Low Nocturnal Temperature Alters Tomato Foliar and Root Phosphorus Fractions Allocation by Reducing Soil Phosphorus Availability
Abstract
1. Introduction
2. Materials and Methods
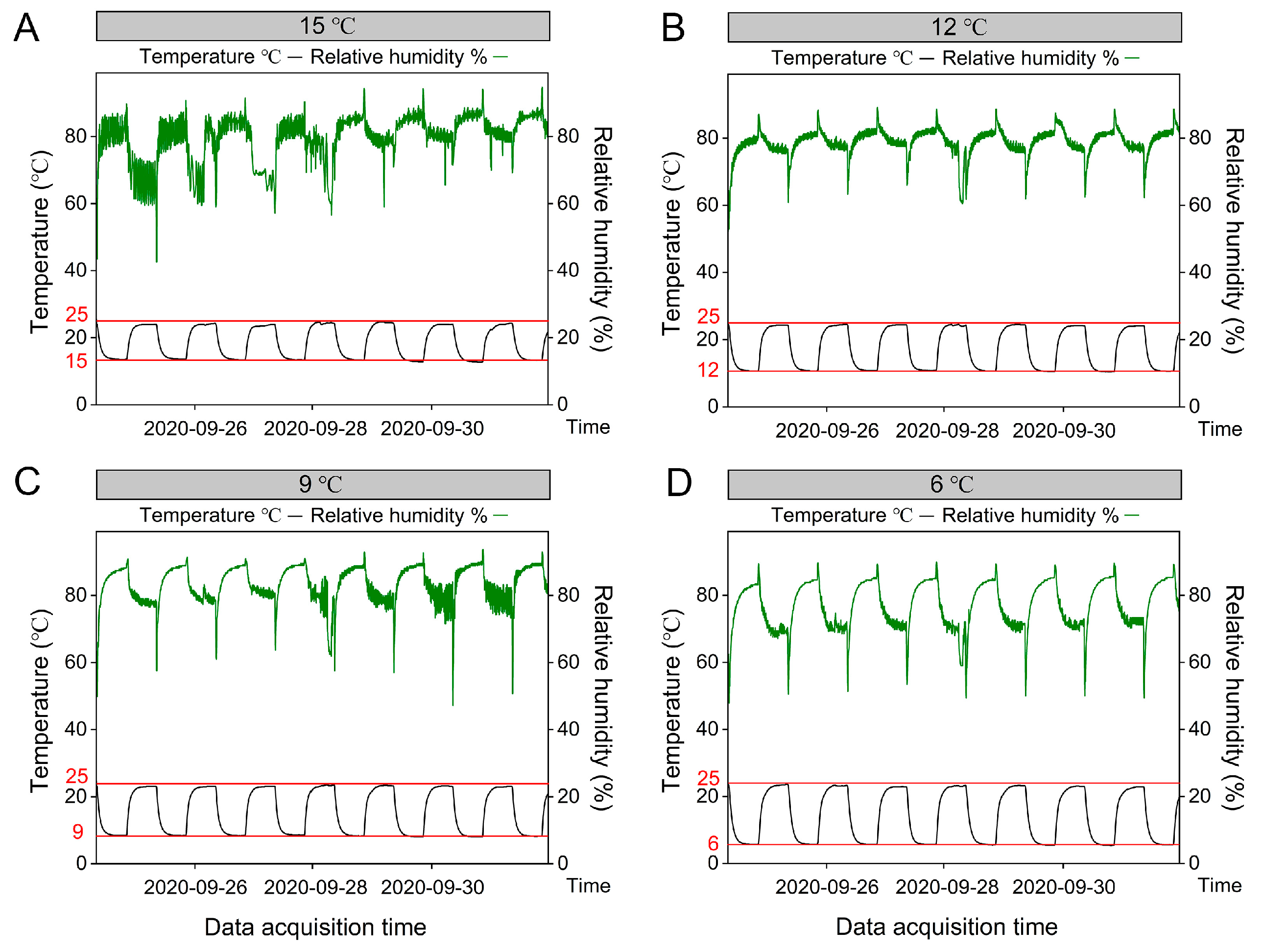
2.1. Experimental Design and Plant Materials
2.2. Soil and Plant Sampling and Chemical Analysis
2.3. Statistical Analyses
3. Results
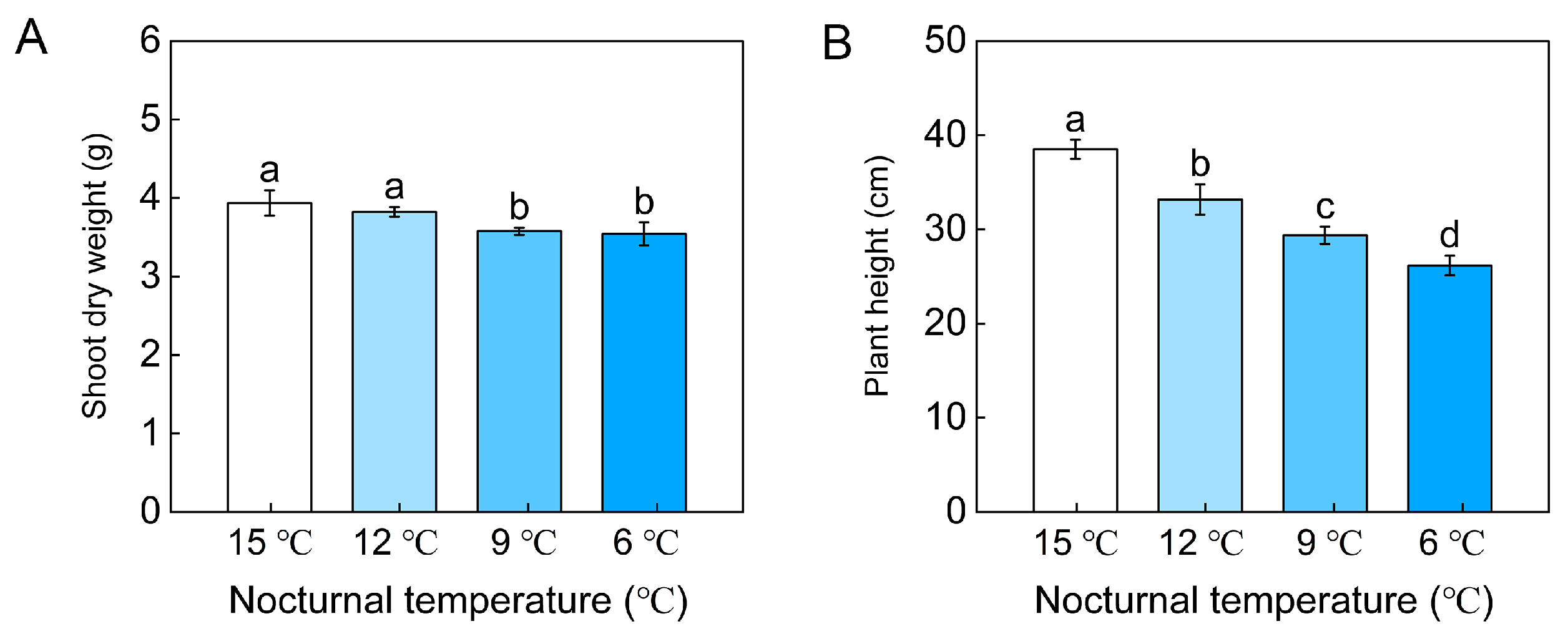
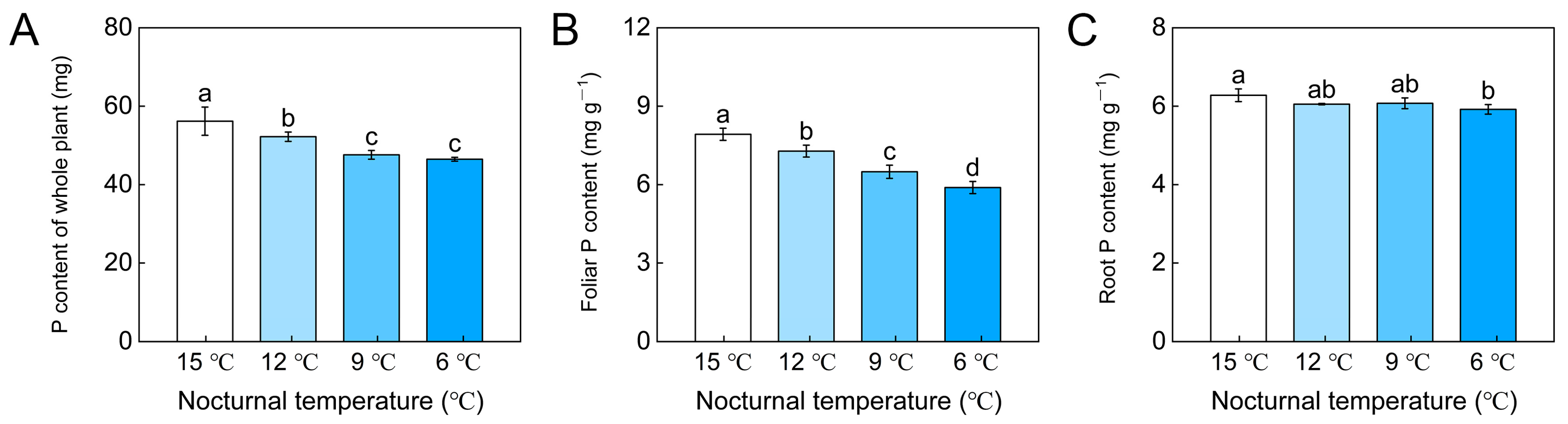
3.1. P absorption of Whole Tomato Plant, Foliar P Content and Root P Content
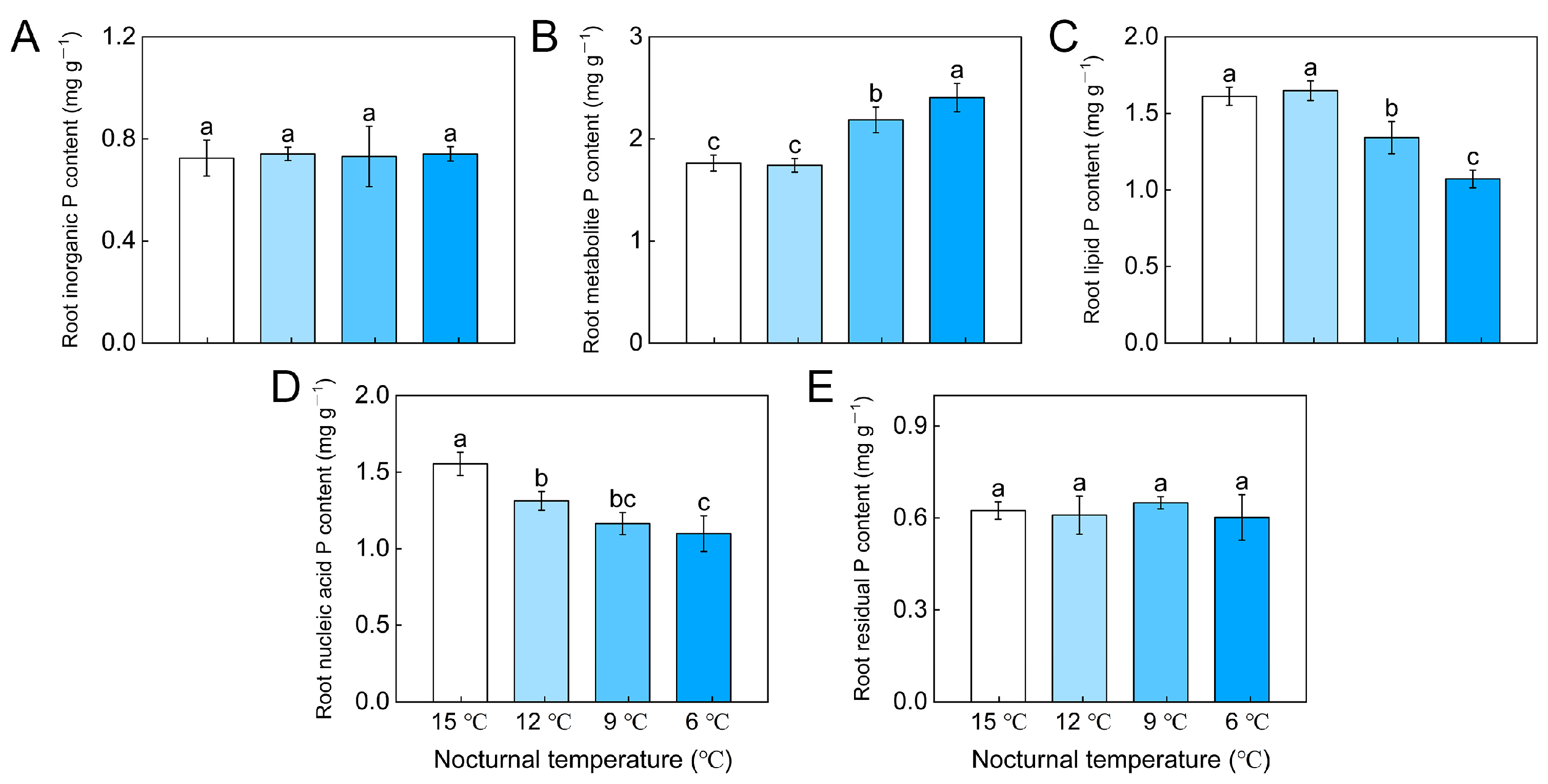
3.2. Tomato Foliar and Root P Fractions
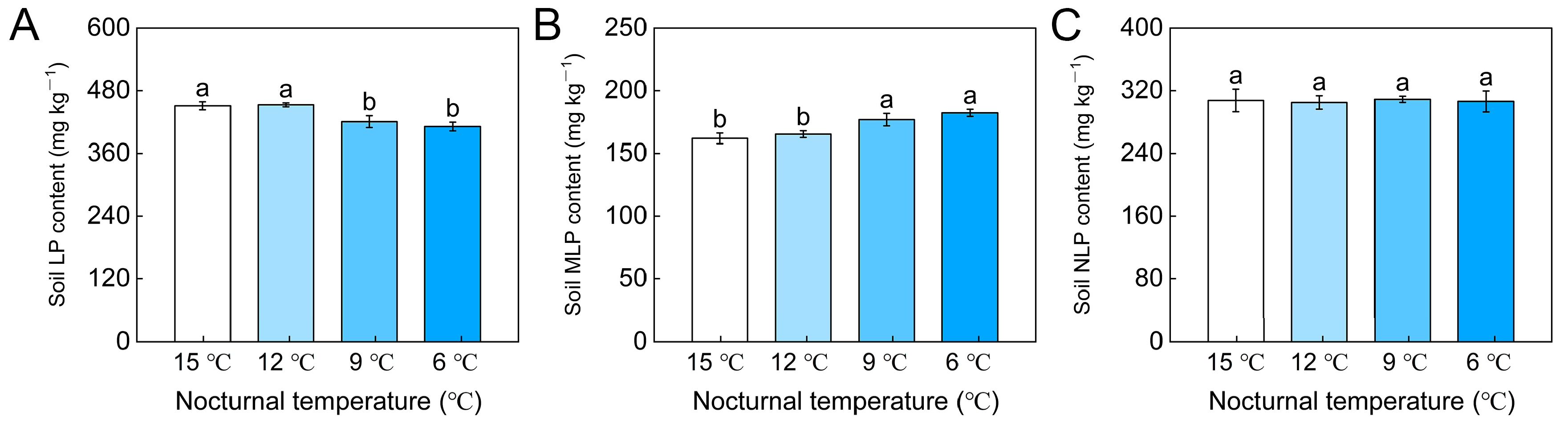
3.3. Soil P fractions
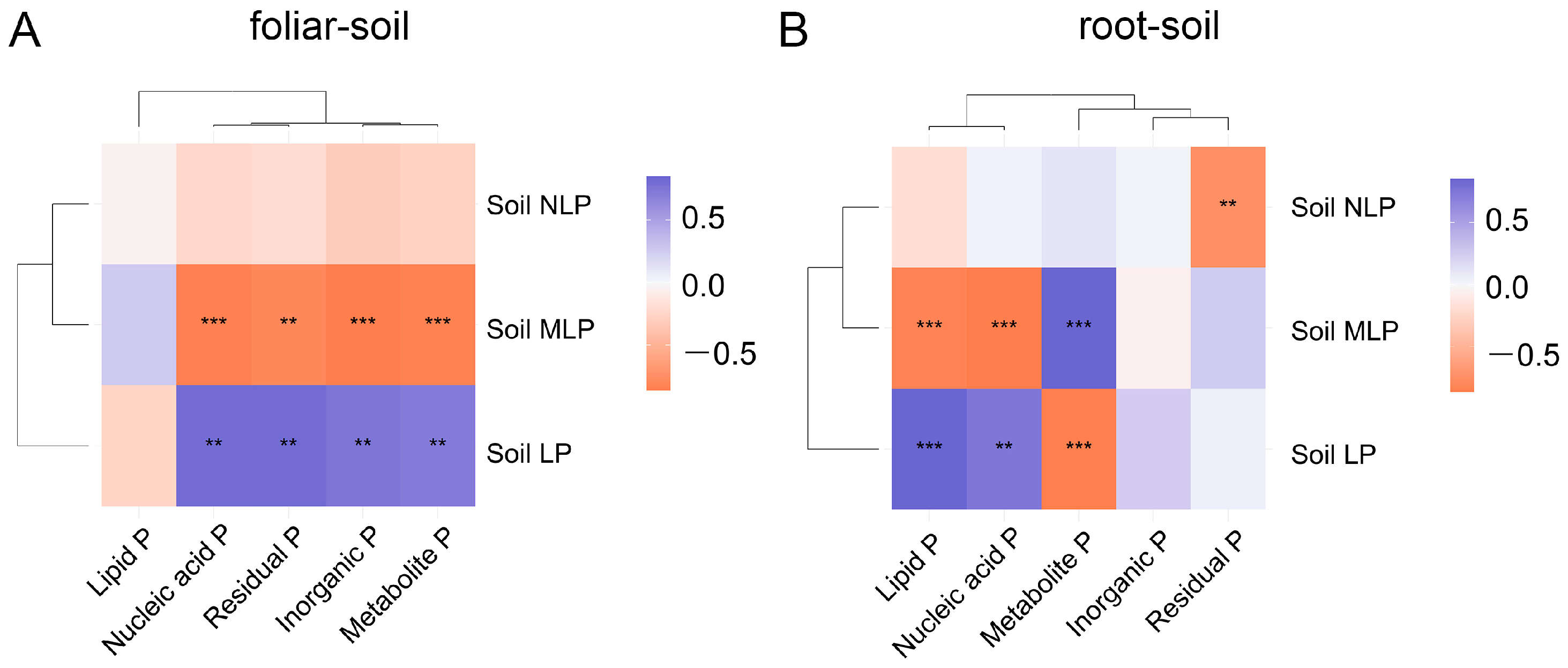
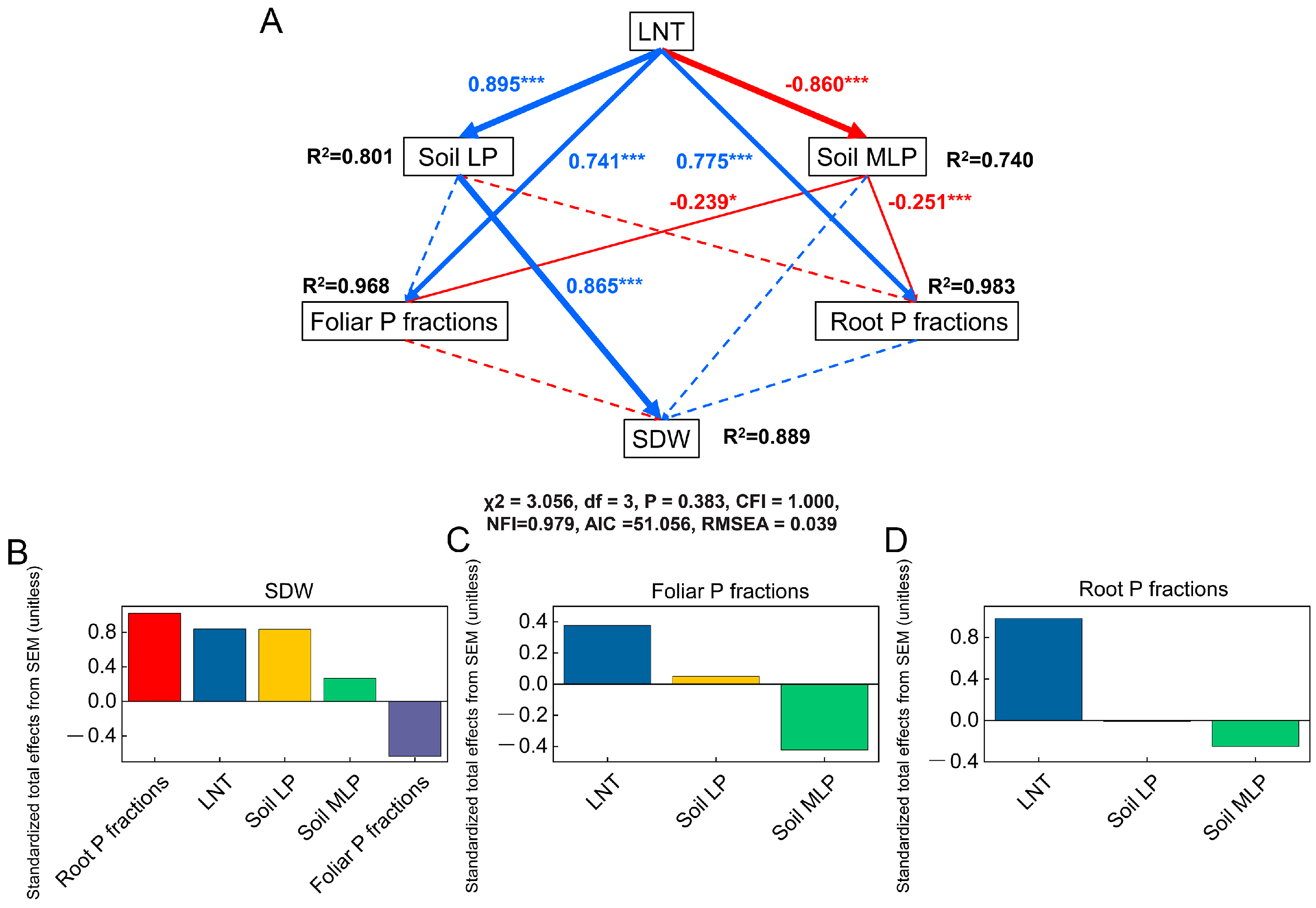
3.4. Linkages between Soil P Fractions and Plant P Fractions
4. Discussion
4.1. Tomato P Fractions Allocation Responds to LNT
4.2. LNT Changes Tomato P Allocation by Reducing Soil P Availability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Wang, Z.; Yang, X.; Wang, F.; Qi, M.; Li, T.; Liu, Y. Cyclic Electron Flow Protects Photosystem I Donor Side under Low Night Temperature in Tomato. Environ. Exp. Bot. 2020, 177, 104151. [Google Scholar] [CrossRef]
- Yan, L.; Sunoj, V.S.J.; Short, A.W.; Lambers, H.; Elsheery, N.I.; Kajita, T.; Wee, A.K.S.; Cao, K.F. Correlations between Allocation to Foliar Phosphorus Fractions and Maintenance of Photosynthetic Integrity in Six Mangrove Populations as Affected by Chilling. New Phytol. 2021, 232, 2267–2282. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Ning, X.; Ni, Y.; Li, T. Effects of Low Temperature on Phosphorus Absorption and Transport of Tomato Seedlings. Acta Bot. Boreali-Occident. Sin. 2017, 37, 0097–0104. [Google Scholar]
- Sun, F.; Chang, R.; Tariq, A.; Sardans, J.; Penuelas, J.; Jiang, H.; Zhou, X.; Li, N. Livestock Grazing-Exclusion under Global Warming Scenario Decreases Phosphorus Mineralization by Changing Soil Food Web Structure in a Tibetan Alpine Meadow. Sci. Total Environ. 2023, 873, 162313. [Google Scholar] [CrossRef]
- Wen, Z.; Pang, J.; Wang, X.; Gille, C.E.; De Borda, A.; Hayes, P.E.; Clode, P.L.; Ryan, M.H.; Siddique, K.H.M.; Shen, J.; et al. Differences in Foliar Phosphorus Fractions, Rather than in Cell-Specific Phosphorus Allocation, Underlie Contrasting Photosynthetic Phosphorus Use Efficiency among Chickpea Genotypes. J. Exp. Bot. 2022, 74, 1974–1989. [Google Scholar] [CrossRef]
- Niederberger, J.; Kohler, M.; Bauhus, J. Distribution of Phosphorus Fractions with Different Plant Availability in German Forest Soils and Their Relationship with Common Soil Properties and Foliar P Contents. Soil 2019, 5, 189–204. [Google Scholar] [CrossRef]
- Motavalli, P.P.; Miles, R.J. Soil Phosphorus Fractions after 111 Years of Animal Manure and Fertilizer Applications. Biol. Fertil. Soils 2002, 36, 35–42. [Google Scholar] [CrossRef]
- Maranguit, D.; Guillaume, T.; Kuzyakov, Y. Land-Use Change Affects Phosphorus Fractions in Highly Weathered Tropical Soils. Catena 2017, 149, 385–393. [Google Scholar] [CrossRef]
- Helfenstein, J.; Tamburini, F.; von Sperber, C.; Massey, M.S.; Pistocchi, C.; Chadwick, O.A.; Vitousek, P.M.; Kretzschmar, R.; Frossard, E. Combining Spectroscopic and Isotopic Techniques Gives a Dynamic View of Phosphorus Cycling in Soil. Nat. Commun. 2018, 9, 3226–3234. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Shah, J.A.; Chu, G. Polyphosphate Fertilizers Increased Maize (Zea Mays L.) P, Fe, Zn, and Mn Uptake by Decreasing P Fixation and Mobilizing Microelements in Calcareous Soil. J. Soils Sediments 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, F.; Islam, W.; Zhang, Z.; Du, Y.; Zhang, Y.; Chai, X.; Liu, Y. Coexistence Desert Plants Respond to Soil Phosphorus Availability by Altering the Allocation Patterns of Foliar Phosphorus Fractions and Acquiring Different Forms of Soil Phosphorus. J. Plant Growth Regul. 2022, 1–15. [Google Scholar] [CrossRef]
- Galván-Tejada, N.C.; Peña-Ramírez, V.; Mora-Palomino, L.; Siebe, C. Soil P Fractions in a Volcanic Soil Chronosequence of Central Mexico and Their Relationship to Foliar P in Pine Trees. J. Plant Nutr. Soil Sci. 2014, 177, 792–802. [Google Scholar] [CrossRef]
- Hei, J.; Xie, H.; Yang, L.; Wang, W.; Sardans, J.; Wang, C.; Tariq, A.; Zeng, F.; Peñuelas, J. Effects of Contrasting N-Enriched Biochar Applications on Paddy Soil and Rice Leaf Phosphorus Fractions in Subtropical China. Sci. Total Environ. 2023, 877, 162949. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, X.; Han, Z.; Pang, J.; Lambers, H.; Finnegan, P.M. Responses of Foliar Phosphorus Fractions to Soil Age Are Diverse along a 2 Myr Dune Chronosequence. New Phytol. 2019, 223, 1621–1633. [Google Scholar] [CrossRef]
- Jiang, M.; Caldararu, S.; Zaehle, S.; Ellsworth, D.S.; Medlyn, B.E. Towards a More Physiological Representation of Vegetation Phosphorus Processes in Land Surface Models. New Phytol. 2019, 222, 1223–1229. [Google Scholar] [CrossRef]
- Hidaka, A.; Kitayama, K. Relationship between Photosynthetic Phosphorus-Use Efficiency and Foliar Phosphorus Fractions in Tropical Tree Species. Ecol. Evol. 2013, 3, 4872–4880. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for Improving Phosphorus-Use Efficiency in Crop Plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, X.; Lambers, H.; Zhang, G.; Liu, N.; Zang, X.; Xiao, M.; Wen, D. Effects of Elevated CO2 Concentration and Nitrogen Addition on Foliar Phosphorus Fractions of Mikania Micranatha and Chromolaena Odorata under Low Phosphorus Availability. Physiol. Plant 2021, 173, 2068–2080. [Google Scholar] [CrossRef]
- Gao, Y.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, Z.; Sardans, J.; Peñuelas, J. Allocation of Foliar-P Fractions of Alhagi Sparsifolia and Its Relationship with Soil-P Fractions and Soil Properties in a Hyperarid Desert Ecosystem. Geoderma 2022, 407, 115546. [Google Scholar] [CrossRef]
- Mo, Q.; Li, Z.; Sayer, E.J.; Lambers, H.; Li, Y.; Zou, B.; Tang, J.; Heskel, M.; Ding, Y.; Wang, F. Foliar Phosphorus Fractions Reveal How Tropical Plants Maintain Photosynthetic Rates despite Low Soil Phosphorus Availability. Funct. Ecol. 2019, 33, 503–513. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L. Chilling-Dependent Photoinhibition, Nutrition and Growth Analysis of Eucalyptus Nitens Seedlings during Establishment. Tree Physiol. 2003, 23, 217–226. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014 (Update 2015). International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. In World Soil Resources Reports; No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Khan, A.; Guo, S.; Wang, R.; Zhang, S.; Yang, X.; He, B.; Li, T. An Assessment of Various Pools of Organic Phosphorus Distributed in Soil Aggregates as Affected by Long-term P Fertilization Regimes. Soil Use Manag. 2023, 39, 833–848. [Google Scholar] [CrossRef]
- Nurhasanah Ritonga, F.; Chen, S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Nucleic Acid Requirement of Plants from Low Phosphorus Habitats. A Commentary on: Foliar Nutrient-Allocation Patterns in Banksia Attenuata and Banksia Sessilis Differing in Growth Rate and Adaptation to Low-Phosphorus Habitats. Ann. Bot. 2021, 128, IV–V. [Google Scholar] [CrossRef] [PubMed]
- Tombesi, S.; Cincera, I.; Frioni, T.; Ughini, V.; Gatti, M.; Palliotti, A.; Poni, S. Relationship among Night Temperature, Carbohydrate Translocation and Inhibition of Grapevine Leaf Photosynthesis. Environ. Exp. Bot. 2019, 157, 293–298. [Google Scholar] [CrossRef]
- Lambers, H.; Finnegan, P.M.; Jost, R.; Plaxton, W.C.; Shane, M.W.; Stitt, M. Phosphorus Nutrition in Proteaceae and Beyond. Nat. Plants 2015, 1, 15109. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold Stress Regulation of Gene Expression in Plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots Withstanding Their Environment: Exploiting Root System Architecture Responses to Abiotic Stress to Improve Crop Tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef]
- Feng, H.; Yan, M.; Fan, X.; Li, B.; Shen, Q.; Miller, A.J.; Xu, G. Spatial Expression and Regulation of Rice High-Affinity Nitrate Transporters by Nitrogen and Carbon Status. J. Exp. Bot. 2011, 62, 2319–2332. [Google Scholar] [CrossRef]
- Zhou, Y.; Sommer, M.L.; Hochholdinger, F. Cold Response and Tolerance in Cereal Roots. J. Exp. Bot. 2021, 72, 7474–7481. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Y.; Pang, J.; Yong, J.W.H.; Chen, Y.; Bai, C.; Han, X.; Liu, X.; Sun, Z.; Zhang, S.; et al. Exogenous Calcium Alleviates Nocturnal Chilling-Induced Feedback Inhibition of Photosynthesis by Improving Sink Demand in Peanut (Arachis Hypogaea). Front. Plant Sci. 2020, 11, 607029. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xu, X.; Chen, X.; Xue, L.; Cao, B. Cucumber Carbohydrate Metabolism and Translocation under Chilling Night Temperature. J. Plant Physiol. 2007, 164, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, X.L.; Peng, F.; Li, C.; Lai, C.; You, Q.; Xue, X.; Wu, Y.; Sun, H.; Chen, Y.; et al. Mobilization of Soil Phosphate after 8 Years of Warming Is Linked to Plant Phosphorus-Acquisition Strategies in an Alpine Meadow on the Qinghai-Tibetan Plateau. Glob. Chang. Biol. 2021, 27, 6578–6591. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, F.; Song, K.; Lin, Y.; Bai, Y.; Zhu, Y.; Giesy, J.P. Competitive Interaction between Soil-Derived Humic Acid and Phosphate on Goethite. Appl. Geochem. 2013, 36, 125–131. [Google Scholar] [CrossRef]
- Chassé, A.W.; Ohno, T. Higher Molecular Mass Organic Matter Molecules Compete with Orthophosphate for Adsorption to Iron (Oxy)Hydroxide. Environ. Sci. Technol. 2016, 50, 7461–7469. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Han, T.; Chen, M.; Zhao, D.; Chao, J.; Zhang, Y. High Mobilization of Phosphorus in Black-Odor River Sediments with the Increase of Temperature. Sci. Total Environ. 2021, 775, 145595. [Google Scholar] [CrossRef]
- Čapek, P.; Kotas, P.; Manzoni, S.; Šantrůčková, H. Drivers of Phosphorus Limitation across Soil Microbial Communities. Funct. Ecol. 2016, 30, 1705–1713. [Google Scholar] [CrossRef]
- Bünemann, E.K. Assessment of Gross and Net Mineralization Rates of Soil Organic Phosphorus—A Review. Soil Biol. Biochem. 2015, 89, 82–98. [Google Scholar] [CrossRef]
- De Sena, A.; Madramootoo, C.A.; Whalen, J.K.; von Sperber, C. Nucleic Acids Are a Major Pool of Hydrolyzable Organic Phosphorus in Arable Organic Soils of Southern Ontario, Canada. Biol. Fertil. Soils 2022, 58, 7–16. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Ma, R.; Sun, Z.; Liu, Y.; Fu, H.; Li, T. Low Nocturnal Temperature Alters Tomato Foliar and Root Phosphorus Fractions Allocation by Reducing Soil Phosphorus Availability. Horticulturae 2023, 9, 536. https://doi.org/10.3390/horticulturae9050536
Shi Q, Ma R, Sun Z, Liu Y, Fu H, Li T. Low Nocturnal Temperature Alters Tomato Foliar and Root Phosphorus Fractions Allocation by Reducing Soil Phosphorus Availability. Horticulturae. 2023; 9(5):536. https://doi.org/10.3390/horticulturae9050536
Chicago/Turabian StyleShi, Qingwen, Ru Ma, Zhouping Sun, Yufeng Liu, Hongdan Fu, and Tianlai Li. 2023. "Low Nocturnal Temperature Alters Tomato Foliar and Root Phosphorus Fractions Allocation by Reducing Soil Phosphorus Availability" Horticulturae 9, no. 5: 536. https://doi.org/10.3390/horticulturae9050536
APA StyleShi, Q., Ma, R., Sun, Z., Liu, Y., Fu, H., & Li, T. (2023). Low Nocturnal Temperature Alters Tomato Foliar and Root Phosphorus Fractions Allocation by Reducing Soil Phosphorus Availability. Horticulturae, 9(5), 536. https://doi.org/10.3390/horticulturae9050536




