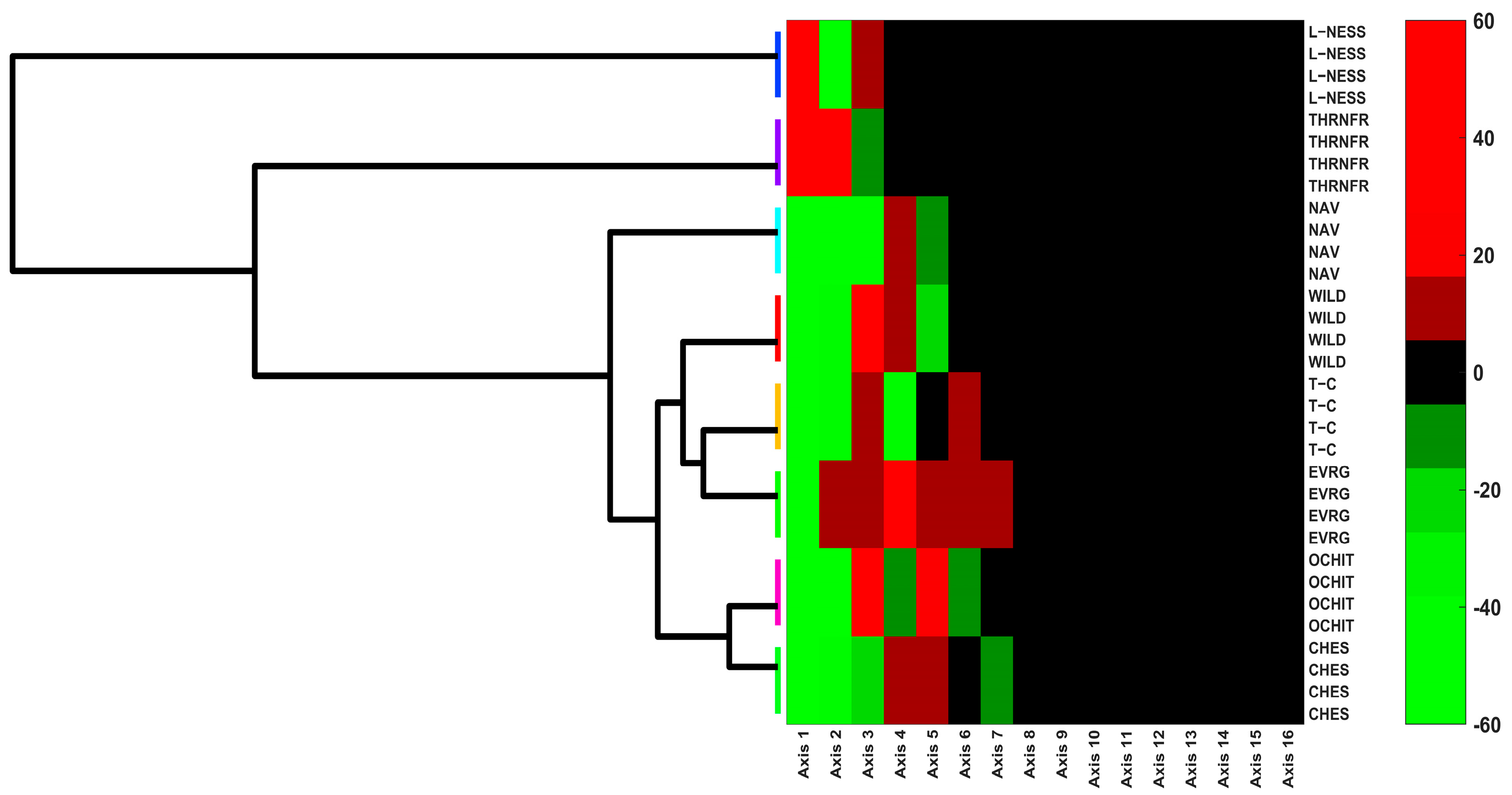
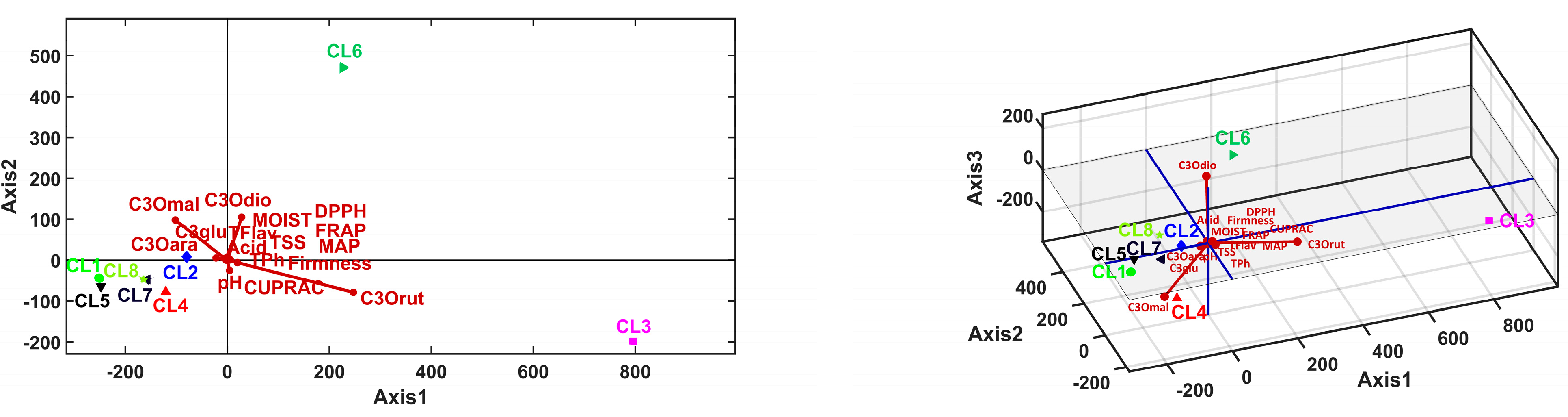
3.5. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strik, B.C.; Clark, J.R.; Finn, C.E.; Banados, M.P. Worldwide Blackberry Production. HortTechnology 2007, 17, 205–213. [Google Scholar] [CrossRef]
- Cuevas-Rodríguez, E.O.; Yousef, G.G.; García-Saucedo, P.A.; López-Medina, J.; Paredes-López, O.; Lila, M.A. Characterization of Anthocyanins and Proanthocyanidins in Wild and Domesticated Mexican Blackberries (Rubus spp.). J. Agric. Food Chem. 2010, 58, 7458–7464. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Zhao, Y. Berry Fruit: Value-Added Products for Health Promotion; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-0614-8. [Google Scholar]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.E.; Moga, M. Rubus fruticosus L.: Constituents, Biological Activities and Health Related Uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef]
- Kiple, K.F.; Ornelas, K.C. The Cambridge World History of Food; Cambridge University Press: Cambridge, UK, 2000; ISBN 978-0-521-40215-6. [Google Scholar]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and Anticancer Properties of Berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Vega, E.N.; Molina, A.K.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Stojković, D.; Soković, M.; et al. Anthocyanins from Rubus fruticosus L. and Morus nigra L. Applied as Food Colorants: A Natural Alternative. Plants 2021, 10, 1181. [Google Scholar] [CrossRef]
- Battino, M.; Beekwilder, J.; Denoyes-Rothan, B.; Laimer, M.; McDougall, G.J.; Mezzetti, B. Bioactive Compounds in Berries Relevant to Human Health. Nutr. Rev. 2009, 67, S145–S150. [Google Scholar] [CrossRef]
- Yilmaz, S. Effects of Dietary Blackberry Syrup Supplement on Growth Performance, Antioxidant, and Immunological Responses, and Resistance of Nile Tilapia, Oreochromis Niloticus to Plesiomonas Shigelloides. Fish Shellfish Immunol. 2019, 84, 1125–1133. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef]
- Oszmiański, J.; Nowicka, P.; Teleszko, M.; Wojdyło, A.; Cebulak, T.; Oklejewicz, K. Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruits. Int. J. Mol. Sci. 2015, 16, 14540–14553. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Veberic, R.; Hudina, M.; Zorenc, Z.; Koron, D.; Senica, M. Fruit Quality Characteristics and Biochemical Composition of Fully Ripe Blackberries Harvested at Different Times. Foods 2021, 10, 1581. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar] [CrossRef] [PubMed]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry Antioxidants: Small Fruits Providing Large Benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Koron, D.; Zorenc, Z.; Veberic, R. Do Optimally Ripe Blackberries Contain the Highest Levels of Metabolites? Food Chem. 2017, 215, 41–49. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant Activities of Flavonoids as Bioactive Components of Food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef]
- Kuhnau, J. The Flavonoids. A Class of Semi-Essential Food Components: Their Role in Human Nutrition. In World Review of Nutrition and Dietetics; Bourne, G.H., Ed.; S. Karger AG: Basel, Switzerland, 1976; Volume 24, pp. 117–191. ISBN 978-3-8055-2344-8. [Google Scholar]
- Siriwoharn, T.; Wrolstad, R.E. Polyphenolic Composition of Marion and Evergreen Blackberries. J. Food Sci. 2004, 69, FCT233–FCT240. [Google Scholar] [CrossRef]
- Rudolf, P.S. Zonarea Speciilor Pomicole. pp. 96–104. Available online: https://www.academia.edu/33315476/Zonarea_speciilor_pomicole (accessed on 28 March 2022).
- Krzykowski, A.; Dziki, D.; Rudy, S.; Polak, R.; Biernacka, B.; Gawlik-Dziki, U.; Janiszewska-Turak, E. Effect of Air-Drying and Freeze-Drying Temperature on the Process Kinetics and Physicochemical Characteristics of White Mulberry Fruits (Morus alba L.). Processes 2023, 11, 750. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Popa, E.E.; Drăghici, M.C.; Popescu, P.A.; Popa, V.I.; Bujor, O.-C.; Ion, V.A.; Popa, M.E. Latest Developments in Edible Coatings on Minimally Processed Fruits and Vegetables: A Review. Foods 2021, 10, 2821. [Google Scholar] [CrossRef]
- Bunea, A.; Rugina, O.D.; Pintea, A.M.; Sconţa, Z.; Bunea, C.I.; Socaciu, C. Comparative Polyphenolic Content and Antioxidant Activities of Some Wild and Cultivated Blueberries from Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 70–76. [Google Scholar] [CrossRef]
- Memete, A.R.; Teuşdea, A.; Adrian, T.; Vicaş, S.; Vlad, A. Effect of Solvent Composition on the Extraction of Anthocyanins from Bilberry Fruits (Vaccinium myrtillus L.). Nat. Resour. Sustain. Dev. 2021, 11, 11–22. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 978-0-12-182200-2. [Google Scholar]
- Memete, A.R.; Teusdea, A.C.; Timar, A.V.; Vuscan, A.N.; Mintaș, O.S.; Cavalu, S.; Vicas, S.I. Effects of Different Edible Coatings on the Shelf Life of Fresh Black Mulberry Fruits (Morus nigra L.). Agriculture 2022, 12, 1068. [Google Scholar] [CrossRef]
- Memete, A.R.; Miere (Groza), F.; Laslo, V.; Purcarea, C.; Vicas, L.; Ganea, M.; Antonescu, A.; Vicas, S.I. An In Vitro Study of the Healing Potential of Black Mulberry (Morus nigra L.) Extract in a Liposomal Formulation. Appl. Sci. 2023, 13, 1041. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated Anthocyanins from Edible Sources and Their Applications in Food Systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Budau, R.; Memete, A.; TIMOFTE, A.; VICAS, S. Phytochemical Screening and Antioxidant Capacity of Two Berry Cultivars, ‘Ruben’ and ‘Duke’, Depending on Their Harvesting Time. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2022, 1, 27. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Rugină, D.; Socaciu, C. High-Purity Anthocyanins Isolation Using Solid Phase Extraction Tehniques. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73, 107–113. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Bandici, L.; Teusdea, A.; Milian, O.P.; Memete, A.R.; Vicas, S.I.; Timar, A.V.; Bandici, G.E. The Use of Microwaves in the Process of Reducing the Browning of Apple Slices. In Proceedings of the 2021 16th International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 10 June 2021; pp. 1–4. [Google Scholar]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Kleszken, E.; Purcarea, C.; Pallag, A.; Ranga, F.; Memete, A.R.; Miere (Groza), F.; Vicas, S.I. Phytochemical Profile and Antioxidant Capacity of Viscum album L. Subsp. album and Effects on Its Host Trees. Plants 2022, 11, 3021. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the Bioactive Compounds, Antioxidant Activity and Chemical Composition of Brazilian Blackberry, Red Raspberry, Strawberry, Blueberry and Sweet Cherry Fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Bovi, G.G.; Rux, G.; Caleb, O.J.; Herppich, W.B.; Linke, M.; Rauh, C.; Mahajan, P.V. Measurement and Modelling of Transpiration Losses in Packaged and Unpackaged Strawberries. Biosyst. Eng. 2018, 174, 1–9. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water Loss of Fresh Fruit: Influencing Pre-Harvest, Harvest and Postharvest Factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Garazhian, M.; Gharaghani, A.; Eshghi, S. Genetic Diversity and Inter-Relationships of Fruit Bio-Chemicals and Antioxidant Activity in Iranian Wild Blackberry Species. Sci. Rep. 2020, 10, 18983. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lin, H.S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Guseynova, B.M. Nutrition value of wild-growing fruits from mountain Dagestan and its safety after fast freezing and cold storage. Vopr. Pitan. 2016, 85, 76–81. [Google Scholar]
- Kolniak-Ostek, J.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Characterization of Phenolic Compounds of Thorny and Thornless Blackberries. J. Agric. Food Chem. 2015, 63, 3012–3021. [Google Scholar] [CrossRef]
- Sariburun, E.; Sahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic Content and Antioxidant Activity of Raspberry and Blackberry Cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Liu, W.; Li, C. Survey of Antioxidant Capacity and Phenolic Composition of Blueberry, Blackberry, and Strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef]
- Rojas-Ocampo, E.; Torrejón-Valqui, L.; Muñóz-Astecker, L.D.; Medina-Mendoza, M.; Mori-Mestanza, D.; Castro-Alayo, E.M. Antioxidant Capacity, Total Phenolic Content and Phenolic Compounds of Pulp and Bagasse of Four Peruvian Berries. Heliyon 2021, 7, e07787. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Stampar, F.; Schmitzer, V.; Cunja, V.; Zupan, A.; Koron, D.; Mikulic-Petkovsek, M. Changes in the Contents of Anthocyanins and Other Compounds in Blackberry Fruits Due to Freezing and Long-Term Frozen Storage. J. Agric. Food Chem. 2014, 62, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Wada, L.; Ou, B. Antioxidant Activity and Phenolic Content of Oregon Caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef] [PubMed]
- Dragović-Uzelac, V.; Savić, Z.; Brala, A.; Levaj, B.; Bursać Kovačević, D.; Biško, A. Evaluation of Phenolic Content and Antioxidant Capacity of Blueberry Cultivars (Vaccinium corymbosum L.) Grown in the Northwest Croatia. Food Technol. Biotechnol. 2010, 48, 214–221. [Google Scholar]
- Okan, O.T.; Deniz, İ.; Yayli, N.; Şat, İ.G.; Öz, M.; Serdar, G.H. Antioxidant Activity, Sugar Content and Phenolic Profiling of Blueberries Cultivars: A Comprehensive Comparison. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 639–652. [Google Scholar] [CrossRef]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of Anthocyanin Content and Profile Throughout Fruit Development and Ripening of Highbush Blueberry Cultivars Grown at Two Different Altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Chen, H.; Yu, W.; Chen, G.; Meng, S.; Xiang, Z.; He, N. Antinociceptive and Antibacterial Properties of Anthocyanins and Flavonols from Fruits of Black and Non-Black Mulberries. Molecules 2017, 23, 4. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Wrolstad, R.E. A Novel Zwitterionic Anthocyanin from Evergreen Blackberry (Rubus laciniatus Willd). J. Agric. Food Chem. 2002, 50, 396–399. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Connor, A.M.; Finn, C.E.; McGhie, T.K.; Alspach, P.A. Genetic and Environmental Variation in Anthocyanins and Their Relationship to Antioxidant Activity in Blackberry and Hybridberry Cultivars. J. Am. Soc. Hortic. Sci. 2005, 130, 680–687. [Google Scholar] [CrossRef]
- Fan-Chiang, H.-J.; Wrolstad, R.E. Anthocyanin Pigment Composition of Blackberries. J. Food Sci. 2005, 70, C198–C202. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid Glycosides and Antioxidant Capacity of Various Blackberry, Blueberry and Red Grape Genotypes Determined by High-Performance Liquid Chromatography/Mass Spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Connor, A.M.; Finn, C.E.; Alspach, P.A. Genotypic and Environmental Variation in Antioxidant Activity and Total Phenolic Content among Blackberry and Hybridberry Cultivars. J. Am. Soc. Hortic. Sci. 2005, 130, 527–533. [Google Scholar] [CrossRef]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-Phase Extraction of Berries’ Anthocyanins and Evaluation of Their Antioxidative Properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin Extraction from Plant Tissues: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Gopinath, H.; Karthikeyan, K.; Meghana, V. For the Love of Color: Plant Colors and the Dermatologist. Indian J. Dermatol. Venereol. Leprol. 2020, 86, 622–629. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Beta, T. Saskatoon and Wild Blueberries Have Higher Anthocyanin Contents than Other Manitoba Berries. Available online: https://pubs.acs.org/doi/pdf/10.1021/jf072529m (accessed on 5 August 2021).
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Litescu, S.C.; Eremia, S.; Radu, G.L. Methods for the Determination of Antioxidant Capacity in Food and Raw Materials. In Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors; Giardi, M.T., Rea, G., Berra, B., Eds.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 2010; pp. 241–249. ISBN 978-1-4419-7347-4. [Google Scholar]
- Tabart, J.; Kevers, C.; Dardenne, N.; Schini-Kerth, V.; Albert, A.; Dommes, J.; Defraigne, J.-O.; Pincemail, J. Deriving a Global Antioxidant Score for Commercial Juices by Multivariate Graphical and Scoring Techniques. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; King’s College London: London, UK, 2014; pp. 301–307. ISBN 978-0-12-404738-9. [Google Scholar]
- Ciorchina, N.; Cutcovschii-Muştuc, A.; Lozinschii, M.; Mîrza, A.; Trofim, M. Blackberry–Importance, Origin and Value. Rev. Bot. 2017, 15, 15–22. [Google Scholar]
- Souza, R.S.D.; Bilharva, M.G.; Marco, R.D.; Antunes, L.E.C.; Martins, C.R.; Malgarim, M.B. Phenological and Productive Characteristics of Blackberry Genotypes Grown in an Organic Production System. An. Acad. Bras. Ciênc. 2021, 93, 825–833. [Google Scholar] [CrossRef]
- Tatar, M.; Varedi, M.; Naghibalhossaini, F. Epigenetic Effects of Blackberry Extract on Human Colorectal Cancer Cells. Nutr. Cancer 2022, 74, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant Activity of Berry Phenolics on Human Low-Density Lipoprotein and Liposome Oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E.; Kwolek, M.; Średnicka-Tober, D.; Kazimierczak, R. GeneticDifferentiation in AnthocyaninContent among Berry Fruits. Curr. Issues Mol. Biol. 2021, 43, 36–51. [Google Scholar] [CrossRef] [PubMed]
| Cultivars | Aspect of Fruits | General Aspects |
|---|---|---|
| CHEST |  | Is a vigorous shrub, semi-erect and fast-growing:
|
| T_C |  | Is a semi-erect blackberry variety and self-fertile:
|
| NAV |  | Is an erect shrub, and prefers sunny areas and less wet and cold soils:
|
| L_NESS |  | Is a vigorous shrub with semi-erect shoots, prefers very sunny areas and is particularly suitable for smaller gardens as it is compact and does not produce suckers:
|
| THRNFR |  | Is a very vigorous shrub, and during the vegetation period, they produce side shoots; the inflorescences are long, with a large number of flowers:
|
| OCHIT |  | Is a very vigorous, fast-growing, upright, and erect shrub, and is a sun lover and self-pollinating:
|
| EVRG |  | Is a semi-vigorous and productive variety, vulnerable to various diseases, very resistant to drought conditions, and its leaves remain green even in winter:
|
| Samples | CHES | T_C | EVRG | NAV | L_NESS | THRNFR | OCHIT | WILD | |
|---|---|---|---|---|---|---|---|---|---|
| Parameters | |||||||||
| Moisture content (%) | 68.8 ± 0.6 a | 75.6 ± 0.02 b | 73.1 ± 0.05 c | 71.8 ± 0.08 d | 68.3 ± 0.02 a, c | 82.7 ± 0.02 f | 64.1 ± 0.1 g | 79.9 ± 0.03 h | |
| GrP (g) | 5.89 ± 0.14 a | 6.67 ± 0.52 b | 5.87 ± 0.14 a | 7.14 ± 0.12 b | 4.55 ± 0.08 c | 9.11 ± 1.16 d | 5.55 ± 0.18 a | 1.72 ± 0.001 e | |
| Height (cm) | 2.33 ± 0.35 a | 2.4 ± 0.26 a | 2.47 ± 0.11 a | 2.43 ± 0.49 a | 2.33 ± 0.38 a | 2.77 ± 0.15 a | 2.30 ± 0.26 a | 1.03 ± 0.47 b | |
| Diameter (cm) | 6.67 ± 0.25 a, d, e | 6.97 ± 0.23 a, d, e | 6.93 ± 0.11 a, d, e | 6.77 ± 0.32 a, d, e | 5.67 ± 0.51 b | 7.20 ± 0.26 d | 6.17 ± 0.30 e | 3.60 ± 0.37 c | |
| Samples | CHES | T_C | EVRG | NAV | L_NESS | THRNFR | OCHIT | WILD | |
|---|---|---|---|---|---|---|---|---|---|
| Parameters | |||||||||
| pH | 3.27 ± 0.014 f | 3.65 ± 0.012 d | 2.85 ± 0.17 g | 3.01 ± 0.012 e | 3.84 ± 0.012 b,c | 3.98 ± 0.008 a, b, c | 4.066 ± 0.018 a | 3.95 ± 0.014 a, c | |
| Acidity (% malic acid) | 6.21 ± 0.60 b | 4.37 ± 0.30 d | 7.86 ± 0.30 a | 7.46 ± 0.6 a | 3.18 ± 0.44 e | 2.55 ± 0.44 e | 2.53 ± 0.054 e, f | 3.83 ± 0.12 d, e | |
| Firmness | 5.28 ± 1.06 a | 5.33 ± 1.49 a | 4.94 ± 0.58 a | 8.56 ± 2.36 b | 7.17 ± 1.85 a | 5.23 ± 0.91 a | 6.06 ± 1.22 a | 5.78 ± 1.88 a | |
| TSS | 14.13 ± 0.05 d | 14.33 ± 0.1 c, d | 14.4 ± 0.08 c | 13.85 ± 0.05 e | 11.18 ± 0.09 f | 12.2 ± 0.08 f | 14.7 ± 0.18 b | 18.18 ± 0.1 a | |
| SAMPLES | CHES | T_C | EVRG | NAV | L_NESS | THRNFR | OCHIT | WILD |
|---|---|---|---|---|---|---|---|---|
| Cyanidin-3-glucoside | 7.51 ± 0.17 a | 58.73 ± 4.34 b, c | 54.48 ± 4.01 c, f | 13.54 ± 0.88 a, f | 87.76 ± 5.74 d | 329.26 ± 7.64 e | 21.48 ± 1.71 a | 43.99 ± 3.15 f |
| Cyanidin-3-O-arabinoside | 1.45 ± 0.1 a, f | 1.02 ± 0.1 a | 5.69 ± 0.19 b, e | 2.34 ± 0.02 f | 8.04 ± 0.60 c | 17.40 ± 0.09 d | 1.54 ± 0.01 a, f | 5.87 ± 0.39 e |
| Cyanidin-3-O-(malonyl)glucoside | 0.64 ± 0.04 f | 1.21 ± 0.01 e | 2.04 ± 0.02 c | 1.69 ± 0.01 d | 3.46 ± 0.02 b | 12.83 ± 0.09 a | nd | nd |
| Cyanidin-3-O-(dioxalyl)glucoside | 0.75 ± 0.001 g | 1.81 ± 0.01 e | 3.39 ± 0.02 c | nd | 5.58 ± 0.03 b | 17.98 ± 0.13 a | 1.05 ± 0.01 f | 2.37 ± 0.04 d |
| Cyanidin-3-rutinoside | 0.93 ± 0.0001 a, g | 2.89 ± 0.02 b | 4.34 ± 0.02 c | 3.33 ± 0.02 d | 17.76 ± 0.13 e | 14.92 ± 0.11 f | 0.84 ± 0.002 g | 2.13 ± 0.01 h |
| TOTAL | 11.28 ± 0.07 a | 65.67 ± 1.93 b, c, f | 69.95 ± 1.80 c, f | 20.84 ± 0.39 a | 122.60 ± 2.49 d | 392.42 ± 3.37 e | 24.92 ± 0.76 a | 54.36 ± 1.37 f |
| R | pH | Acid | Firm | TPh | TFlav | TSS | MAP | FRAP | DPPH | CUPRAC | Moisture | C3glu | C3Oara | C3Omal | C3Odio | C3Orut |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.000 | −0.961 | −0.124 | 0.290 | 0.228 | 0.022 | 0.357 | 0.341 | 0.349 | 0.446 | −0.176 | 0.394 | 0.342 | 0.254 | 0.389 | 0.313 |
| Acid | −0.961 | 1.000 | 0.107 | −0.347 | −0.288 | 0.124 | −0.441 | −0.351 | −0.374 | −0.499 | 0.086 | −0.469 | −0.403 | −0.344 | −0.467 | −0.413 |
| Firm | −0.124 | 0.107 | 1.000 | 0.128 | 0.092 | −0.178 | −0.154 | 0.213 | 0.210 | 0.059 | 0.009 | −0.145 | −0.072 | −0.068 | −0.150 | 0.168 |
| TPh | 0.290 | −0.347 | 0.128 | 1.000 | 0.696 | −0.505 | 0.549 | 0.916 | 0.952 | 0.920 | 0.323 | 0.603 | 0.648 | 0.596 | 0.605 | 0.849 |
| TFlav | 0.228 | −0.288 | 0.092 | 0.696 | 1.000 | −0.560 | 0.804 | 0.662 | 0.691 | 0.818 | 0.704 | 0.780 | 0.833 | 0.823 | 0.813 | 0.798 |
| TSS | 0.022 | 0.124 | −0.178 | −0.505 | −0.560 | 1.000 | −0.494 | −0.281 | −0.356 | −0.472 | −0.526 | −0.447 | −0.396 | −0.567 | −0.469 | −0.749 |
| MAP | 0.357 | −0.441 | −0.154 | 0.549 | 0.804 | −0.494 | 1.000 | 0.468 | 0.497 | 0.746 | 0.783 | 0.988 | 0.919 | 0.983 | 0.987 | 0.680 |
| FRAP | 0.341 | −0.351 | 0.213 | 0.916 | 0.662 | −0.281 | 0.468 | 1.000 | 0.984 | 0.913 | 0.173 | 0.541 | 0.573 | 0.503 | 0.525 | 0.709 |
| DPPH | 0.349 | −0.374 | 0.210 | 0.952 | 0.691 | −0.356 | 0.497 | 0.984 | 1.000 | 0.934 | 0.235 | 0.568 | 0.629 | 0.539 | 0.563 | 0.793 |
| CUPRAC | 0.446 | −0.499 | 0.059 | 0.920 | 0.818 | −0.472 | 0.746 | 0.913 | 0.934 | 1.000 | 0.431 | 0.797 | 0.806 | 0.765 | 0.795 | 0.862 |
| Moisture | −0.176 | 0.086 | 0.009 | 0.323 | 0.704 | −0.526 | 0.783 | 0.173 | 0.235 | 0.431 | 1.000 | 0.743 | 0.785 | 0.845 | 0.770 | 0.553 |
| C3glu | 0.394 | −0.469 | −0.145 | 0.603 | 0.780 | −0.447 | 0.988 | 0.541 | 0.568 | 0.797 | 0.743 | 1.000 | 0.939 | 0.977 | 0.993 | 0.700 |
| C3Oara | 0.342 | −0.403 | −0.072 | 0.648 | 0.833 | −0.396 | 0.919 | 0.573 | 0.629 | 0.806 | 0.785 | 0.939 | 1.000 | 0.923 | 0.963 | 0.780 |
| C3Omal | 0.254 | −0.344 | −0.068 | 0.596 | 0.823 | −0.567 | 0.983 | 0.503 | 0.539 | 0.765 | 0.845 | 0.977 | 0.923 | 1.000 | 0.975 | 0.729 |
| C3Odio | 0.389 | −0.467 | −0.150 | 0.605 | 0.813 | −0.469 | 0.987 | 0.525 | 0.563 | 0.795 | 0.770 | 0.993 | 0.963 | 0.975 | 1.000 | 0.738 |
| C3Orut | 0.313 | −0.413 | 0.168 | 0.849 | 0.798 | −0.749 | 0.680 | 0.709 | 0.793 | 0.862 | 0.553 | 0.700 | 0.780 | 0.729 | 0.738 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memete, A.R.; Sărac, I.; Teusdea, A.C.; Budău, R.; Bei, M.; Vicas, S.I. Bioactive Compounds and Antioxidant Capacity of Several Blackberry (Rubus spp.) Fruits Cultivars Grown in Romania. Horticulturae 2023, 9, 556. https://doi.org/10.3390/horticulturae9050556
Memete AR, Sărac I, Teusdea AC, Budău R, Bei M, Vicas SI. Bioactive Compounds and Antioxidant Capacity of Several Blackberry (Rubus spp.) Fruits Cultivars Grown in Romania. Horticulturae. 2023; 9(5):556. https://doi.org/10.3390/horticulturae9050556
Chicago/Turabian StyleMemete, Adriana Ramona, Ioan Sărac, Alin Cristian Teusdea, Ruben Budău, Mariana Bei, and Simona Ioana Vicas. 2023. "Bioactive Compounds and Antioxidant Capacity of Several Blackberry (Rubus spp.) Fruits Cultivars Grown in Romania" Horticulturae 9, no. 5: 556. https://doi.org/10.3390/horticulturae9050556
APA StyleMemete, A. R., Sărac, I., Teusdea, A. C., Budău, R., Bei, M., & Vicas, S. I. (2023). Bioactive Compounds and Antioxidant Capacity of Several Blackberry (Rubus spp.) Fruits Cultivars Grown in Romania. Horticulturae, 9(5), 556. https://doi.org/10.3390/horticulturae9050556







