Isolating, Identifying, and Analyzing the Biological Characteristics of Pathogens Causing Postharvest Disease in Fresh Radix Astragali
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Purification
2.2. Morphological Observation
2.3. Molecular Biological Identification
2.3.1. Genomic DNA Extraction
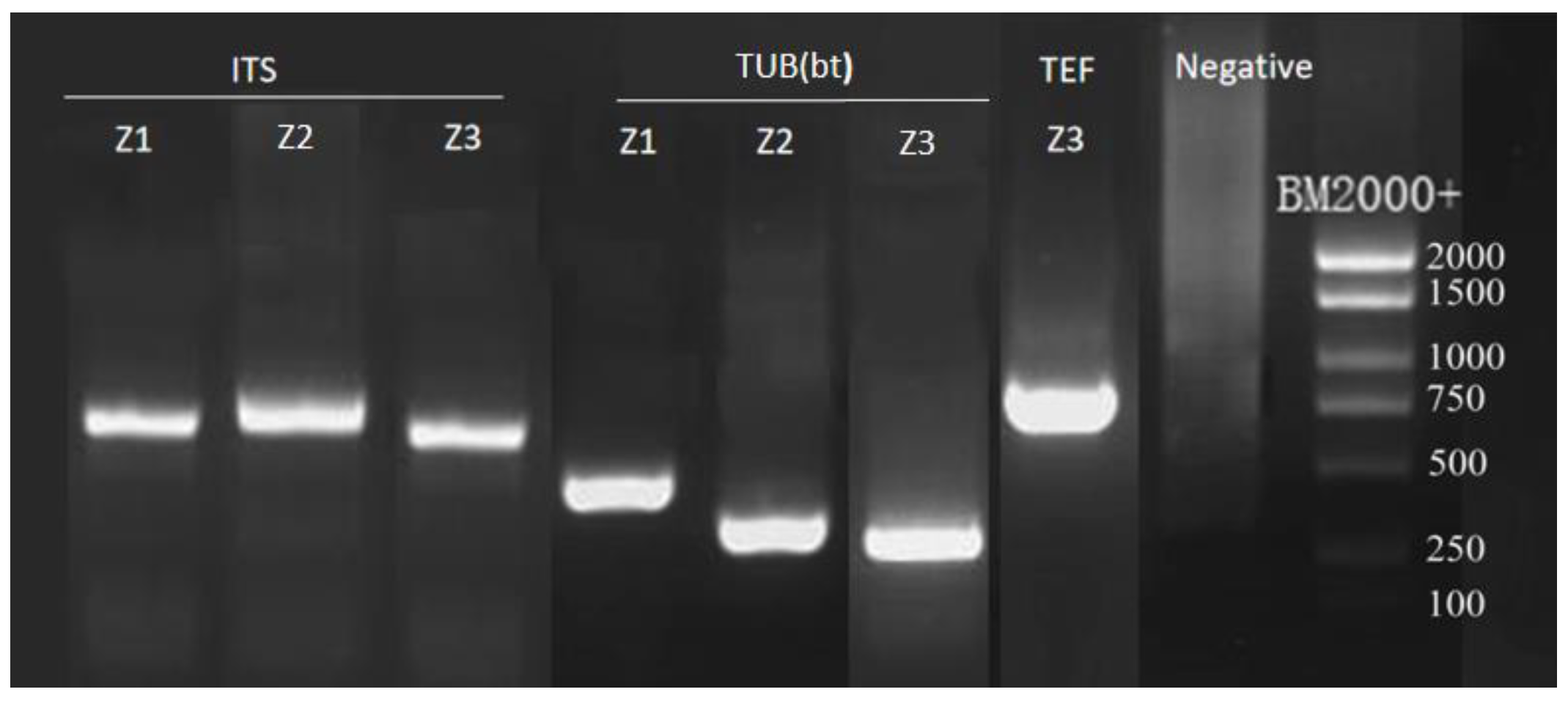
2.3.2. PCR Amplification and Sequencing
2.4. The Pathogenicity Test
2.5. Effects of Temperature, pH, Light, and Carbon and Nitrogen Sources on Mycelial Growth and Spore Production
2.6. Determination of Total Carbohydrates, Flavonoids, and Saponins
2.7. Statistical Analysis
3. Results
3.1. The Occurrence of RA Mildew
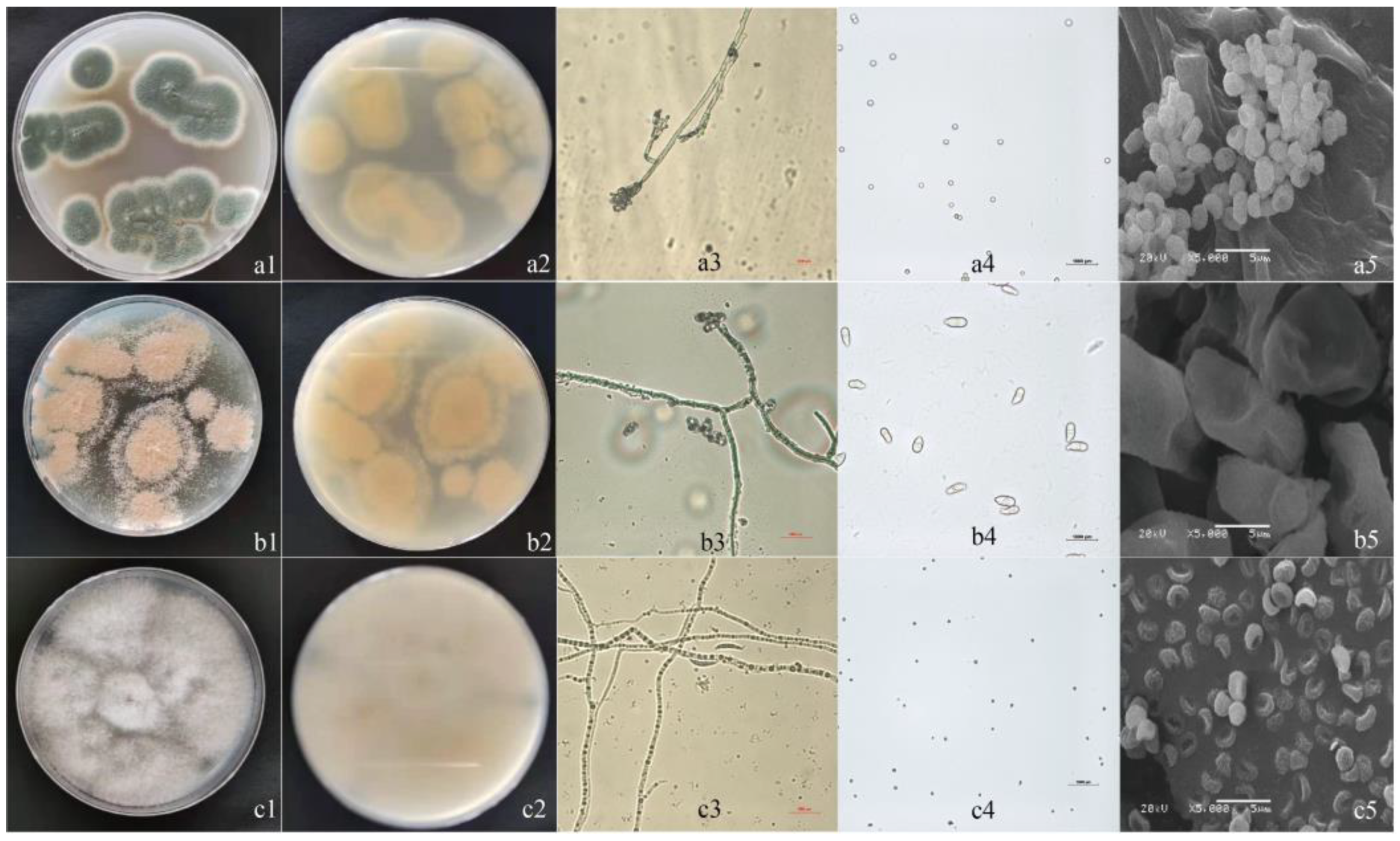
3.2. Isolation and Morphological Identification
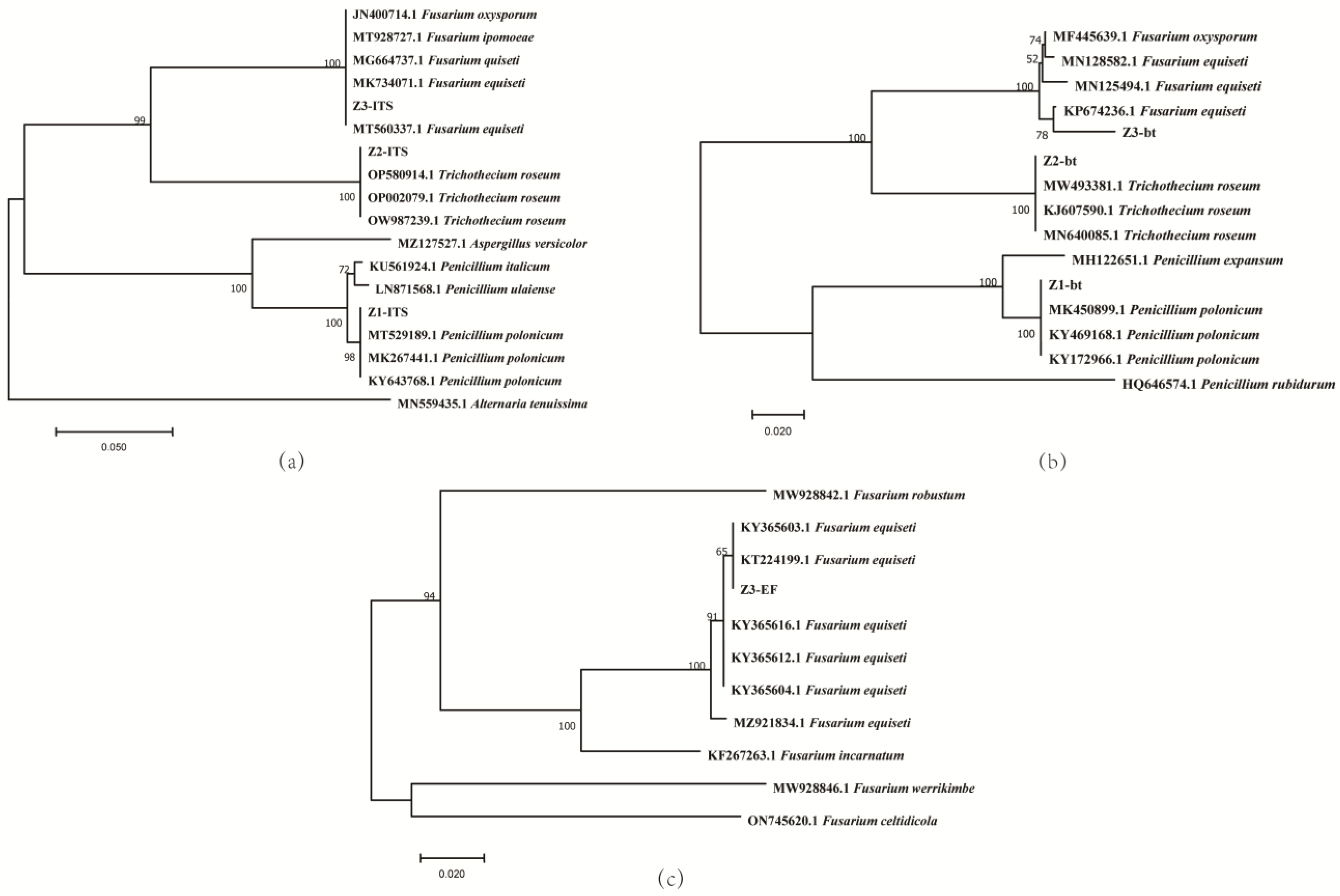
3.3. Phylogenetic Analysis
3.4. Pathogenicity Test
3.5. Effects of Temperature, pH, and Light on Mycelial Growth and Spore Production
3.5.1. Temperature Conditions
3.5.2. pH Conditions
3.5.3. Lighting Conditions
3.6. Effects of Carbon and Nitrogen Sources on Mycelial Growth and Spore Production
3.6.1. Carbon Sources
3.6.2. Nitrogen Sources
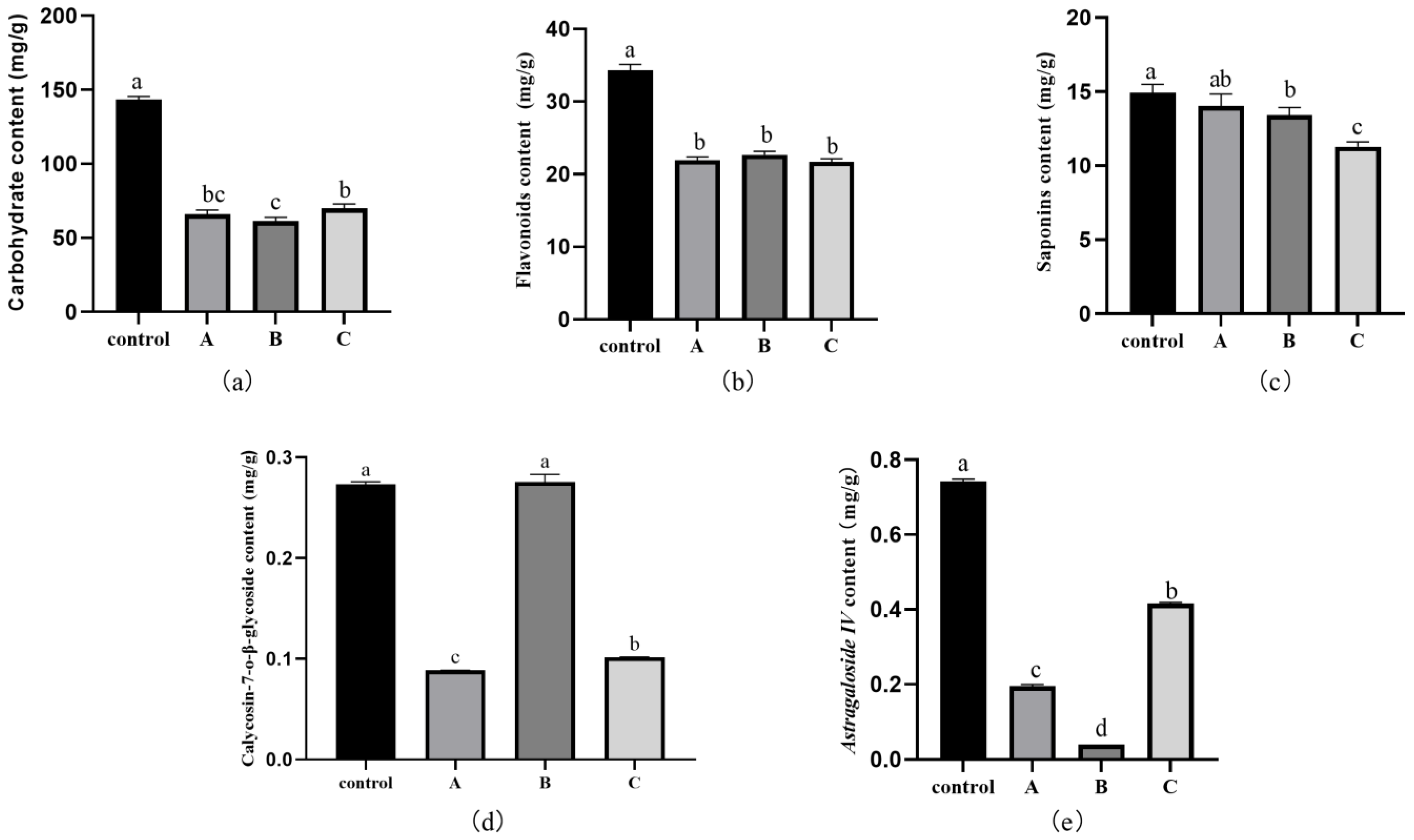
3.7. Effect of Fungal Infection on the Active Ingredients, Total Carbohydrates, Flavonoids, and Saponins in RA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix A.1. Determination of Total Carbohydrates
Appendix A.1.1. Preparation of Glucose Standard Solutions
Appendix A.1.2. Preparation of Standard Curves for Total Carbohydrates
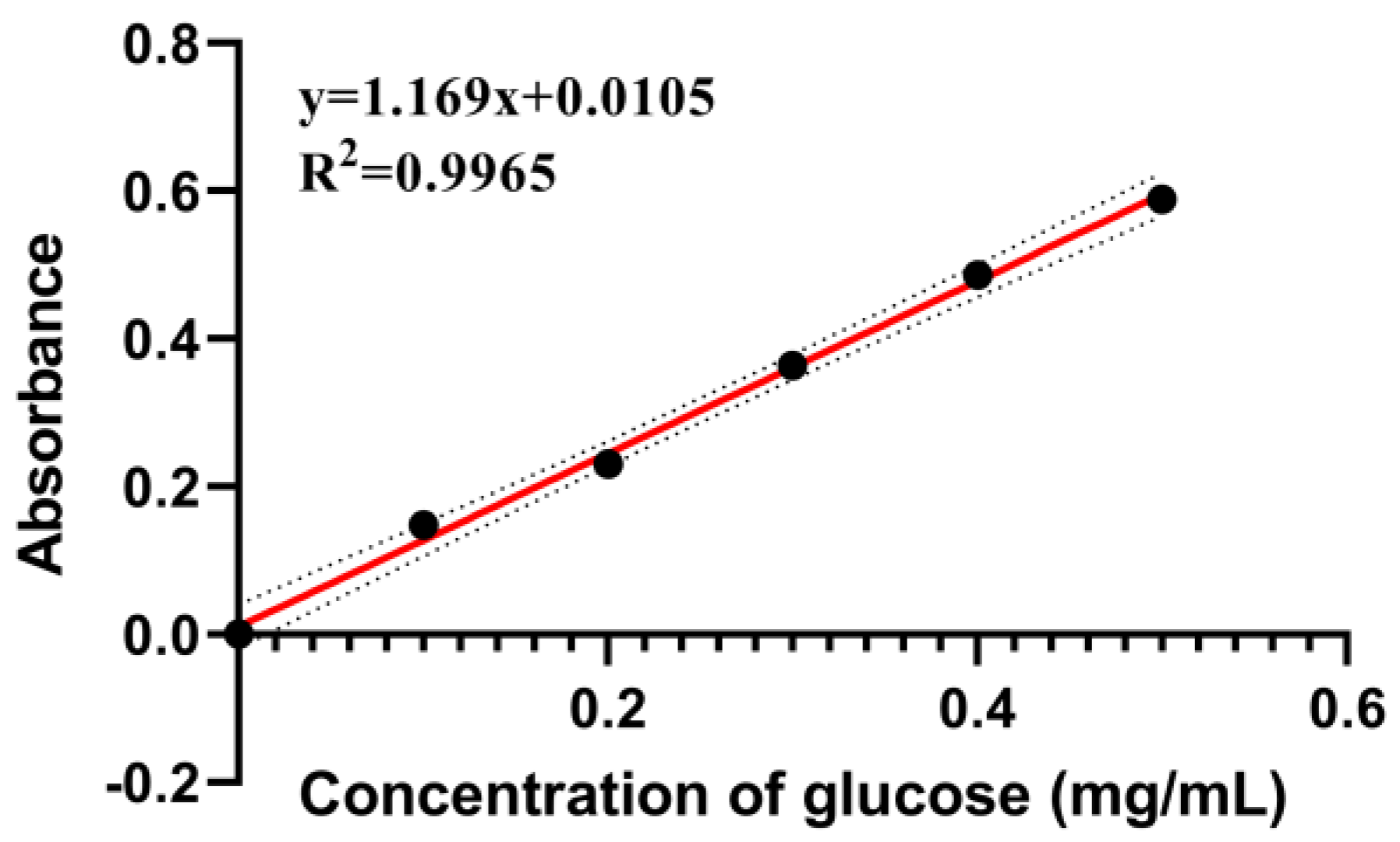
Appendix A.1.3. Sample Test
Appendix A.2. Determination of Total Flavonoids
Appendix A.2.1. Preparation of Rutin Standard Solution
Appendix A.2.2. Preparation of Standard Curves for Total Flavonoids
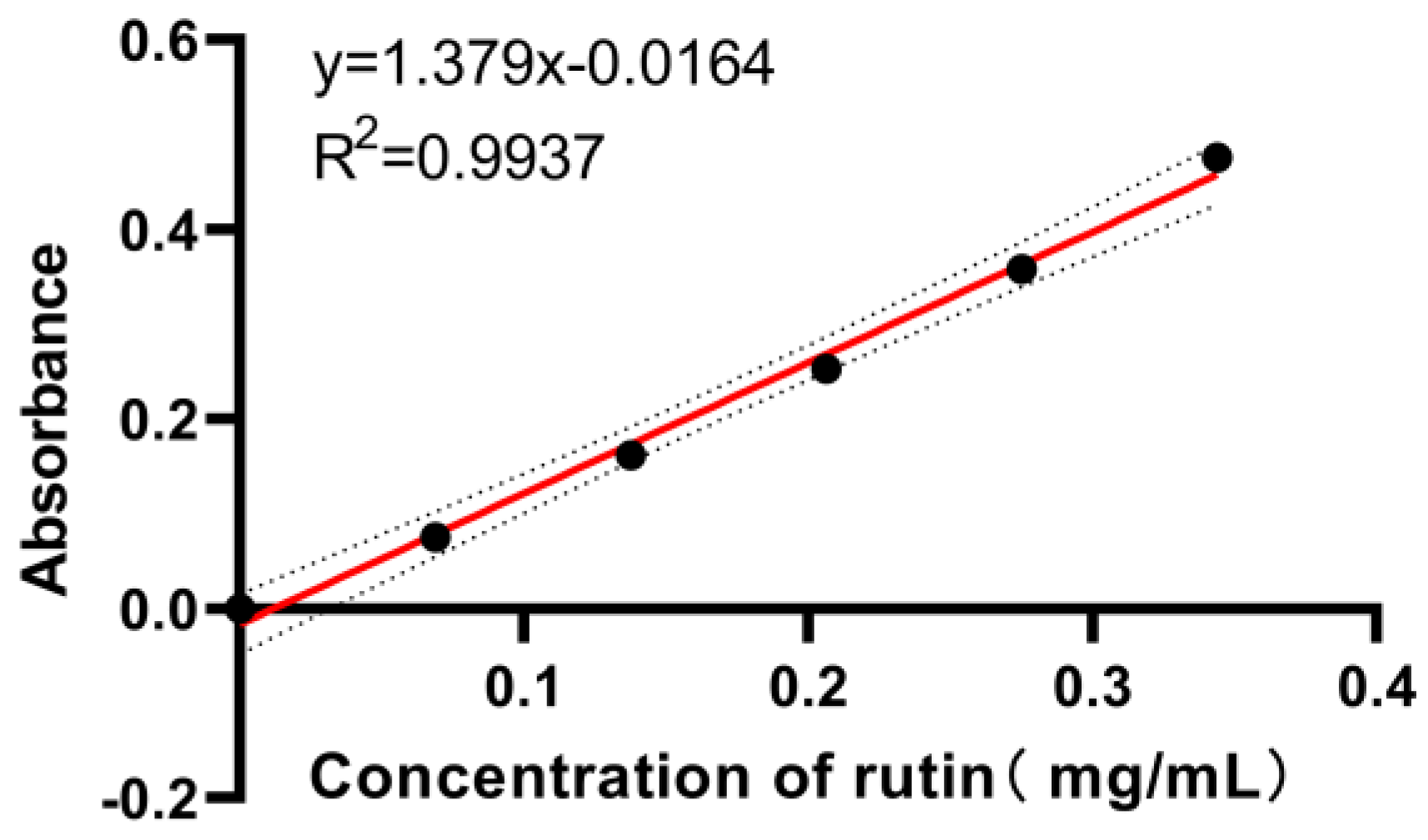
Appendix A.2.3. Sample Tests
Appendix A.3. Determination of Total Saponins
Appendix A.3.1. Preparation of Standard Solutions of Astragaloside IV
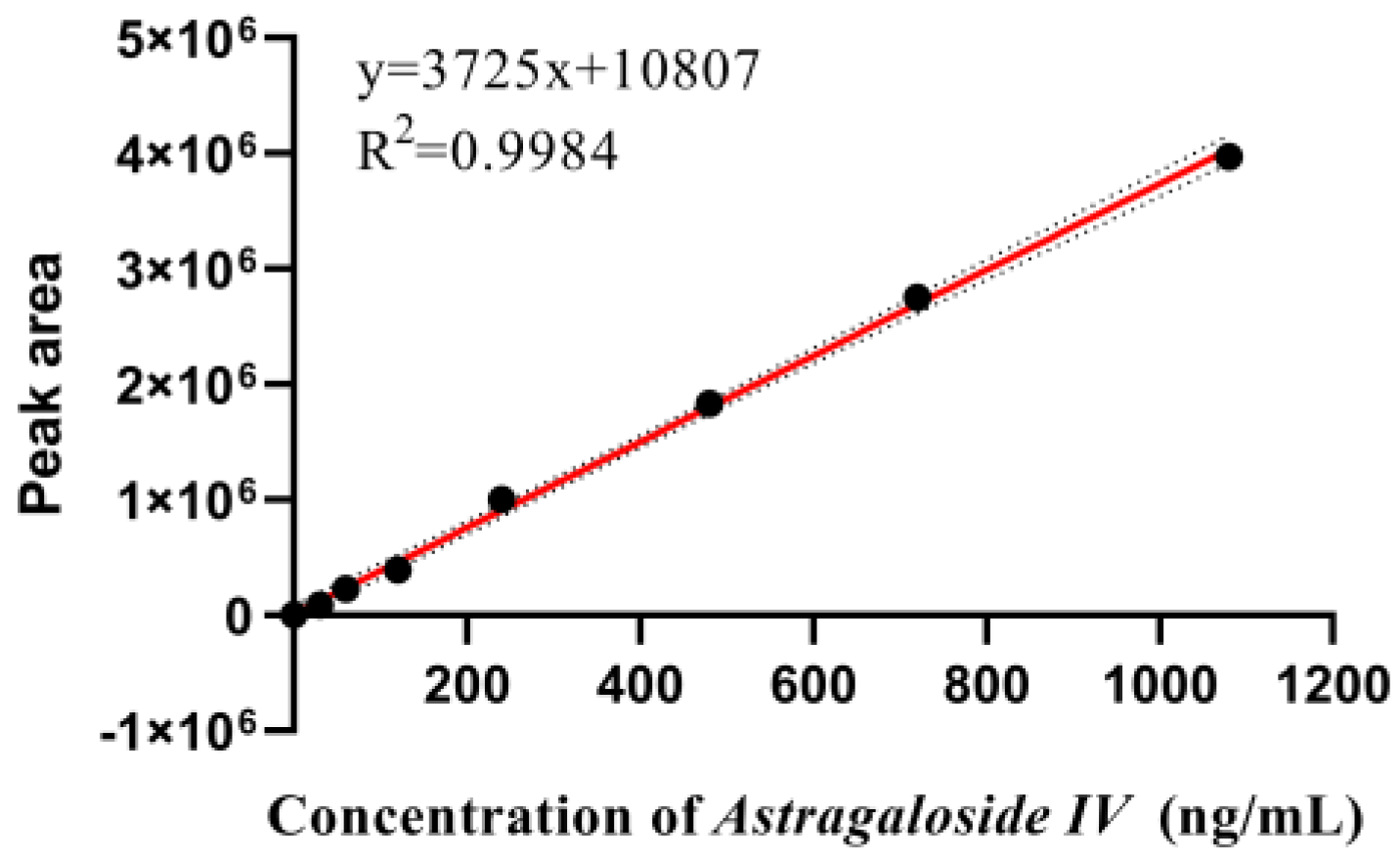
Appendix A.3.2. Preparation of Standard Curves for Total Saponins

Appendix A.3.3. Sample Tests
Appendix A.4. Determination of Calycosin-7-O-β-Glycoside

Appendix A.5. Determination of Astragaloside IV
References
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef]
- Rangsipanuratn, W.; Kammarnjassadakul, P.; Janwithayanuchit, I.; Paungmoung, P.; Ngamurulert, S.; Sriprapun, M.; Yangen, S.; Soottitantawat, V.; Sandee, A. Detection of microbes, aflatoxin and toxic heavy metals in Chinese medicinal herbs commonly consumed in Thailand. Pharm. Sci. Asia 2017, 44, 162–171. Available online: https://pharmacy.mahidol.ac.th/journal/_files/2017-44-3_5.pdf (accessed on 20 January 2023). [CrossRef]
- Commission, S.P. Chinese Pharmacopoeia; Chemistry and Industry Press: Beijing, China, 2015; p. 261. [Google Scholar]
- Wang, Y.; Zhang, C.; Zhang, L.; Zhao, M.; OuYang, Z.; Yang, M. A comprehensive strategy for screening and exploring multi-class mycotoxins contamination status in Astragali radix. Microchem. J. 2020, 158, 105294. [Google Scholar] [CrossRef]
- Su, C.; Hu, Y.; Gao, D.; Luo, Y.; Chen, A.J.; Jiao, X.; Gao, W. Occurrence of Toxigenic Fungi and Mycotoxins on Root Herbs from Chinese Markets. J. Food Prot. 2018, 81, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, I.; Chen, R.; Cheng, Y.; Chen, J. First Report of Powdery Mildew on Astragalus sinicus (Chinese Milk Vetch) Caused by Erysiphe trifoliorum in China. Plant Dis. 2022, 106, 2535. [Google Scholar] [CrossRef]
- Jin, M.; Yang, C.; Yang, L.; Cui, L.; Wei, L. Isolation and identification of a new Colletotrichum species causing anthracnose of Astragalus membranaceus. Crop Prot. 2020, 143, 105470. [Google Scholar] [CrossRef]
- Guan, Y.M.; Zhang, S.N.; Ma, Y.Y.; Zhang, Y.Y. Occurrence of Dactylonectria torresensis Causing Root Rot on Astragalus membranaceus in Northeastern China. Plant Dis. 2021, 105, 1219. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, X.; Qin, X.; Lei, Z. Analysis of dominant pathogen community causing Astragalus membranaceus var. mongholicus root rot in Shanxi Province. J. Plant Prot. 2018, 45, 878–885. (In Chinese) [Google Scholar]
- Zhou, H.; Yang, H.; Tang, W. Occurrence of Fusarium root rot in Astragalus adsurgens and identification of the pathogens. Acta Agrestia Sinica 2004, 12, 285. (In Chinese) [Google Scholar]
- Zhu, Y.; Wang, S.; Xu, X.; Wang, L.; Zhou, H.; Ying, X.; Hu, Q.; Wang, X.; Ji, S.; Cai, Q. Exposure assessment and risk-based limit levels evaluation of ochratoxin A in Astragali radix in China. Ecotoxicol. Environ. Saf. 2022, 237, 113517. [Google Scholar] [CrossRef]
- Frisvad, J.; Hubka, V.; Ezekiel, C.; Hong, S.-B.; Nováková, A.; Chen, A.; Arzanlou, M.; Larsen, T.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Miao, M.-S. Application characteristics of fresh traditional Chinese medicine. In Proceedings of the 2017 2nd International Conference on Biological Sciences and Technology (BST 2017), Zhuhai, China, 17–19 November 2017; Atlantis Press: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Wei, J.C. Fungal Identification Manual; Shanghai Scientific and Technical Publishers: Shanghai, China, 1979. (In Chinese) [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. Available online: https://sci-hub.ru/10.1016/b978-0-12-372180-8.50042-1 (accessed on 24 January 2023).
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Qu, W.; Chang, S.; Li, C.; Xu, F.; Ju, M.; Zhao, R.; Wang, H.; Zhang, H.; Miao, H. Identification of Pathogenicity Groups and Pathogenic Molecular Characterization of Fusarium oxysporum f. sp. sesami in China. Phytopathology 2020, 110, 1093–1104. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual, 1st ed.; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 47–53. Available online: https://link.springer.com/chapter/10.1007/978-1-4419-1463-7_6 (accessed on 29 January 2023).
- Zhang, W.-H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr. Polym. 2019, 227, 115332. [Google Scholar] [CrossRef]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic profiles and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef]
- Wan, Z.; Zhu, J.; Tian, R.; Yang, W.; Chen, Z.; Hu, Q.; Zeng, Z. Quality evaluation for Dipacus asperoides from Enshi areas and optimization extraction of saponins and organic acids and its application. Arab. J. Chem. 2021, 14, 103107. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Bartholomew, H.P.; Lichtner, F.; Gaskins, V.L.; Jurick, W.M. First Report of Blue Mold Caused by Penicillium polonicum on Apple in the United States. Plant Dis. 2022, 106, 762. [Google Scholar] [CrossRef]
- Duduk, N.; Vasić, M.; Vico, I. First report of Penicillium polonicum causing blue mold on stored onion (Allium cepa) in Serbia. Plant Dis. 2014, 98, 1440. [Google Scholar] [CrossRef]
- Hamid, M.I.; Hussain, M.; Ghazanfar, M.U.; Raza, M.; Liu, X.Z. Trichothecium roseum Causes Fruit Rot of Tomato, Orange, and Apple in Pakistan. Plant Dis. 2014, 98, 1271. [Google Scholar] [CrossRef]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Kew, UK, 1971. [Google Scholar]
- Guo, M.; Li, B.; Wang, R.; Liu, P.; Chen, Q. Occurrence of dieback disease caused by Fusarium equiseti on Dendrobium officinale in China. Crop Prot. 2020, 137, 105209. [Google Scholar] [CrossRef]
- Available online: https://www.ncbi.nlm.nih.gov/home/learn/ (accessed on 18 October 2022).
- Su, J.; Guo, C.; Zhang, J.; Qi, Y.; Cao, S.; Li, M. Isolation and identification of penicilliosis pathogens in onion during storage period. J. Gansu Agric. Univ. 2016, 51, 95–101. (In Chinese) [Google Scholar]
- Dai, P.; Liang, X.; Wang, Y.; Gleason, M.L.; Zhang, R.; Sun, G. High Humidity and Age-Dependent Fruit Susceptibility Promote Development of Trichothecium Black Spot on Apple. Plant Dis. 2019, 103, 259–267. [Google Scholar] [CrossRef]
- Slavov, S.; Mayama, S.; Atanassov, A. Some aspects of epidemiology of Alternaria alternata tobacco pathotype. Biotechnol. Biotechnol. Equip. 2004, 18, 85–89. [Google Scholar] [CrossRef]
- Li, X.; Li, B.-H.; Lian, S.; Dong, X.-L.; Wang, C.-X.; Liang, W.-X. Effects of temperature, moisture and nutrition on conidial germination, survival, colonization and sporulation of Trichothecium roseum. Eur. J. Plant Pathol. 2018, 153, 557–570. [Google Scholar] [CrossRef]
- Frans, M.; Aerts, R.; Van Laethem, S.; Ceusters, J. Environmental effects on growth and sporulation of Fusarium spp. causing internal fruit rot in bell pepper. Eur. J. Plant Pathol. 2017, 149, 875–883. [Google Scholar] [CrossRef]
- Rotem, J.; Cohen, Y.; Bashi, E. Host and Environmental Influences on Sporulation In Vivo. Annu. Rev. Phytopathol. 1978, 16, 83–101. [Google Scholar] [CrossRef]
- Li, F.; Matloob, M.; Nzabanita, C.; Li, Y. Growth, sporulation and germination of Verticillium alfalfae on media. Eur. J. Plant Pathol. 2021, 161, 383–395. [Google Scholar] [CrossRef]
- Yun, Y.H.; Son, S.Y.; Choi, C.W.; Hong, J.K.; Kim, Y.S.; Kim, S.H. The occurrence of pink mold rot fungus Trichothecium roseum on tomatoes in Korea. Afr. J. Microbiol. Res. 2013, 7, 1128–1135. [Google Scholar] [CrossRef]
- Bertonha, L.C.; Neto, M.L.; Garcia, J.A.A.; Vieira, T.F.; Castoldi, R.; Bracht, A.; Peralta, R.M. Screening of Fusarium sp. for xylan and cellulose hydrolyzing enzymes and perspectives for the saccharification of delignified sugarcane bagasse. Biocatal. Agric. Biotechnol. 2018, 16, 385–389. [Google Scholar] [CrossRef]
- Vaishnav, N.; Singh, A.; Adsul, M.; Dixit, P.; Sandhu, S.K.; Mathur, A.; Puri, S.K.; Singhania, R.R. Penicillium: The next emerging champion for cellulase production. Bioresour. Technol. Rep. 2018, 2, 131–140. [Google Scholar] [CrossRef]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between Secondary Metabolism and Fungal Development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, B.; Tian, S. Effects of carbon, nitrogen and ambient pH on patulin production and related gene expression in Penicillium expansum. Int. J. Food Microbiol. 2015, 206, 102–108. [Google Scholar] [CrossRef]
- Abd Elkader, H.T.A.E.; Essawy, A.E.; Al-Shami, A.S. Astragalus species: Phytochemistry, biological actions and molecular mechanisms underlying their potential neuroprotective effects on neurological diseases. Phytochemistry 2019, 103, 2692. [Google Scholar] [CrossRef] [PubMed]



| Culture Conditions | P. polonicum | T. roseum | F. equiseti | ||||
|---|---|---|---|---|---|---|---|
| Colony Diameter (mm) | Spore Production (×107/mL) | Colony Diameter (mm) | Spore Production (×107/mL) | Colony Diameter (mm) | Spore Production (×107/mL) | ||
| Temperature (°C) | 15 | 28.33 ± 0.58 b | 46.7 ± 1.52 d | 42.33 ± 0.58 c | 1.33 ± 0.06 d | 0.00 ± 0.00 e | 0.00 ± 0.00 c |
| 20 | 29.00 ± 1.00 b | 61.50 ± 1.73 c | 53.00 ± 1.80 b | 15.8 ± 0.75 b | 47.67 ± 2.08 c | 29.98 ± 3.93 b | |
| 25 | 34.67 ± 0.58 a | 87.00 ± 3.00 a | 64.67 ± 2.08 a | 19.8 ± 2.27 a | 66.17 ± 0.58 a | 42.42 ± 5.39 a | |
| 30 | 21.67 ± 0.58 c | 79.00 ± 2.84 b | 44.67 ± 0.58 c | 4.67 ± 0.61 c | 57.17 ± 0.76 b | 29.58 ± 5.64 b | |
| 35 | 9.50 ± 0.50 d | 0.50 ± 0.10 e | 13.00 ± 1.00 d | 2.13 ± 0.21 d | 13.70 ± 1.20 d | 4.25 ± 0.08 c | |
| pH | 5 | 25.50 ± 0.71 d | 39.40 ± 0.32 d | 68.50 ± 0.50 b | 8.67 ± 0.50 b | 68.67 ± 1.44 b | 51.60 ± 4.80 b |
| 6 | 27.50 ± 0.71 c | 52.40 ± 0.96 c | 77.00 ± 0.50 a | 11.47 ± 1.00 a | 71.00 ± 0.87 a | 64.27 ± 2.80 a | |
| 7 | 30.00 ± 0.00 ab | 84.70 ± 2.70 a | 66.67 ± 0.29 b | 6.53 ± 0.69 c | 62.33 ± 1.15 c | 50.83 ± 3.33 b | |
| 8 | 30.75 ± 0.35 a | 67.50 ± 0.64 b | 60.00 ± 0.50 c | 5.93 ± 0.70 c | 59.50 ± 0.50 d | 40.67 ± 2.10 c | |
| 9 | 28.75 ± 0.35 bc | 64.80 ± 1.91 b | 56.83 ± 3.33 d | 5.33 ± 0.57 cd | 54.50 ± 0.50 e | 22.50 ± 0.87 d | |
| 11 | 27.50 ± 0.71 c | 54.80 ± 3.34 c | 53.67 ± 0.76 e | 4.07 ± 0.30 d | 48.33 ± 0.29 f | 8.40 ± 0.60 e | |
| Light condition | Full illumination | 30.47 ± 0.35 b | 50.83 ± 0.76 c | 54.92 ± 0.72 c | 8.25 ± 0.25 c | 57.47 ± 0.45 c | 33.33 ± 1.91 c |
| 12 h light/12 h dark | 31.70 ± 0.62 b | 53.70 ± 0.26 b | 56.75 ± 1.25 b | 12.67 ± 1.37 b | 59.47 ± 0.45 b | 40.42 ± 0.72 b | |
| Total darkness | 35.75 ± 0.79 a | 65.37 ± 0.35 a | 61.25 ± 1.25 a | 15.58 ± 0.14 a | 65.47 ± 0.50 a | 50.00 ± 1.25 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Nan, M.; Xue, H. Isolating, Identifying, and Analyzing the Biological Characteristics of Pathogens Causing Postharvest Disease in Fresh Radix Astragali. Horticulturae 2023, 9, 1019. https://doi.org/10.3390/horticulturae9091019
Zhang Y, Nan M, Xue H. Isolating, Identifying, and Analyzing the Biological Characteristics of Pathogens Causing Postharvest Disease in Fresh Radix Astragali. Horticulturae. 2023; 9(9):1019. https://doi.org/10.3390/horticulturae9091019
Chicago/Turabian StyleZhang, Yuan, Mina Nan, and Huali Xue. 2023. "Isolating, Identifying, and Analyzing the Biological Characteristics of Pathogens Causing Postharvest Disease in Fresh Radix Astragali" Horticulturae 9, no. 9: 1019. https://doi.org/10.3390/horticulturae9091019
APA StyleZhang, Y., Nan, M., & Xue, H. (2023). Isolating, Identifying, and Analyzing the Biological Characteristics of Pathogens Causing Postharvest Disease in Fresh Radix Astragali. Horticulturae, 9(9), 1019. https://doi.org/10.3390/horticulturae9091019





