Abstract
The availability of water and nitrogen in the soil affect the metabolism of onion bulbs. The synthesis of metabolites and bioactive compounds are the most affected, along with the quality of the onion bulbs However, it is necessary to know the effects of different water levels and nitrogen fertilization to optimize the quality of the onion. The objective of this research was to study the effects of the different conditions of hydric stress and nitrogen fertilization during the development of onion (Allium cepa L.) crop, regarding its physicochemical and bioactive properties. Onions were grown using four available irrigation regimes (25, 50, 75 and 100%) and four doses of nitrogen fertilization (100, 150, 200 and 250 kg N ha−1). Onion without any treatment was considered as a control. The treatments low in irrigation and nitrogen fertilization increased the pH level (5.7 to 5.9) and bulb coloration in bright white/yellowish tones. An increase was observed compared to control in titratable acidity (0.13%) just in the nitrogen content, ascorbic acid (46%) and antioxidant capacity with DPPH (12.3%) and ABTS (93.7%). A decrease was shown in soluble solids (14.6%), firmness (3.5 kg cm−2), dry matter (6.6%), total phenols (50%) and FRAP (33.2%) values. Pyruvic acid remained constant (1.5 µmol g−1 FW). The onion bulb extracts showed an erythroprotective effect with a hemolysis inhibition percentage higher than 95%. Finally, the onions had low pungency, and were soft and extra sweet. The treatments with 25% usable humidity and nitrogen fertilization of 150 and 250 kg ha−1, favored the physical, chemical and bioactive quality of the onion bulb.
1. Introduction
The onion (Allium cepa L.) is one of the most important and most widely grown commercial crop products in the world [1], with a global production of about 100 million tons annually [2]. Onion consumption is generalized in almost every tradition and culture, as a complement in culinary preparations due to its nutritional properties as well as for medicinal uses [3]. Among the nutritional properties of this crop are a wide source of A and C vitamins, and minerals such as iron, thiamine, niacin and manganese [4]. Onion is also known to have large amounts of antioxidant compounds due to the synthesis of phenolic elements and flavonoids [5]. Flavor and pungency are biochemical attributes due to several of its bioactive organosulfur and phenolic compounds [6]. In addition to sulfur compounds such as sulfoxides, mono, di and trisulfurs, onion contains phytonutrients such as terpenoids, tannins, alkaloids, thiosulfinates and fructoligosaccharides [7].
Onion owes its biological properties to its flavonoids, phenolic acid content and organosulfur compounds, which are very important in treating and preventing a series of chronic diseases [8,9]. These properties have antimicrobial, antidiabetic, analgesic, anti-inflammatory, hypolipidemic, antihypertensive, immunoprotecting and antioxidant effects [10,11,12]. Thus, onions are a natural source of antioxidants [13]. The measuring of antioxidant activity in onions has been estimated using a series of in vitro testing that includes 2,2′-acid azyno-bis-(3-ethylbenzoathiazoline-6-sulfonic) (ABTS), 1,1-diphenyl-2-pycrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), lipid peroxidation, oxygen radical absorbance capacity (ORAC) and total antioxidant capacity (TAC), as well as Trolox equivalent antioxidant capacity (TEAC) [14]. Some of the factors that influence the antioxidant activity in onions are related to the variety, agronomical management, storage conditions, extraction methods and processing technologies applied to onions [15,16,17,18,19].
On the other hand, some authors mention that plants grown under abiotic stress conditions biosynthesize secondary metabolites, including a wide array of bioactive compounds acting as functional molecules for crop adaptation, but which are also of great interest to human health [20]. Among the types of abiotic stress, hydric stress is the main factor responsible for the high metabolite content in plants [21]. Likewise, limited nitrogen supply has been associated with high levels of phenolic compounds in crops [22]. The amount of irrigation water and application of nitrogen in onions not only affects performance, but also their biochemical qualities [23].
Some research reports that quality attributes, such as firmness and color, can be influenced by onion genetic makeup, agronomic practices and water management [24] and [25]. Similarly, ref. [26] mentions that the chemical composition of the onion is determined by genetic factors, while the changes in the concentration of substances, such as organic acids [27], could be determined by the environment and agricultural practices. As a result, this study intends to analyze the effect of different hydric stress conditions and nitrogen fertilization during the development of onion (Allium cepa L.) crop in terms of physicochemical and bioactive properties in an arid environment.
2. Materials and Methods
2.1. Study Site
Field studies were conducted during the period of January to May 2021 in the experimental area of the Institute of Agricultural Sciences of the Autonomous University of Baja California (32°24′34″ N, 115°11′16″ W). The climate, according to the Köppen classification, is hot desert with scarce precipitation in winter (BW [h’] hs [x’] [e’]) [28]. The environmental conditions that occurred during the experimental period are shown in Table 1. The soil properties were clayey texture, EC 5.2 ds m−1, SAR 1.7, pH 8.0, chlorine 1063 ppm, bicarbonate 732 ppm, magnesium 388.8 ppm, calcium 240.5 ppm, sodium 181 ppm, potassium 78 ppm, sulfate 2.39 ppm and nitrate 2.28 ppm.

Table 1.
Environmental conditions recorded during the experimental period between January and May 2021. Average monthly values of meteorological data recorded with an automatic weather station.
2.2. Crop Establishment and Management
The white onion variety Sierra Blanca hybrid of intermediate photoperiod was transplanted on January 1st, when the seedlings presented from 3 to 4 leaves. The seedlings were planted in double rows with a spacing of 80 × 20 × 15 cm between furrow, row and plants, respectively. The water without fertilizer was applied weekly through a drip irrigation system during the first 30 days of the crop (before the beginning of bulb formation). Water contained 0.9 ds m−1 and a pH of 8.01. Weeds were controlled manually.
2.3. Treatments and Experimental Design
At the beginning of the onion bulb formation, treatments were applied. Four nitrogen fertilization dosages (100, 150, 200 and 250 Kg N ha−1) as well as four levels of available soil moisture (25, 50, 75 and 100%) were analyzed. The combination of both factors can be seen in Table 2. Onion without any treatment was considered as a control. The available soil moisture was established based on the soil water characteristic curve of clayey texture. Soil moisture regimes were determined with a watermark sensor to a depth 30 cm. A reading of 10 ± 5 cb corresponded to a 100% Aw, 35 ± 5 cb to 75% Aw, 70 ± 5 cb to 50% Aw, 70 ± 5 cb to 50% Aw and 110 cb to 25% Aw. The experiment was conducted in 4 × 4 split plot in randomized complete block design (RCBD) with three repetitions.

Table 2.
Description of treatments of nitrogen fertilization and soil moisture in onion cultivation.
2.4. Physicochemical Parameters
These parameters were studied using the Association of Official Agricultural Chemists (AOAC) [29] methodology. Ten grams of fresh onion tissue was homogenized in a commercial Osterizer blender with 50 mL of distilled water (pH 7.0). The extract was filtered and used to determine pH, titratable acidity (%) and total soluble solids (°Brix). The color was measured in two planes opposite the equatorial section of the onion, using a chroma meter CR-410 (Konica Minolta, Inc., Tokyo, Japan) (Figure 1). Color values were expressed using a tridimensional coordinate system L* a* b*, where the vertical axis L* is the luminosity (black = 0 and white = 100), the horizontal axis a* is the trend from red to green and b* is the trend from blue to yellow. Additionally, the values of C* (Chroma) and h (hue) were determined [30]. In addition to this, firmness (expressed in Newton (N)) was measured using a digital Chatillon DFE-100 texture meter (AETEK Inc., New Taipei, Taiwan) with a cylindrical 8 mm point. Finally, we determined the dry matter percentage by placing the bulb cut up into pieces in a drying stove at 60 °C for a period of 72 h [31].

Figure 1.
Measurement of onion color using a chroma meter CR-410 (Konica Minolta, Inc., Japan).
2.5. Pyruvic Acid Determination
This was quantified by using the method proposed by Anthon and Barret [32]. Five grams of vegetable tissue were homogenized with 5 mL of distilled water. After 10 min at room temperature, the mixture was filtered by using 2 layers of organza fabric. The filtrate was centrifuged at 3000 rpm at 4 °C for 10 min. An aliquot of 25 µL of the supernatant was taken and combined with 1 mL of deionized water and 1 mL of 2,4-dinitrophenylhydrazine (DNPH 0.25 g L−1 in HCl 1 M) solution. The mixture was incubated at 37 °C in a water bath for 10 min, and 1 mL of NaOH (1.5 M) was added. Afterwards, the absorbance at 515 nm was recorded and the triplicated results were calculated through a pyruvate pattern curve and expressed in µmol·g−1 of fresh weight.
2.6. Ascorbic Acid Determination
The ascorbic acid contents were determined by using Tillman’s method, which is also known as the 2,6-dichlorophenolindophenol volumetric method. This is based on the reduction of 2,6-dichlorophenolindophenol by the ascorbic acid. Five grams of fresh tissue was homogenized with 50 mL of oxalic acid at 5%. Afterwards, an aliquot of 5 mL was taken and treated with Tillman solution until a permanent pink color was visible for 1 min. The triplicated results were calculated by comparing to a standard curve using pure ascorbic acid and expressed in mg of ascorbic acid 100 g−1 of fresh weight [29].
2.7. Preparation of Onion Extracts
The extraction was carried out according to Downes et al. [33] with some modifications. The onion samples were previously dehydrated at 32 °C (150 ± 0.5 mg) for 48 h, pulverized and filtered by using a 30-mesh sieve (0.595 nm). Samples were dissolved in 3 mL of acidified methanol composed of 70:29.5:0.5 (v/v/v) methanol (reactive grade): water (Milli Q): HCl (reactive grade). Afterwards, the samples were placed in a water bath at 35 °C for 90 min, shaking every 15 min during the extraction. After they cooled down, the samples were centrifuged at 10,000 rpm for 15 min and adjusted at 0.06 g mL−1. Then, they were stored at −20 °C until they were analyzed. The extracts were used to determine total phenols, antioxidant activity and erythroprotective activity.
2.8. Total Phenol Determination
Total phenols were determined by using the Folin–Ciocalteu technique [34]. An aliquot of 10 µL of the extract was added to 25 µL of Folin solution 1 N. After 5 min at room temperature, 25 µL of Na2CO3 solution at 20% and 140 µL of distilled water were added to reach a final volume of 200 µL. The absorbance was determined after 39 min at 760 nm. A calibration curve was produced between 0 and 1 mg mL−1 by using gallic acid as a standard. Assays were performed in triplicate. All results were expressed in milligrams of gallic acid equivalent per gram of sample (mg GAE g−1).
2.9. Antioxidant Capacity Determination
2.9.1. Ferric Reducing Antioxidant Power (FRAP)
The antioxidant power due to reduction from a ferric to ferrous ion was determined by using the Rubio et al. [35] methodology with a few modifications. Firstly, an acidified stock solution of a sodium acetate buffer (300 mmol L−1, pH 3.6) was prepared. In addition, a second solution containing a TPTZ iron complex (2,4,6-tripyridyl-s-triazine) using 20 mmol of FeCl3·6H2O in a TPTZ solution in 40 mmol of HCl was mixed. Once the stock solutions were prepared, a FRAP work solution was made in a 10:1:1 relation (Buffer: FeCl3·6H2O: TPTZ·HCl). In a plate of 96 wells, 20 µL of extract and 280 µL of the FRAP solution were added. Absorbance was determined 30 min later with a Thermo Fisher Scientific Inc. Multiskan GO, NY, USA microplate reader. Ferric ion reduction was determined through a change in color from yellow to blue. A Trolox standard curve was produced in a concentration range from 0 to 200 µM. The results were expressed as µM ET g−1 of sample.
2.9.2. DPPH (2,2-Diphenyl-2-Pycrylhydrazyl)
This step was conducted with 1.5 mg of the DPPH• radical dissolved in 50 mL of methanol. This solution was adjusted to 0.7 ± 0.01 at 515 nm [36]. A total of 200 µL of DPPH• radical was mixed with 20 µL of extract. The sample was kept in darkness for 30 min, and the absorbance at 515 nm was determined. A Trolox standard curve was made (0 to 200 µM) and the results were expressed in µM of Trolox equivalents per gram of sample (µM TE g−1).
2.9.3. ABTS (acid 2,2′-Azyno-bis-3 Ethylbenzothiazoline-6-Sulfonic)
This step was conducted following the Re et al. [37] methodology. The radical was prepared by dissolving 19.3 mg of ABTS in 5 mL of distilled water. Independently, potassium persulfate (0.0378 g) was dissolved in 1 mL of water. A total of 88 µL of the persulfate solution was added to the ABTS solution and left in the dark for 12 h. This last solution was adjusted to 0.7 ± 0.05 at 734 nm. Subsequently, the prepared ABTS•+ cationic radical solution (270 µL) was added to 20 µL of the sample, and after 30 min at room temperature, the absorbance at 734 nm was determined. A Trolox calibration curve was made between 0 and 1 mg mL−1. The results were expressed in µM of Trolox equivalents per gram of sample (µM ET g−1).
2.10. Erythroprotective Activity
When cells (erythrocytes) are exposed to free radicals, they attack the phospholipids of the membranes, causing their destruction or hemolysis. The presence of ascorbic acid and phenolic compounds contributes to the protection of erythrocytes against free radicals [38]. For this analysis, erythrocytes were obtained through the venipuncture technique following the Mexican regulations (NOM-253-SSA1-2012) [39]. Erythrocytes (O blood group Rh+) were extracted from healthy subjects who signed their respective informed consent forms. Blood was deposited in a sterile vial containing anticoagulant (EDTA). Erythrocyte suspension was prepared at 10% with a saline solution containing phosphate (PBS) pH 7.4. It was centrifuged at 2000× g for 10 min at 4 °C and washed three times. The red blood cell packs (erythrocytes) were recovered, and after that, an erythrocyte suspension in PBS was prepared with a 5:95 (v/v) ratio, respectively. The erythroprotective effect was measured by quantifying the anti-hemolytic activity of the extract. Firstly, hemolysis was induced by using AAPH (2,2′-azobis(2-methylpropionamidine), which generates free radicals in the erythrocyte membrane. In order to conduct the analysis, we proceeded to use 100 µL of erythrocytes + 100 µL of the AAPH + 100 µL of each extract (0.06 g mL−1). We considered the erythrocyte suspension and the AAPH with erythrocytes as controls. Following this, we incubated at 37 °C for 3 h, shaking continuously (30 rpm), and we added 1 mL of PBS prior to centrifuging at 2000× g for 10 min at 4 °C. The supernatant was read at 540 nm in a 96-well microplate. The hemolysis inhibition percentage was obtained by using Equation (1):
where AAPH is the hemolysis absorbance caused by the AAPH, and HS is the absorbance of the hemolysis inhibition for each treatment according to the Lu et al. [40] methodology.
% Hemolysis inhibition = (AAPH − HS)/AAPH × 100
2.11. Statistical Analysis
The data from each variable of the study were subjected to analysis of variance (ANOVA) and Tukey’s multiple comparison test (p ≤ 0.05), using the statistical software SAS 9.4. Principal component analysis was used to identify grouping variables in the treatments.
3. Results and Discussions
3.1. Physicochemical Parameters
Table 3 shows the physicochemical parameters of onion regarding available soil moisture and nitrogen fertilization. The levels of pH varied because of the available moisture (p < 0.05) and nitrogen fertilization (p < 0.05). However, this difference was minimal between the untreated sample and those with the different treatments maintaining an acid pH (5.7–5.9). This behavior was similar to that reported by Wakchaure et al. [41] in white onions under severe hydric stress or excessive irrigation, recording pH values of 5.8 to 5.6. However, Venâncio et al. [42] reported that, regarding the soil fertilization and salinity, the pH of the bulbs did not record any significant differences. It is likely that the pH variation of the bulb relies on the agronomical management of the crop, cultural practices and environmental conditions, as indicated by Klunklin and Savage [43].

Table 3.
Physicochemical parameters of onion bulbs (Allium cepa L.) in response to available soil moisture and nitrogen fertilization.
Titratable acidity was a characteristic which showed a response to irrigation (p < 0.05) and fertilizers (p < 0.05). Treatment with 100% moisture and 250 Kg N ha−1 obtained the highest acidity, registering 0.135%. With a difference in pH, there was a slight increase in titratable acidity in the treatments of 75 and 100% available moisture, whereas in fertilization, the same increase appeared in the 150 kg N ha−1 treatment. Acidity measures both free protons as well as those connected, as opposed to pH, which measures only free protons. Therefore, pH remained with a constant number of free protons in the different treatments while acidity did not. This might be because onion bulbs have different organic acid contents. Organic acids are distributed unevenly in onion bulbs with higher levels of malate in the external scales and citrate in the internal ones [44].
Total soluble solids showed a decrease of about 14.6% compared to untreated onion, showing no significant differences in 25% available moisture and in 100 kg N ha−1 of fertilizer. Similar results were reported by Pejic et al. [45] and Ghodke et al. [46]. Yoo et al. [47] mention that the crop used in this research (“Sierra Blanca”) is sweet. On the other hand, Chávez-Mendoza et al. [48] indicated that it contains 12.9± 0.8 °Brix, a similar amount to that obtained in our research (12.3 °Brix). Regarding color, particularly at 0 and 25% of available moisture, “L*” showed values close to 100, which indicates higher color clarity; “b*” showed a slighter tendency more towards a yellow color; and “C*” high brightness in the bulb surface. The value a*, since it is too small, tends to be white in color. The values of h obtained indicate white/yellow hues. It is possible that the 0 and 25% treatments of usable humidity showed a higher whiteness, luminosity and brightness in the bulbs due to restricted irrigation conditions. Plants under hydric stress protect themselves by reducing the photosynthesis rate [46,49] and the production of chlorophyl or color [50], redirecting most of the energy from the plant to the production of more proline (40%) and phenols (26%), thus promoting its special coloration [51].
Firmness was slightly higher in untreated samples of onion (4.5 kg cm−2) compared to treated bulbs (3.5 kg cm−2). Mallor et al. [24] and Agnieszka et al. [25] mention that the quality attributes such as firmness can be influenced by agronomical practices and water management. Randle [52] indicates that high doses of nitrogen in crops can produce soft bulbs and decrease the useful post-harvest life. On the other hand, Di Miceli et al. [53] reported higher firmness (8.44 kg cm−2 and 7.94 kg cm−2) in onions with high nitrogen concentrations (160 and 220 kg N ha−1, respectively). These controversies among researchers suggest that the genetic composition of the onion is related to the firmness of the bulb.
The onion dry matter (Figure 2) had a significant response to soil moisture and fertilization (p < 0.05). The highest dry weight (Figure 2A) was recorded in the control treatment (12.7%), followed by 25 and 50 usable humidity percentage (6.6% and 5%, respectively). The 75 and 100% levels of humidity recorded less dry matter and showed no significant differences. We observed a similar trend in fertilization treatments (Figure 2B) with 0 nitrogen (12.7%), 100 (5.3%) and 150 (5%) kg N ha−1. Hence, in both factors, there was a decrease of about 40–48% dry matter in comparison to the control. Fatideh and Asil [54] and Abdelkhalik et al. [55] reported that the maximum production of dry matter in onions was recorded with minimum humidity treatments, a behavior which was similar to our results. Khokhar [56] mentioned that too much nitrogen can not only reduce the amount of dry matter, but it can also cause an excessive vegetative growth and delayed maturity, among other variables.
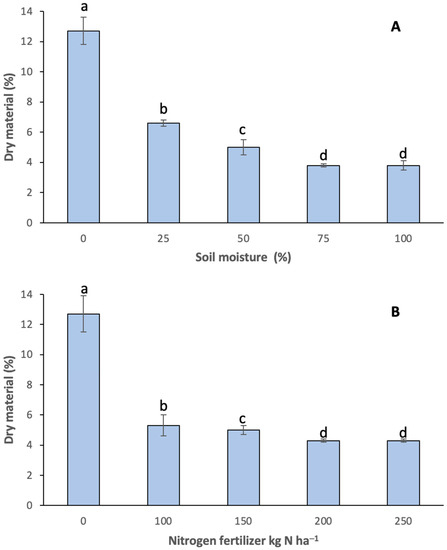
Figure 2.
Onion dry matter at different (A) soil moistures and (B) nitrogen fertilization. Different letters indicate significant difference (p < 0.05). Media ± standard deviation of three repetitions.
The interaction that presented the highest percentage of dry matter was that of the treatments of 25% available soil moisture and 100 Kg N ha−1, registering 7.4%, followed by the interaction of 25% moisture and 150 Kg N ha−1, with 6.9% for these variables.
3.2. Pyruvic Acid Content
This parameter is directly related to the pungency of the onion bulb. The “Sweet Onion Industry” in Georgia (USA), indicates the scale of pyruvic acid values varying from 0 to 18 µmol g−1 FW. When values are between 0 and 3, they are considered to be low-pungency onions, between 3 and 7 is considered to be medium pungency and if they are higher than 7, they are considered to be high-pungency onions. Another more specific scale is the one presented by “Vidalia Labs International” in Georgia (USA, 2005), where the scale is between the values of 0 and 10 µmol g−1 FW. If the pyruvic acid values are lower than 3, they are considered to be weak flavored onions, those between 3 and 5.5 are classified as being slightly pungent, those between 5.5 and 6 are pungent and if the value is higher than 6, they are considered very pungent. Therefore, onions with a level of pyruvic acid lower than 3.5 are called extra sweet onions, those between 3.6 and 5.5 are sweet onions and those with values higher than 5.6 are hot onions [24]. All samples had 1.5 µmol g−1 FW of pyruvic acid. Therefore, they fall within the classification of low pungency, weak flavored and extra sweet onions. Similar amounts have been reported by Abdissa et al. [57] in red Bombay onions, and by Ghodke et al. [46] and Gonçalves et al. [58] in sweet onions.
3.3. Ascorbic Acid Content
Ascorbic acid content held a significant relation with irrigation and fertilizers (p < 0.05). Ascorbic acid contents increased compared to the control (Figure 3). From 50% to 100% available soil moisture levels, there was an approximate increase of 46% of ascorbic acid. There were no significant differences between treatments (Figure 3A). In terms of fertilization response, the level of 200 kg N ha−1 recorded higher ascorbic acid contents (15.13 mg/100 g FW) with a 47.4% increase compared to the control (Figure 3B). As opposed to other bioactive compounds, the effect of hydric deficit on the accumulation of ascorbic acid in agricultural crops is, in some cases, inconsistent [59]. For example, there was an increase in ascorbic acid reported by Wichrowska et al. [60] and Golubkina et al. [61] in ‘Efekt’ onions when growing with hydric deficit. However, Ncayiyana et al. [62] reported no significant differences in the ascorbic acid content of red and white onions at different nitrogen fertilization doses. Ascorbic acid is considered an antioxidant that induces responses related to plant growth to cope with stress. Stress increases the level of cell oxidation state, which induces an increase in the synthesis of antioxidants to counteract the effects of that oxidation. This may explain the ascorbic acid increase in the different treatments applied to the onions.
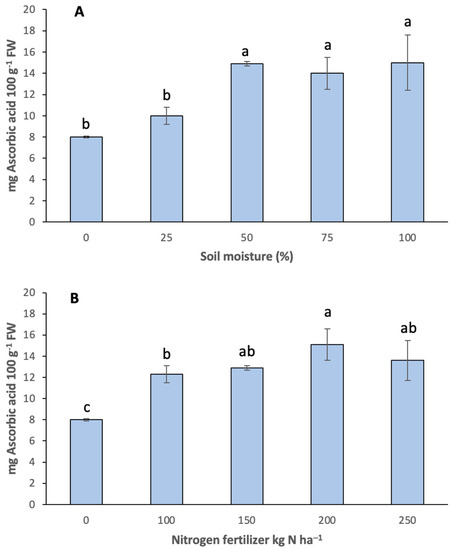
Figure 3.
Onion ascorbic acid content at different (A) soil moistures and (B) nitrogen fertilization. Different letters indicate significant difference (p < 0.05). Media ± standard deviation of three repetitions. FW = fresh weight.
3.4. Total Phenols
Phenolic compounds showed a decrease with the different treatments compared to the control both in available soil moisture as well as with the use of nitrogen fertilization (Table 4). The more the doses of the treatments increased, the lower the number of phenolic compounds in the onion bulbs, thus showing a negative correlation (r2 = −0.8227). It has been reported that the proportion of phenols in onions varies depending on the color. Red and yellow onions have the main polyphenols: gallic and ferulic acids, particularly quercetin as QDG (quercetin-3,4′-diglucoside) and Q4′G (quercetin-4′-glucoside) [63]. However, even though phenols protect cells from potential oxidative damage caused by oxidative stress [41], this time, this role was mitigated by ascorbic acid, which showed an increase in the different treatments, and also has functions similar to those of phenolic compounds. On the other hand, this behavior could be due to environmental or genetic crop factors [64].

Table 4.
Total phenol content and antioxidant activity (FRAP, DPPH and ABTS) in onion bulbs (Allium cepa L.) grown at different levels of available soil moisture and nitrogen fertilization.
3.5. Antioxidant Capacity
The potential of a substance or compound to inhibit or hinder the oxidation of a substrate is known as antioxidant capacity. There are two main mechanisms to carry this out: by electron transfer (SET) or by proton transfer (HAT). The test to obtain FRAP is about an SET electron transfer reaction based on the reduction of a yellow complex of ferric tripyridyl triazine or Fe3+-TPTZ (2,4,6-trypyridyls-triazine) to the blue-colored reduced ferrous complex Fe2+-TPTZ by an acid medium antioxidant [35]. According to the results, the reducing antioxidant power in onions also decreased with the different treatments compared to the control (Table 4). This could have been due to the decrease in the number of phenolic compounds, since there was a positive correlation between phenolic compounds and the FRAP (r2 = 0.8742), indicating that most of the reducing power was due to this type of compound. Pulido et al. [65] state that quercetin has a higher capacity than ascorbic acid to reduce Fe3+ in the FRAP test. The amounts obtained in the present research (66.8 µM TE g−1) were higher than those reported by Gorinstein et al. [66] in white onions (15.44 a 23.39 µM TE g−1).
On the other hand, DPPH and ABTS are synthetic free radicals that help us measure antioxidant activity known as antiradical activity. Free radicals are unstable molecules since they have one or more unpaired electrons, which can cause the oxidation of other molecules, thus inducing cellular damage and cellular death, which is why it is so important to study them [16,36]. In this research, onion bulb extracts showed an increase in the inhibition of both radicals in all the treatments studied compared to the control (Table 2). Even in ABTS, there was double the activity in the treatments at 25% of available moisture and 100 kg N ha−1. Phenolic compounds and ascorbic acid showed a high positive correlation in both radicals DPPH (r2 = 0.9124 and r2 = 0.8843, respectively) and ABTS (r2 = 0.9424 and r2 = 0.9043, respectively) indicating that both groups of compounds present in the onion bulb have an antiradical activity. Ibrahim et al. [67] mention that low levels of nitrogen fertilization could be related to antioxidant activities higher than those exposed to more favorable conditions due to the accumulation of polyphenolic compounds in vegetable tissues. This behavior was proven in the antiradical activity with DPPH and ABTS.
3.6. Erythroprotection Effect
Cellular membranes (such as those of erythrocytes) formed by phospholipids are widely susceptible to oxidation and damage by free radicals. For this reason, the ability of antioxidants present in onions to prevent hemolysis of erythrocytes was evaluated in this study [38]. The AAPH (2,2′-azobis(2-methylpropionamidine)) compound is an inductor of peroxyl (ROO•) radicals in the erythrocyte membrane causing lipidic peroxidation and, consequently, hemolysis. Therefore, in this study, onion bulb extracts were used to inhibit this hemolysis. Both in treatments with available soil moisture as well as those with nitrogen fertilization, there was an increase in the hemolysis percentage compared to the control (Figure 4). Treatments starting at 50% available moisture showed a hemolysis percentage higher than 90% (Figure 4A), whereas in conditions starting at 100 kg N ha−1, percentages higher than 85% were observed (Figure 4B). According to correlations conducted with ascorbic acid and the number of phenolic compounds with regard to the hemolysis percentage, we observed a higher correlation of both compounds (r2 = 0.9362 and r2 = 0.9643, respectively), thus indicating that both groups of compounds contribute to the protection of erythrocytes against free radicals.
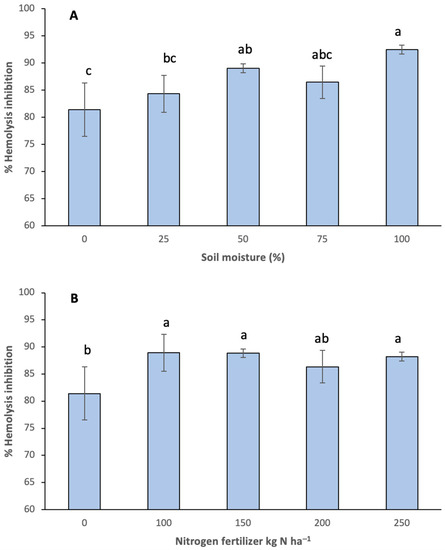
Figure 4.
Erythroprotective effect reported in percentage inhibition of hemolysis of onion bulb extracts from different (A) soil moistures and (B) nitrogen fertilization. Different letters indicate significant difference (p < 0.05). Media ± standard deviation of three repetitions.
In this context, with this technique, it is possible to also attribute an antioxidant capacity because it neutralizes peroxyl radicals. It is generally through the HAT mechanism because of the transfer of a hydrogen atom (H•) from a phenol (Ar-OH) or any other antioxidant compound of the radical ROO•, thus neutralizing this radical and avoiding hemolysis [14]. Among the phenolic compounds in onions (quercetin and its glucosides, kaempferol, isorhamnetin, myricetin, gallic acid, ferulic acid) [6,68], quercetin has a high antiradical activity and acts as a strong antioxidant due to its structural properties [69]. Therefore, onion bulb extracts with the different treatments generate compounds that may have erythroprotective properties.
3.7. Combined Variable Analysis with Principal Components
In the principal component analysis of the irrigation regime and nitrogen fertilization combination, it was found that 69% of the treatments variation is associated with the first two principal components (PC). PC1 contributed 55.48 % and PC2 with 13.72 %. The most important variables in PC1 were pH, dry matter (%), light (L), phenols and FRAP. In PC2, color parameters (a, h) and DPPH were the outstanding ones.
The treatments dispersion according to CP1 and CP2 (Figure 5), shows that in PC1 the treatment 1 (control: without water and nitrogen fertilizer) was outstanding in dry matter percentage, phenols production, FRAP and in color the luminosity. In descending order, treatments 2, 3, 4 and 5 were identified as those with the highest expression of the aforementioned variables. In these treatments, the minimum irrigation regime condition was maintained in combination with different nitrogen doses. In the rest of the treatments a similar behavior is maintained. Based on these results, it is evident that the minimum moisture condition has a greater impact on the physicochemical and bioactive levels in the onion.
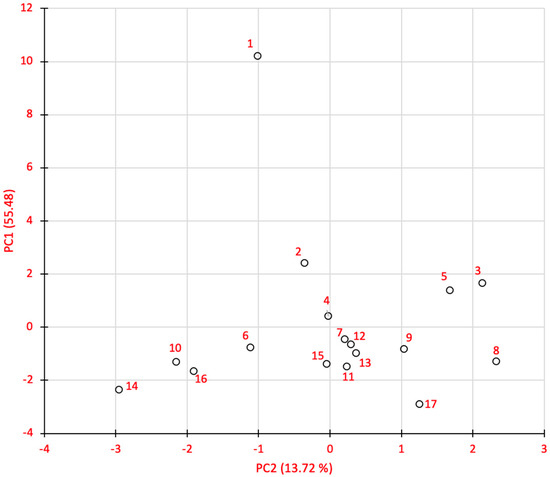
Figure 5.
Principal component analysis (PCA) of physical, chemical and bioactive characteristics of the onion bulb.
Regarding CP2, it is identified that treatments 1, 6, 10, 14 and 16, present numerically lower values in the attributes of color (a) and tonality (h). There is an intermediate group integrated by 2, 4, 7, 11, 12, 13 and 15. Another group that can be distinguished is the format for treatments 3, 5 and 8, which registered the highest values. The same behavior was observed in DPPH.
In the joint analysis of CP1 and CP2, treatments 3 and 5 were identified as the best for producing good quality onions in physicochemical and bioactive properties. In both treatments an irrigation regime of 25% available soil moisture and nitrogen fertilization of 150 and 250 kg ha−1, respectively are maintained. With these results it is evident that water stress condition improves the bulb quality, although the risk of inducing a reduction in yield due to water availability.
4. Conclusions
The factors available soil moisture and nitrogen fertilization mainly had an influence on the increase in titratable acidity, ascorbic acid and antioxidant capacity by DPPH and ABTS. In contrast, these factors influenced the reduction in pH, soluble solids, firmness, dry matter, total phenol levels and FRAP reducing power. Pungency was not affected by any of the treatments, leaving onions in a low-pungency classification, weak flavored and extra sweet. However, the extracts from the onion bulbs have a good erythroprotective effect and high antioxidant capacity to prevent lipidic peroxidation, which can benefit consumers, giving it added value, and with this property, they can prevent cellular damage and prevent chronic degenerative diseases. The treatments with 25% usable humidity and nitrogen fertilization of 150 and 250 kg ha−1, favored the physical, chemical and bioactive quality of the onion bulb.
Therefore, this study provides the necessary basis to continue carrying out and delving into more studies on the bioactive properties of onions, manipulating their composition and other factors besides soil moisture and nitrogen content such as lighting and some others. In general terms, most of the measured parameters were mainly fostered by low soil moisture (50%) and nitrogen (100 kg N ha−1) levels, which are a financially and environmentally feasible advantage for onion crops.
Author Contributions
Conceptualization, R.D.I.-G. and R.F.D.-M.; methodology, R.S.-O., Á.M.S.-H. and D.G.-M.; formal analysis, R.I.G.-V.; resources, J.D.-R. and A.M.G.-L.; writing—original draft preparation, S.M.B.-H.; supervision, C.L.D.-T.-S.; project administration, O.G.-J.; graphical abstract, C.L.D.-T.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was carried out in accordance with the Declaration of Helsinki of 1975. The clinical laboratory is accredited by ISO-IEC 17.025 (NMX-EC-17025) and ISO 15.189 prepared by the technical committee ISO/TC 212 (Clinical Laboratory Testing and In vitro Diagnostic Systems) taking as reference the ISO/IEC 17.025 and ISO 9001 standards.
Informed Consent Statement
All subjects gave their informed consent for inclusion in the study prior to their participation.
Data Availability Statement
The original contribution data presented in this research are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples are available from the corresponding authors.
References
- Mishra, P.; Sarkar, C.; Vishwajith, K.P.; Dhekale, B.S.; Sahu, P.K. Instability and forecasting using ARIMA model in area, production and productivity of onion in India. J. Crop Weed 2013, 9, 96–101. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). The State of Food and Agriculture. Available online: https://www.fao.org/3/ca6030en/ca6030en.pdf (accessed on 9 July 2019).
- Suleria, H.A.R.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66. [Google Scholar] [CrossRef]
- Alabi, K.P.; Olaniyan, A.M.; Odewole, M.M. Characteristics of onion under different process pretreatments and different drying conditions. J. Food Process. Technol. 2016, 7, 2. [Google Scholar]
- Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J. Flavonoids changes in fresh-cut onions during storage in different packaging systems. Food Chem. 2011, 124, 652–658. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; Regueiro, J.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Increasing the added-value of onions as a source of antioxidant flavonoids: A critical review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1050–1062. [Google Scholar] [CrossRef]
- Eltaweel, M. Assessment of antimicrobial activity of onion extract (Allium cepa) on Staphylococcus aureus; in vitro study. In Proceedings of the International Conference on Chemical, Agricultural and Medical Sciences. Int. J. Adv. Chem. Eng. Biol. Sci. 2013, 1, 29–30. [Google Scholar]
- Bystrická, J.; Musilová, J.; Vollmannová, A.; Timoracká, M.; Kavalcová, P. Bioactive components of onion (Allium cepa L.)—A Review. Acta Aliment. 2013, 42, 11–22. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.B.; Seo, W.D.; Kang, S.T.; Lim, J.W.; Cho, K.M. Comparative studies of antioxidant activities and nutritional constituents of persimmon juice (Diospyros kaki L. cv. Gapjubaekmok). Prev. Nutr. Food Sci. 2012, 17, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef]
- Razavi-Azarkhiavi, K.; Behravan, J.; Mosaffa, F.; Sehatbakhsh, S.; Shirani, K.; Karimi, G. Protective effects of aqueous and ethanol extracts of rosemary on H2O2-induced oxidative DNA damage in human lymphocytes by comet assay. J. Complement. Integr. Med. 2014, 11, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Mai, Y.; Li, H.; Wang, Z.; Xu, J.; He, X. Health benefits of the flavonoids from onion: Constituents and their pronounced antioxidant and anti-neuroinflammatory capacities. J. Agric. Food Chem. 2020, 68, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Ali, M.; Al-Rashdan, A.; Ahmed, N. Onion (Allium cepa L.) is potentially a good source of important antioxidants. J. Food Sci. Technol. 2019, 56, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoģlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [PubMed]
- Loredana, L.; Giuseppina, A.; Filomena, N.; Florinda, F.; Marisa, D.; Donatella, A. Biochemical, antioxidant properties and antimicrobial activity of different onion varieties in the Mediterranean area. J. Food Meas. Charact. 2019, 13, 1232–1241. [Google Scholar] [CrossRef]
- Manohar, C.M.; Xue, J.; Murayyan, A.; Neethirajan, S.; Shi, J. Antioxidant activity of polyphenols from Ontario grown onion varieties using pressurized low polarity water technology. J. Funct. Foods 2017, 31, 52–62. [Google Scholar] [CrossRef]
- Islam, S.; Khar, A.; Singh, S.; Tomar, B.S. Variability, heritability and trait association studies for bulb and antioxidant traits in onion (Allium cepa L.) varieties. Indian J. Agric. Sci. 2019, 89, 450–457. [Google Scholar] [CrossRef]
- Yang, S.J.; Paudel, P.; Shrestha, S.; Seong, S.H.; Jung, H.A.; Choi, J.S. In vitro protein tyrosine phosphatase 1B inhibition and antioxidant property of different onion peel cultivars: A comparative study. Food Sci. Nutr. 2019, 7, 205–215. [Google Scholar] [CrossRef]
- Toscano-Sagar, N.A.; Pareek, S.; González-Aguilar, G.A. Cuantificación de flavonoides, fenoles totales y propiedades antioxidantes de la piel de cebolla: Un estudio comparativo de quince cultivares indios. Rev. Cienc. Tecnol. Aliment. 2020, 57, 2423–2432. [Google Scholar]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Piri, H.; Naserin, A. Effect of different levels of water, applied nitrogen and irrigation methods on yield, yield components and IWUE of onion. Sci. Hortic. 2020, 268, 109361. [Google Scholar] [CrossRef]
- Mallor, G.C.; Carravedo, F.M.; Estopañán, M.G.; Mallor, G.F. Characterization of genetic resources of onion (Allium cepa L.) from the Spanish secondary centre of diversity. Span. J. Agric. Res. 2011, 9, 144–155. [Google Scholar] [CrossRef]
- Agnieszka, S.; Robert, P.; Del, V.L.; Silvano, S.; Gianluca, C. Interactions among genotype, environment and agronomic practices on production and quality of storage onion (Allium cepa L.)–A review. Hortic. Sci. 2017, 44, 21–42. [Google Scholar]
- Kimura, Y.; Okazaki, K.; Yanagida, D.; Muro, T. Cultivar and regional diferences in the metabolite composition of onion (Allium cepa L.). Sci. Hortic. 2014, 168, 1–8. [Google Scholar] [CrossRef]
- Galdon, B.R.; Rodriguez, C.T.; Rodriguez, E.R.; Ronero, C.D. Organic acid contents in onion cultivars (Allium cepa L.). J. Agric. Food Chem. 2008, 56, 6512–6519. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Instituto de Geografía–UNAM: Ciudad de México, Mexico, 1988. [Google Scholar]
- Association of Official Analytical Chemists-AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists-AOAC: Gaithersburg Montgomery, MD, USA, 1998; p. 23. [Google Scholar]
- Petropoulos, S.A.; Ntatsi, G.; Fernandes, Â.; Barros, L.; Barreira, J.C.M.; Ferreira, I.C.; Antoniadis, V. Long-term storage effect on chemical composition, nutritional value and quality of Greek onion landrace “Vatikiotiko”. Food Chem. 2016, 201, 168–176. [Google Scholar] [CrossRef]
- Coca, A.; Carranza, C.E.; Miranda, D.; Rodríguez, M.H. Efecto del NaCl sobre los parámetros de crecimiento, rendimiento y calidad de la cebolla de bulbo (Allium cepa L.) bajo condiciones controladas. Rev. Colomb. Cienc. Hortícolas 2012, 6, 196–212. [Google Scholar]
- Anthon, G.E.; Barret, M. Modified method for the determination of pyruvic acid with dinitrophenyl hydrazine in the assessment of onion pungency. J. Sci. Food Agric. 2003, 83, 1210–1213. [Google Scholar] [CrossRef]
- Downes, K.; Chope, G.A.; Terry, L.A. Effect of curing at different temperatures on biochemical composition of onion (Allium cepa L.) skin from three freshly cured and cold stored UK-grown onion cultivars. Post Harv. Biol. Technol. 2009, 54, 80–86. [Google Scholar] [CrossRef]
- Eldeen, I.M.S.; Seow, E.M.; Abdullah, R.; Sulaiman, S.F. In vitro antibacterial, antioxidant, total phenolic contents and anti-HIV-1 reverse transcriptase activities of extracts of seven Phyllanthus sp. S. Afr. J. Bot. 2011, 77, 75–79. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable radical dipheylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
- NOM-253-SSA1-2012. Norma Oficial Mexicana, for the Disposal of Human Blood and Its Components for Therapeutic Purposes. Available online: http://www.cnts.salud.gob.mx/descargas/NOM-253-SSA1-2012.pdf (accessed on 26 October 2020).
- Lu, J.; Jin, Y.; Liu, G.; Zhu, N.; Gui, M.; Yu, A.; Li, X. Flavonoids from the leaves of Actinidia kolomikta. Chem. Nat. Compd. 2010, 46, 205–208. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Kumar, S.; Khapte, P.S.; Meena, K.K.; Rane, J.; Pathak, H. Quantification of water stress impacts on canopy traits, yield, quality and water productivity of onion (Allium cepa L.) cultivars in a shallow basaltic soil of water scarce zone. Agric. Water Manag. 2021, 249, 106824. [Google Scholar] [CrossRef]
- Venâncio, J.B.; da Silva Dias, N.; de Medeiros, J.F.; de Moraes, P.L.D.; do Nascimento, C.W.A.; de Sousa Neto, O.N.; da Silva Sá, F.V. Yield and morphophysiology of onion grown under salinity and fertilization with silicon. Sci. Hortic. 2022, 301, 111095. [Google Scholar] [CrossRef]
- Klunklin, W.; Savage, G. Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods 2017, 6, 56. [Google Scholar] [CrossRef]
- Golubkina, N.; Caruso, G. Nutritional Composition and Antioxidant Properties of Fruits and Vegetables, 1st ed.; Jaiswal, A.K., Ed.; Academic Press: London, UK, 2020; Volume 1, pp. 73–87. [Google Scholar]
- Pejic, B.; Gvozdanovi, J.; Mili, S.; Ignjatovi, A.; Krsti, D. Effect of irrigation schedules on yield and water use of onion (Allium cepa L.). Afr. J. Biotechnol. 2011, 10, 2644–2652. [Google Scholar]
- Ghodke, P.H.; Andhale, P.S.; Gijare, U.M.; Thangasamy, A.; Khade, Y.P.; Mahajan, V.; Singh, M. Physiological and biochemical responses in onion crop to drought stress. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2054–2062. [Google Scholar] [CrossRef]
- Yoo, K.S.; Lee, E.J.; Patil, B.S. Changes in flavor precursors, pungency, and sugar content in short-day onion bulbs during 5-month storage at various temperatures or in controlled atmospheres. J. Food Sci. 2012, 77, 216–221. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; Vega-Garcia, M.O.; Guevara-Aguilar, A.; Sánchez, E.; Alvarado-González, M.; Flores-Córdova, M.A. Effect of prolonged storage in controlled atmospheres on the conservation of the onion (Allium cepa L.) quality. Emir. J. Food Agric. 2016, 28, 842–852. [Google Scholar] [CrossRef]
- Hanci, F.; Cebeci, E. Improvement of abiotic stress tolerance in onion: Selection studies under salinity conditions. Int. J. Eng. Sci. 2019, 7, 54–58. [Google Scholar]
- Almaroai, Y.A.; Eissa, M.A. Role of marine algae extracts in water stress resistance of onion under semiarid conditions. J. Soil Sci. Plant Nutr. 2020, 20, 1092–1101. [Google Scholar] [CrossRef]
- Eissa, M.A.; Roshdy, N.M.K. Nitrogen fertilization: Effect on Cd-phytoextraction by the halophytic plant quail bush [Atriplex lentiformis (Torr.) S. Wats]. S. Afr. J. Bot. 2018, 115, 126–131. [Google Scholar] [CrossRef]
- Randle, W.M. Increasing nitrogen concentration in hydroponic solutions affects onion flavor and bulb quality. J. Am. Soc. Hort. Sci. 2000, 125, 254–259. [Google Scholar] [CrossRef]
- Di Miceli, G.; Farruggia, D.; Iacuzzi, N.; Bacarella, S.; La Bella, S.; Consentino, B.B. Planting date and different n-fertilization rates differently modulate agronomic and economic traits of a sicilian onion landrace and of a commercial variety. Horticulturae 2022, 8, 454. [Google Scholar] [CrossRef]
- Fatideh, M.M.; Asil, M.H. Onion yield, quality and storability as affected with different soil moisture and nitrogen regimes. South West. J. Hortic. Biol. Environ. 2012, 3, 145–165. [Google Scholar]
- Abdelkhalik, A.; Pascual, B.; Najera, I.; Baixauli, C.; Pascual, S.N. Regulated deficit irrigation as a water-saving strategy for onion cultivation in Mediterranean Conditions. Agronomy 2019, 9, 521. [Google Scholar] [CrossRef]
- Khokhar, K.M. Mineral nutrient management for onion bulb crops—A review. J. Hortic. Sci. Biotechnol. 2019, 94, 703–717. [Google Scholar] [CrossRef]
- Abdissa, Y.; Tekalign, T.; Pant, L.M. Growth, bulb yield and quality of onion (Allium cepa L.) as influenced by nitrogen and phosphorus fertilization on vertisol I. growth attributes, biomass production and bulb yield. Afr. J. Agric. Res. 2011, 6, 3252–3258. [Google Scholar]
- Gonçalves, F.D.C.; Grangeiro, L.C.; de Sousa, V.D.F.; Santos, J.P.D.; Souza, F.I.D.; da Silva, L.R. Yield and quality of densely cultivated onion cultivars as function of nitrogen fertilization. Rev. Bras. Eng. Agricola Ambient. 2019, 23, 847–851. [Google Scholar] [CrossRef]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of deficit irrigation on biomass, yield, water productivity and fruit quality of processing tomato under semi-arid Mediterranean climate conditions. Sci. Hortic. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Wichrowska, D.; Wojdyła, T.; Rolbiecki, S.; Rolbiecki, R.; Czop, P.; Jagosz, B.; Ptach, W. Effect of nitrogen fertilization on the marketable yield and nutritive value of onion. Acta Sci. Pol. Hortorum Cultus 2017, 16, 125–133. [Google Scholar] [CrossRef]
- Golubkina, N.; Amalfitano, C.; Sekara, A.; Tallarita, A.; Pokluda, R.; Stoleru, V.; Cuciniello, A.; Agafonov, A.F.; Kalisz, A.; Hamburdă, S.B.; et al. Yield and bulb quality of storage onion cultivars as affected by farming system and nitrogen dose. Sci. Hortic. 2022, 293, 110751. [Google Scholar] [CrossRef]
- Ncayiyana, M.; Bertling, I.; Maboko, M.M. Yield and nutritional quality of different short-day onion cultivars as affected by nitrogen application. S. Afr. J. Plant Soil 2018, 35, 215–221. [Google Scholar] [CrossRef]
- Cheng, A.; Chen, X.; Jin, Q.; Wang, W.; Shi, J.; Liu, Y. Comparison of phenolic content and antioxidant capacity of red and yellow onions. Czech J. Food Sci. 2013, 31, 501–508. [Google Scholar] [CrossRef]
- López-Martínez, L.X.; Aguilar-Cisneros, L.M.; Dublán-García, O. Actividad antioxidante e inhibidora de α-glucosidasa y α-amilasa de tres variedades de cebolla (Allium cepa L.). Nova Scientia 2014, 6, 234–347. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Drzewie, M.; Martincová, O.; Katrich, E.; Trakhtenberg, S. Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols. J. Agric. Food Chem. 2008, 56, 4418–4426. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z.; Rahmat, A.; Rahman, Z.A. Involvement of nitrogen on flavonoids, glutathione, anthocyanin, ascorbic acid and antioxidant activities of Malaysian medicinal plant Labisia pumila Blume (Kacip Fatimah). Int. J. Mol. Sci. 2012, 13, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent advances in bioactive compounds, health functions, and safety concerns of onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef] [PubMed]
- Brglez, M.E.; Knez, H.M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).