Reactive Oxygen Species Metabolism Modulation on the Quality of Apple Fruits Inoculated with Penicillium expansum under Different Ambient pHs
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Inoculum with Different pH Values
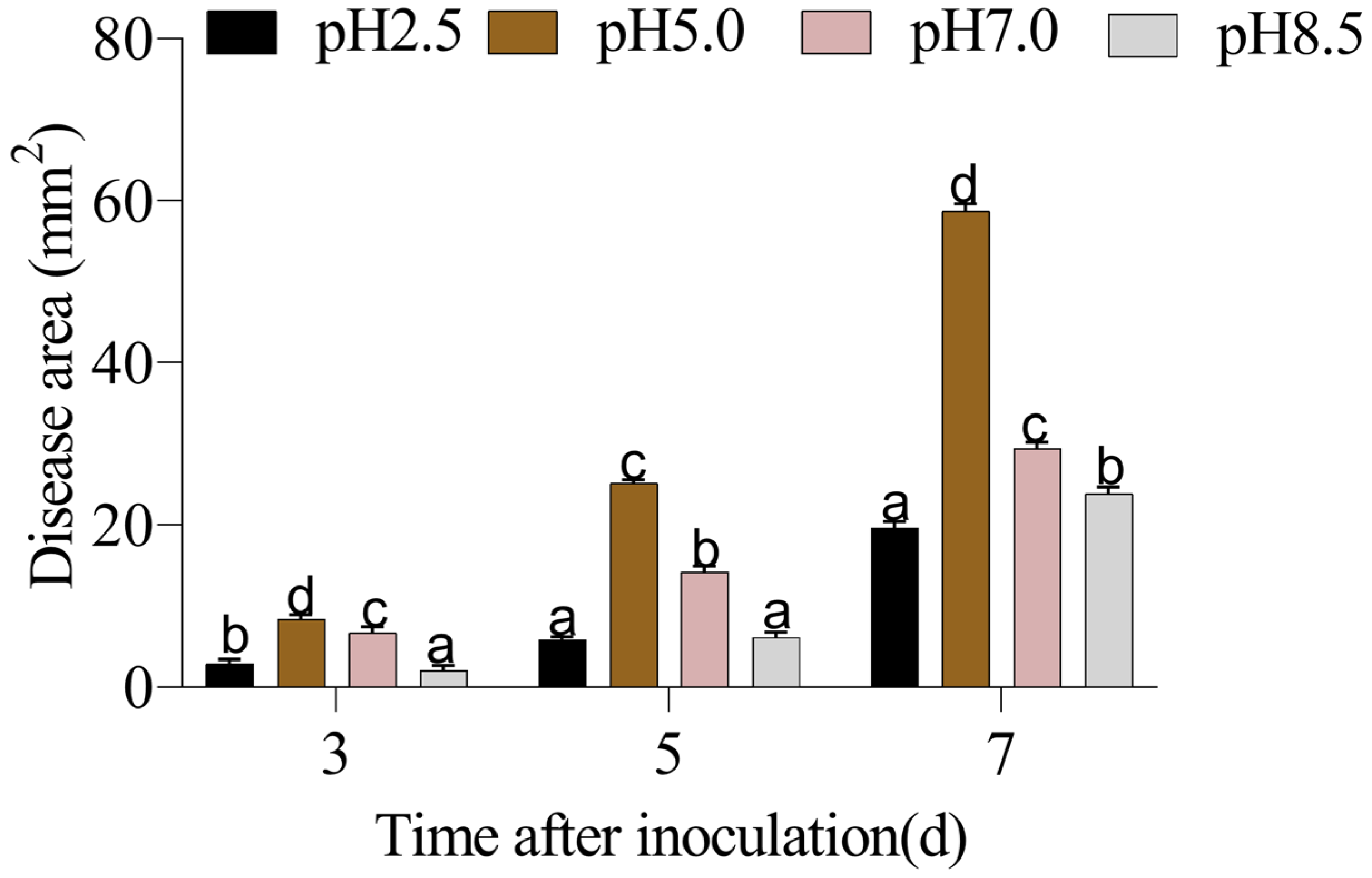
2.2. Fruit Inoculation, Measurement of Disease Area, and Sampling
2.3. Determination of Quality Parameters of Apple Fruits Inoculated with P. expansum under Different Ambient pHs
2.4. The Generation of Superoxide Anion (O2•−) and Hydrogen Peroxide (H2O2)
2.5. Cell Membrane Integrity and Malondialdehyde Content
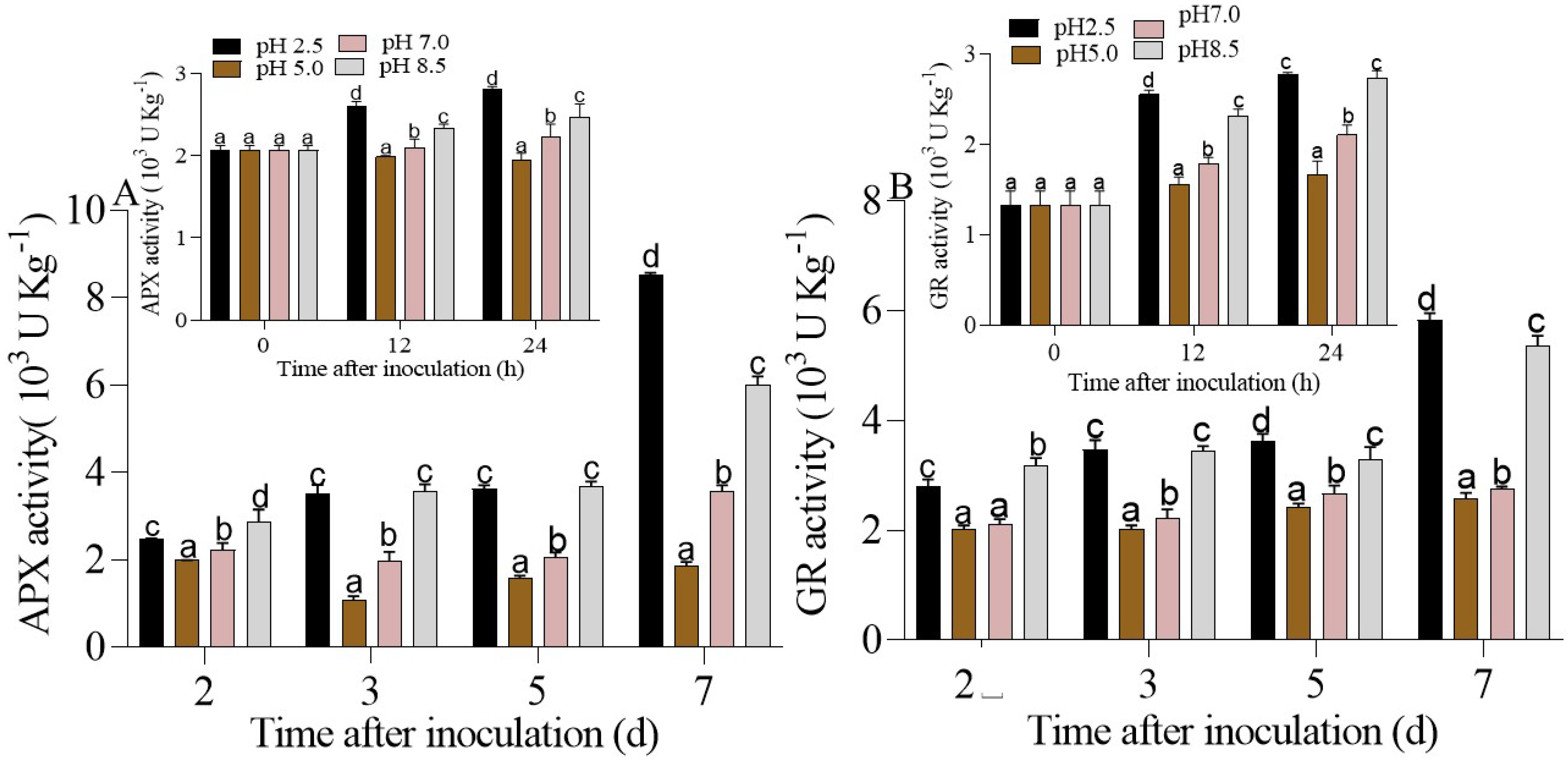
2.6. Enzymatic Activity Assay
2.6.1. Determination of Activities of Enzymes Involved in ROS Production
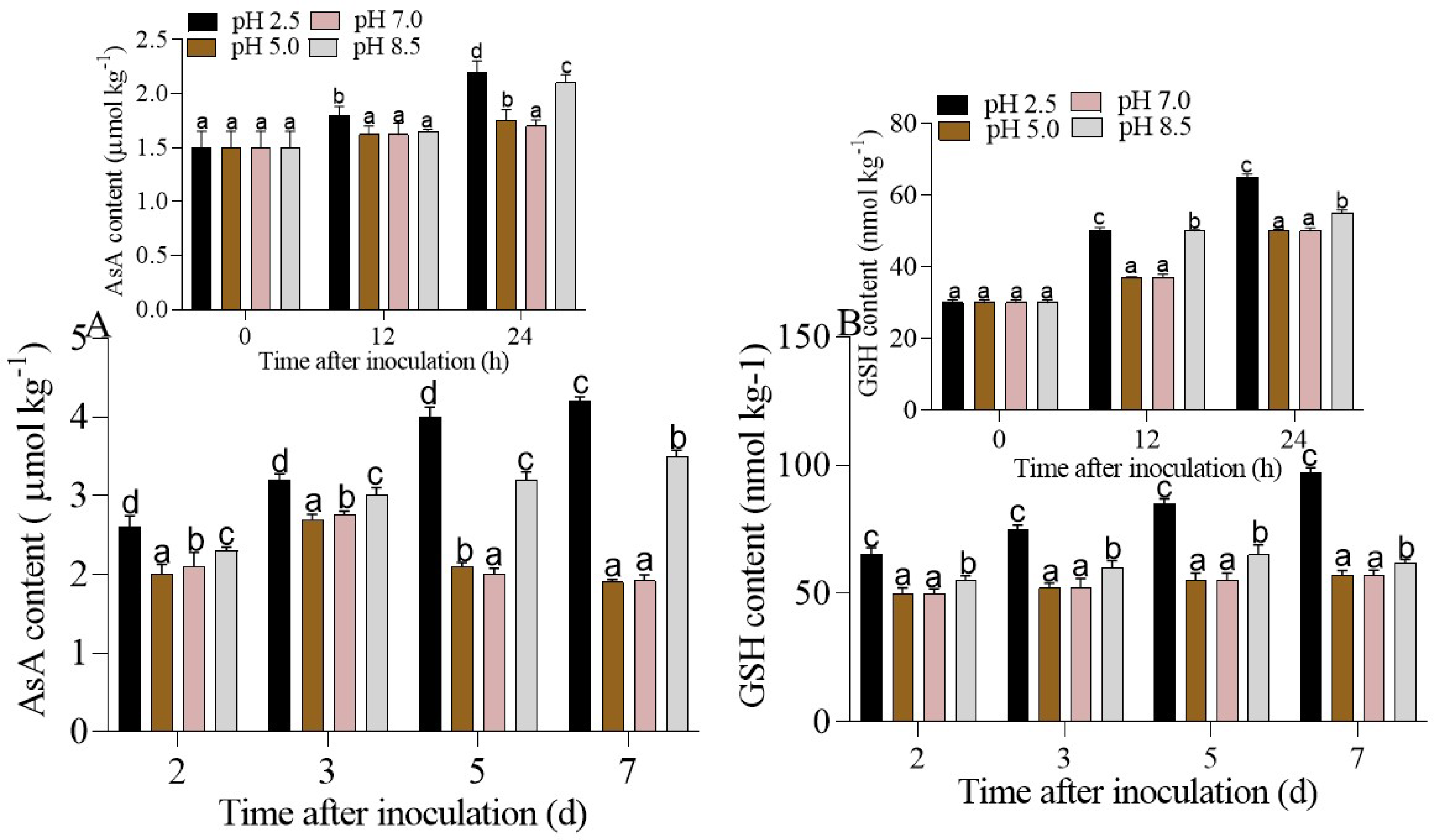
2.6.2. Determination of Key Enzyme Activities Associated with AsA-GSH Cycle
2.6.3. Determination of AsA and GSH Contents
2.7. Statistical Analysis
3. Results
3.1. The Quality of P. expansum-Inoculated Apple Fruit Was Affected by Different Ambient pHs
3.2. Superoxide Anion (O2•−) and Hydrogen Peroxide (H2O2) Production in Apple Fruits Inoculated with P. expansum under Different Ambient pHs
3.3. Cell Membrane Integrity and MDA Content in P. expansum-Inoculated Apple Fruit under Different Ambient pHs
3.4. Regulation of Enzymatic Antioxidant Activities in P. expansum-Inoculated Apple Fruits under Different Ambient pHs
3.4.1. NOX, SOD, POD, CAT Regulation in P. expansum-Inoculated Apples Fruits at Different Ambient pHs
3.4.2. Regulation of the AsA-GSH cycle in P. expansum-Inoculated Apple Fruits under Different Ambient pHs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumari, M.; Kamat, S.; Dixit, R.; Pandey, S.; Giri, V.P.; Mishra, A. Microbial Formulation Approaches in Postharvest Disease Management. In Food Security and Plant Disease Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 279–305. ISBN 978-0-12-821843-3. [Google Scholar]
- Prusky, D.; Barad, S.; Luria, N.; Ment, D. PH Modulation of Host Environment, a Mechanism Modulating Fungal Attack in Postharvest Pathogen Interactions. In Post-Harvest Pathology; Prusky, D., Gullino, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 11–25. ISBN 978-3-319-07700-0. [Google Scholar]
- Prusky, D.; Yakoby, N. Pathogenic Fungi: Leading or Led by Ambient PH?: Pathogenic Fungi: Leading or Led by Ambient PH? Mol. Plant Pathol. 2003, 4, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Manteau, S.; Abouna, S.; Lambert, B.; Legendre, L. Differential Regulation by Ambient PH of Putative Virulence Factor Secretion by the Phytopathogenic Fungus Botrytis Cinerea. FEMS Microbiol. Ecol. 2003, 43, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit–Pathogen Interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S. Environmental PH Modulation by Pathogenic Fungi as a Strategy to Conquer the Host. PLoS Pathog. 2017, 13, e1006149. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.L.; Leverentz, B.; Conway, W.S. Local Modulation of Host PH by Colletotrichum Species as a Mechanism to Increase Virulence. Mol. Plant-Microbe Interact. 2001, 14, 1105–1113. [Google Scholar] [CrossRef]
- Alkan, N.; Espeso, E.A.; Prusky, D. Virulence Regulation of Phytopathogenic Fungi by PH. Antioxid. Redox Signal. 2013, 19, 1012–1025. [Google Scholar] [CrossRef]
- Moss, M.O. Fungi, Quality and Safety Issues in Fresh Fruits and Vegetables. J. Appl. Microbiol. 2008, 104, 1239–1243. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Primary Keys and Miscellaneous Fungi. In Fungi and Food Spoilage; Springer: Boston, MA, USA, 1997; pp. 59–171. ISBN 978-1-4613-7936-2. [Google Scholar]
- Snowdon, A.L. 1: General Introduction and Fruits. In A Colour Atlas of Post-Harvest Diseases and Disorders of Fruits and Vegetables; Wolfe Scientific: London, UK, 1990; ISBN 978-0-7234-0931-1. [Google Scholar]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial Spoilage of Vegetables, Fruits and Cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive Oxygen Species in Phytopathogenic Fungi: Signaling, Development, and Disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef]
- Pilati, S.; Brazzale, D.; Guella, G.; Milli, A.; Ruberti, C.; Biasioli, F.; Zottini, M.; Moser, C. The Onset of Grapevine Berry Ripening Is Characterized by ROS Accumulation and Lipoxygenase-Mediated Membrane Peroxidation in the Skin. BMC Plant Biol. 2014, 14, 87. [Google Scholar] [CrossRef]
- Tian, S.; Torres, R.; Ballester, A.-R.; Li, B.; Vilanova, L.; González-Candelas, L. Molecular Aspects in Pathogen-Fruit Interactions: Virulence and Resistance. Postharvest Biol. Technol. 2016, 122, 11–21. [Google Scholar] [CrossRef]
- Maheshwari, R.; Dubey, R.S. Nickel-Induced Oxidative Stress and the Role of Antioxidant Defence in Rice Seedlings. Plant Growth Regul. 2009, 59, 37–49. [Google Scholar] [CrossRef]
- Overmyer, K.; Brosché, M.; Kangasjärvi, J. Reactive Oxygen Species and Hormonal Control of Cell Death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef]
- Srivastava, S.; Dubey, R.S. Manganese-Excess Induces Oxidative Stress, Lowers the Pool of Antioxidants and Elevates Activities of Key Antioxidative Enzymes in Rice Seedlings. Plant Growth Regul. 2011, 64, 1–16. [Google Scholar] [CrossRef]
- Racchi, M. Antioxidant Defenses in Plants with Attention to Prunus and Citrus Spp. Antioxidants 2013, 2, 340–369. [Google Scholar] [CrossRef]
- Alkan, N.; Davydov, O.; Sagi, M.; Fluhr, R.; Prusky, D. Ammonium Secretion by Colletotrichum Coccodes Activates Host NADPH Oxidase Activity Enhancing Host Cell Death and Fungal Virulence in Tomato Fruits. Mol. Plant-Microbe Interact. 2009, 22, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, Z.; Bi, Y.; Zong, Y.; Gong, D.; Wang, B.; Li, B.; Sionov, E.; Prusky, D. The Effect of Environmental PH during Trichothecium Roseum (Pers.:Fr.) Link Inoculation of Apple Fruits on the Host Differential Reactive Oxygen Species Metabolism. Antioxidants 2021, 10, 692. [Google Scholar] [CrossRef]
- Liu, D.; Liu, M.; Liu, X.-L.; Cheng, X.-G.; Liang, Z.-W. Silicon Priming Created an Enhanced Tolerance in Alfalfa (Medicago sativa L.) Seedlings in Response to High Alkaline Stress. Front. Plant Sci. 2018, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Hussain, M.; Irshad, M.; Mitra, S.; Li, M.; Liu, S.; Qiu, D. Exogenous Melatonin Mitigates Acid Rain Stress to Tomato Plants through Modulation of Leaf Ultrastructure, Photosynthesis and Antioxidant Potential. Molecules 2018, 23, 388. [Google Scholar] [CrossRef]
- Ju, S.; Yin, N.; Wang, L.; Zhang, C.; Wang, Y. Effects of Silicon on Oryza Sativa L. Seedling Roots under Simulated Acid Rain Stress. PLoS ONE 2017, 12, e0173378. [Google Scholar] [CrossRef]
- Luo, S.; Wan, B.; Feng, S.; Shao, Y. Biocontrol of Postharvest Anthracnose of Mango Fruit with Debaryomyces Nepalensis and Effects on Storage Quality and Postharvest Physiology: Biocontrol of Mango with D. Nepalensis. J. Food Sci. 2015, 80, M2555–M2563. [Google Scholar] [CrossRef]
- Hossain, M.A.; Rana, M.M.; Kimura, Y.; Roslan, H.A. Changes in Biochemical Characteristics and Activities of Ripening Associated Enzymes in Mango Fruit during the Storage at Different Temperatures. BioMed Res. Int. 2014, 2014, 232969. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, E.; Gasser, F.; Guggenbühl, B.; Künsch, U. Efficacy of Instrumental Measurements for Determination of Minimum Requirements of Firmness, Soluble Solids, and Acidity of Several Apple Varieties in Comparison to Consumer Expectations. Postharvest Biol. Technol. 2003, 27, 27–37. [Google Scholar] [CrossRef]
- Bao, G.; Bi, Y.; Li, Y.; Kou, Z.; Hu, L.; Ge, Y.; Wang, Y.; Wang, D. Overproduction of Reactive Oxygen Species Involved in the Pathogenicity of Fusarium in Potato Tubers. Physiol. Mol. Plant Pathol. 2014, 86, 35–42. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of Hydrogen Peroxide in Plant Extracts Using Titanium(IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Li, C.; Wang, B.; Ma, L.; Ren, Y.; Bi, Y.; Li, Y.; Xue, H.; Prusky, D. The Effect of Benzo-(1,2,3)-Thiadiazole-7-Carbothioic Acid S-Methyl Ester (BTH) Treatment on Regulation of Reactive Oxygen Species Metabolism Involved in Wound Healing of Potato Tubers during Postharvest. Food Chem. 2020, 309, 125608. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Bi, Y.; Ge, Y.; Wang, Y.; Fan, C.; Li, D.; Deng, H. Postharvest BTH Treatment Induced Disease Resistance and Enhanced Reactive Oxygen Species Metabolism in Muskmelon (Cucumis melo L.) Fruit. Eur. Food Res. Technol. 2012, 234, 963–971. [Google Scholar] [CrossRef]
- Chumyam, A.; Shank, L.; Faiyue, B.; Uthaibutra, J.; Saengnil, K. Effects of Chlorine Dioxide Fumigation on Redox Balancing Potential of Antioxidative Ascorbate-Glutathione Cycle in ‘Daw’ Longan Fruit during Storage. Sci. Hortic. 2017, 222, 76–83. [Google Scholar] [CrossRef]
- Qin, G.; Liu, J.; Cao, B.; Li, B.; Tian, S. Hydrogen Peroxide Acts on Sensitive Mitochondrial Proteins to Induce Death of a Fungal Pathogen Revealed by Proteomic Analysis. PLoS ONE 2011, 6, e21945. [Google Scholar] [CrossRef]
- Xue, H.; Sun, Y.; Li, L.; Bi, Y.; Hussain, R.; Zhang, R.; Long, H.; Nan, M.; Pu, L. Acetylsalicylic Acid (ASA) Induced Fusarium Rot Resistance and Suppressed Neosolaniol Production by Elevation of ROS Metabolism in Muskmelon Fruit. Sci. Hortic. 2020, 265, 109264. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Qiao, F.; Zhang, X.-M.; Liu, X.; Chen, J.; Hu, W.-J.; Liu, T.-W.; Liu, J.-Y.; Zhu, C.-Q.; Ghoto, K.; Zhu, X.-Y.; et al. Elevated Nitrogen Metabolism and Nitric Oxide Production Are Involved in Arabidopsis Resistance to Acid Rain. Plant Physiol. Biochem. 2018, 127, 238–247. [Google Scholar] [CrossRef]
- Bhuyan, M.H.M.B.; Hasanuzzaman, M.; Mahmud, J.A.; Hossain, M.S.; Alam, M.U.; Fujita, M. Explicating Physiological and Biochemical Responses of Wheat Cultivars under Acidity Stress: Insight into the Antioxidant Defense and Glyoxalase Systems. Physiol. Mol. Biol. Plants 2019, 25, 865–879. [Google Scholar] [CrossRef]
- Jimdjio, C.K.; Xue, H.; Bi, Y.; Nan, M.; Li, L.; Zhang, R.; Liu, Q.; Pu, L. Effect of Ambient PH on Growth, Pathogenicity, and Patulin Production of Penicillium Expansum. Toxins 2021, 13, 550. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Tanou, G.; Molassiotis, A.; Diamantidis, G. Induction of Reactive Oxygen Species and Necrotic Death-like Destruction in Strawberry Leaves by Salinity. Environ. Exp. Bot. 2009, 65, 270–281. [Google Scholar] [CrossRef]
- Yan, J.; Tsuichihara, N.; Etoh, T.; Iwai, S. Reactive Oxygen Species and Nitric Oxide Are Involved in ABA Inhibition of Stomatal Opening. Plant Cell Environ. 2007, 30, 1320–1325. [Google Scholar] [CrossRef]
- Majumdar, A.; Kar, R.K. Orchestration of Cu-Zn SOD and Class III Peroxidase with Upstream Interplay between NADPH Oxidase and PM H+-ATPase Mediates Root Growth in Vigna Radiata (L.) Wilczek. J. Plant Physiol. 2019, 232, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant Systems and O2 ·−/H2O2 Production in the Apoplast of Pea Leaves. Its Relation with Salt-Induced Necrotic Lesions in Minor Veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Drought Induces Oxidative Stress and Enhances the Activities of Antioxidant Enzymes in Growing Rice Seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Shanan, N. Optimum PH Value for Improving Postharvest Characteristics and Extending Vase Life of Rosa Hybrida Cv. Tereasa Cut Flowers. Asian J. Adv. Agric. Res. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Cao, B.; Xu, K. Low PH Altered Salt Stress in Antioxidant Metabolism and Nitrogen Assimilation in Ginger (Zingiber officinale) Seedlings. Physiol. Plant. 2020, 168, 648–659. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Bhuyan, M.; Hasanuzzaman, M.; Mahmud, J.; Hossain, M.; Bhuiyan, T.; Fujita, M. Unraveling Morphophysiological and Biochemical Responses of Triticum aestivum L. to Extreme PH: Coordinated Actions of Antioxidant Defense and Glyoxalase Systems. Plants 2019, 8, 24. [Google Scholar] [CrossRef]
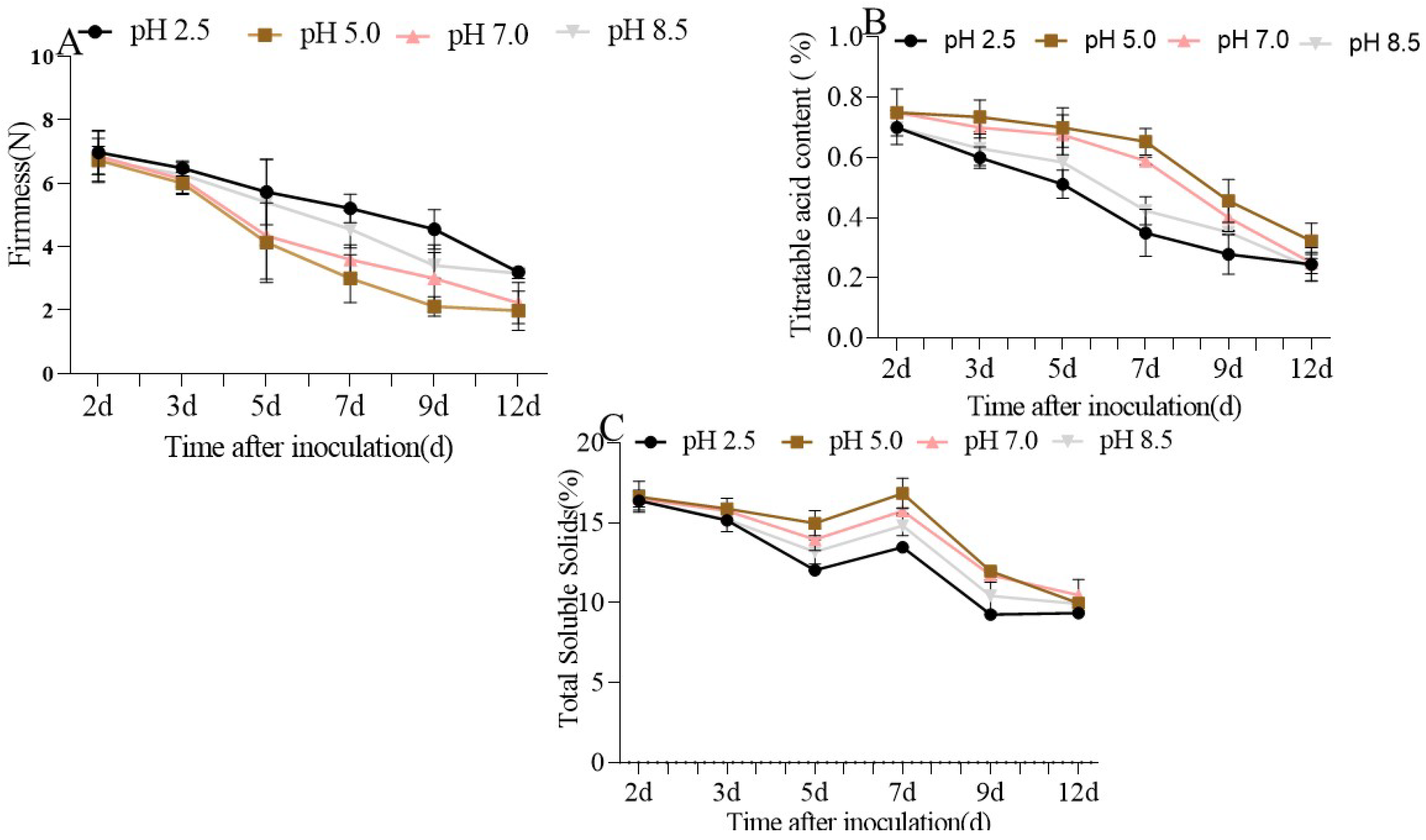





| 2 d | 3 d | 5 d | 7 d | 9 d | 12 d | |
|---|---|---|---|---|---|---|
| pH 2.5 | 6.97 (±0.69) | 6.47 (±023) | 5.72 (±1.0) | 5.21 (±0.45) | 4.55 (±0.62) | 3.20 (±0.12) |
| pH 5.0 | 6.72 (±0.69) | 6.00 (±0.35) | 4.13 (±1.2) | 3.00 (±0.75) | 2.12 (±0.30) | 1.99 (±0.61) |
| pH 7.0 | 6.85 (±0.79) | 6.12 (±0.35) | 4.34 (±1.36) | 3.59 (±0.46) | 3.00 (±1.06) | 2.22 (±0.64) |
| pH 8.5 | 6.72 (±0.43) | 6.27 (±0.37) | 5.4 (±1.35) | 4.55 (±0.59) | 3.42 (±0.4) | 3.16 (±0.16) |
| 2 d | 3 d | 5 d | 7 d | 9 d | 12 d | |
|---|---|---|---|---|---|---|
| pH 2.5 | 0.70 (±0.01) | 0.60 (±0.04) | 0.51 (±0.05) | 0.35 (±0.08) | 0.27 (±0.07) | 0.24 (±0.06) |
| pH 5.0 | 0.75 (±0.01) | 0.73 (±0.06) | 0.70 (±0.07) | 0.73 (±0.06) | 0.65 (±0.04) | 0.32 (±0.06) |
| pH 7.0 | 0.75 (±0.08) | 0.70 (±0.04) | 0.67 (±0.07) | 0.58 (±0.01) | 0.40 (±0.04) | 0.25 (±0.04) |
| pH 8.5 | 0.70 (±0.06) | 0.63 (±0.06) | 0.58 (±0.08) | 0.42 (±0.05) | 0.35 (±0.01) | 0.23 (±0.04) |
| 2 d | 3 d | 5 d | 7 d | 9 d | 12 d | |
|---|---|---|---|---|---|---|
| pH 2.5 | 16.38 (±0.07) | 15.16 (±0.04) | 12.03 (±0.04) | 13.48 (±0.04) | 9.26 (±0.08) | 9.35 (±0.07) |
| pH 5.0 | 16.63 (±0.97) | 15.88 (±0.66) | 14.98 (±0.78) | 16.3884 (±0.94) | 11.98 (±0.09) | 9.98 (±0.06) |
| pH 7.0 | 16.51 (±0.51) | 15.76 (±0.08) | 13.95 (±0.67) | 15.75 (±0.18) | 11.73 (±0.44) | 10.48 (±0.98) |
| pH 8.5 | 16.42 (±0.63) | 15.22 (±0.78) | 13.19 (±0.75) | 14.82 (±0.62) | 10.43 (±0.98) | 9.93 (±0.18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimdjio Kouasseu, C.; Yang, X.; Xue, H.; Bi, Y.; Liu, Z.; Xi, J.; Nan, M.; Prusky, D. Reactive Oxygen Species Metabolism Modulation on the Quality of Apple Fruits Inoculated with Penicillium expansum under Different Ambient pHs. Horticulturae 2023, 9, 538. https://doi.org/10.3390/horticulturae9050538
Jimdjio Kouasseu C, Yang X, Xue H, Bi Y, Liu Z, Xi J, Nan M, Prusky D. Reactive Oxygen Species Metabolism Modulation on the Quality of Apple Fruits Inoculated with Penicillium expansum under Different Ambient pHs. Horticulturae. 2023; 9(5):538. https://doi.org/10.3390/horticulturae9050538
Chicago/Turabian StyleJimdjio Kouasseu, Carelle, Xi Yang, Huali Xue, Yang Bi, Zhiguang Liu, Jihui Xi, Mina Nan, and Dov Prusky. 2023. "Reactive Oxygen Species Metabolism Modulation on the Quality of Apple Fruits Inoculated with Penicillium expansum under Different Ambient pHs" Horticulturae 9, no. 5: 538. https://doi.org/10.3390/horticulturae9050538
APA StyleJimdjio Kouasseu, C., Yang, X., Xue, H., Bi, Y., Liu, Z., Xi, J., Nan, M., & Prusky, D. (2023). Reactive Oxygen Species Metabolism Modulation on the Quality of Apple Fruits Inoculated with Penicillium expansum under Different Ambient pHs. Horticulturae, 9(5), 538. https://doi.org/10.3390/horticulturae9050538







