Effect of Foliar Application of Sodium Selenate on Mineral Relationships in Brassicaceae Crops
Abstract
1. Introduction
2. Material and Methods
2.1. Growing Conditions and Experimental Protocol
2.2. Sample Preparation
2.3. Elemental Composition
2.4. Determination of Selenium
2.5. Statistical Analysis
3. Results and Discussion
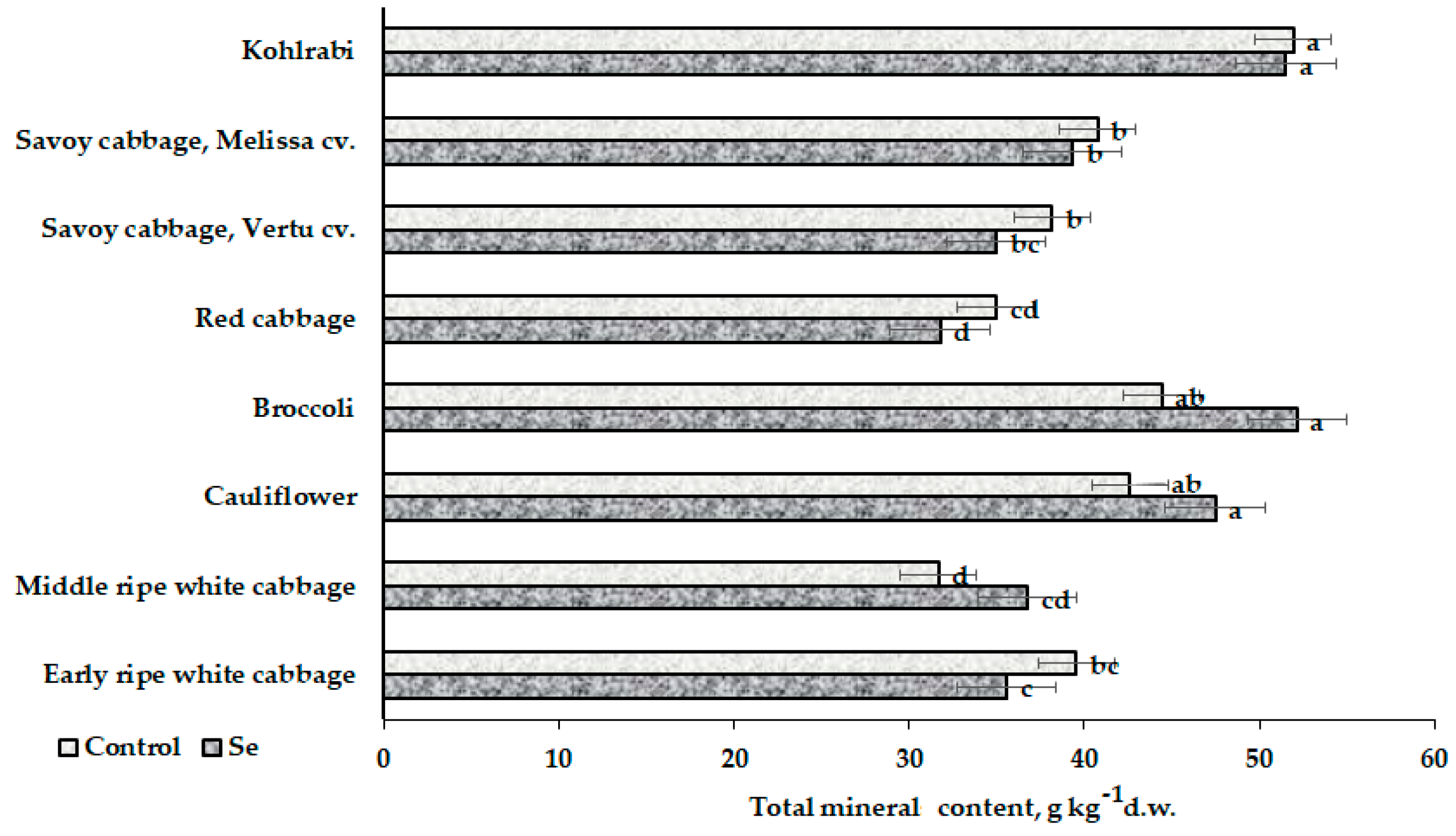
3.1. Mineral Composition
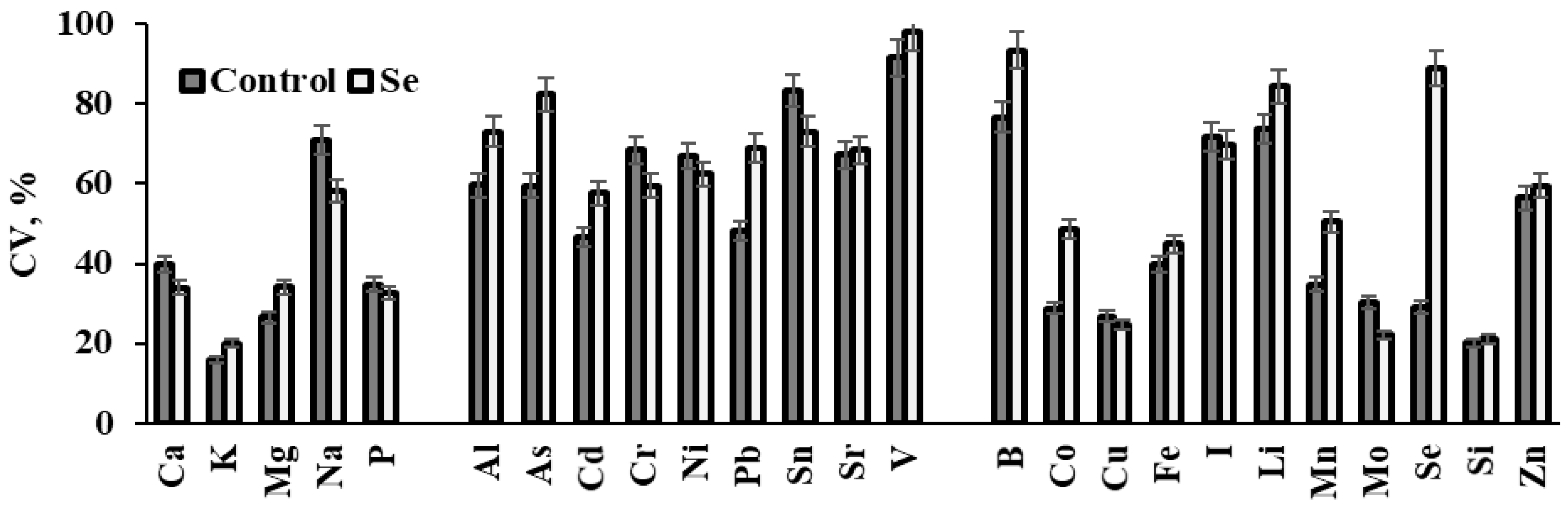
3.2. Changes in Elemental Composition under Se Supply
3.3. Correlations
3.3.1. Stable Interactions
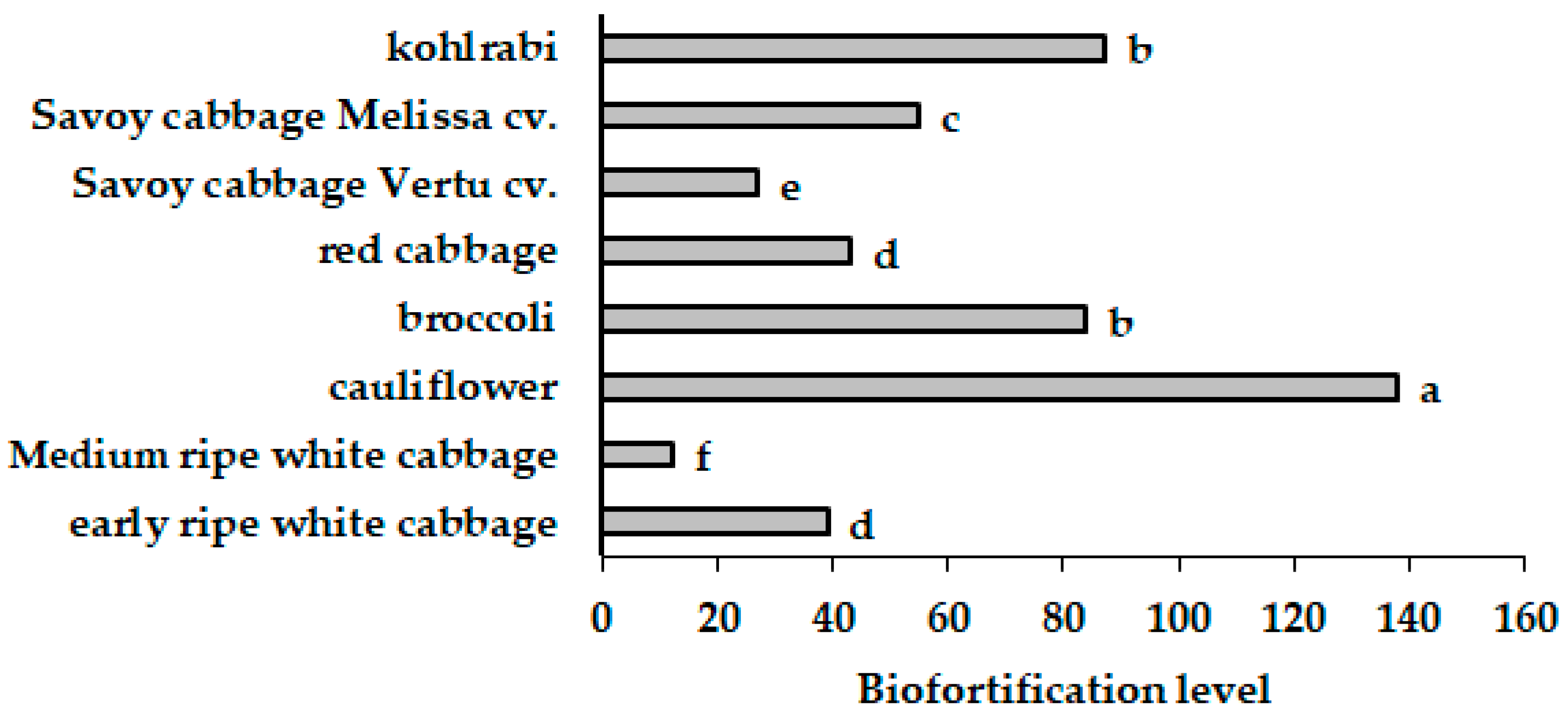
3.3.2. Differences in Se Accumulation
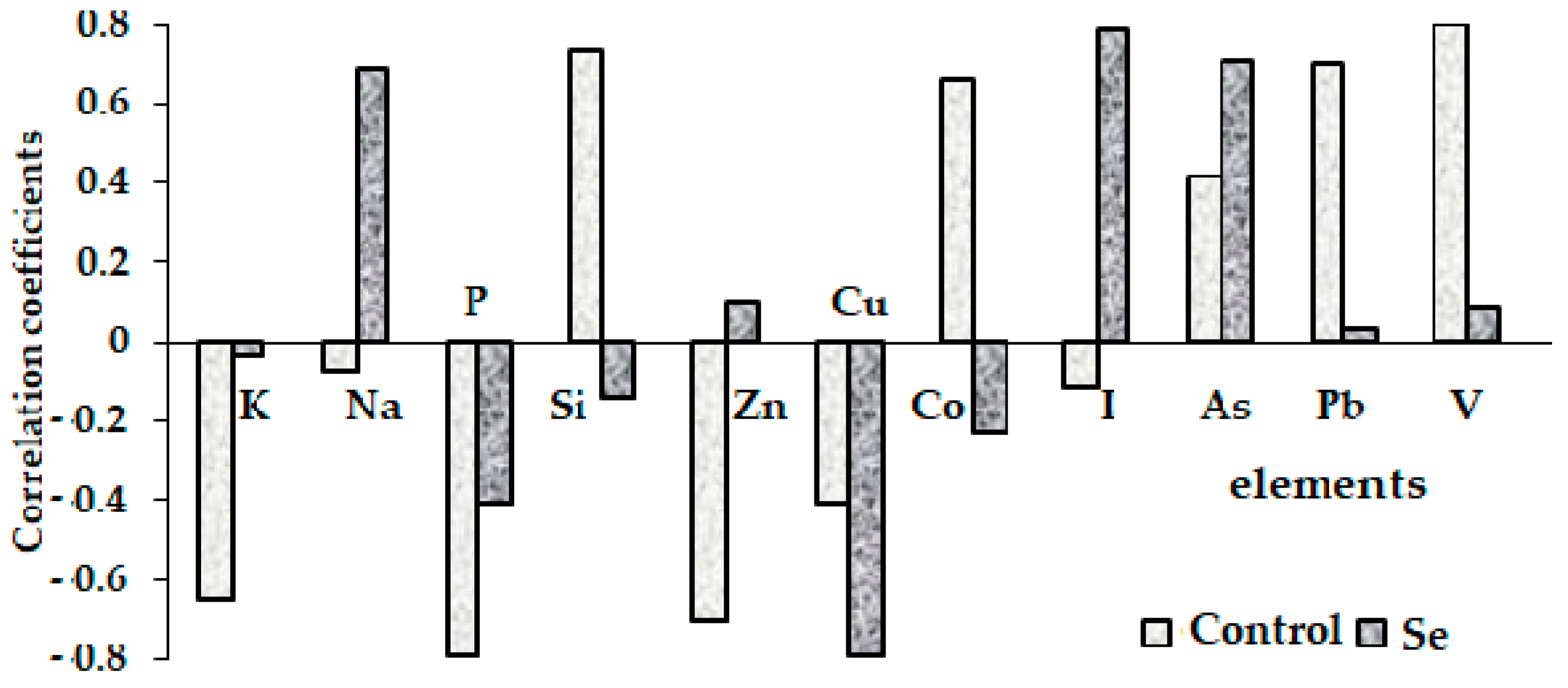
3.3.3. Significant Changes in Element Correlations
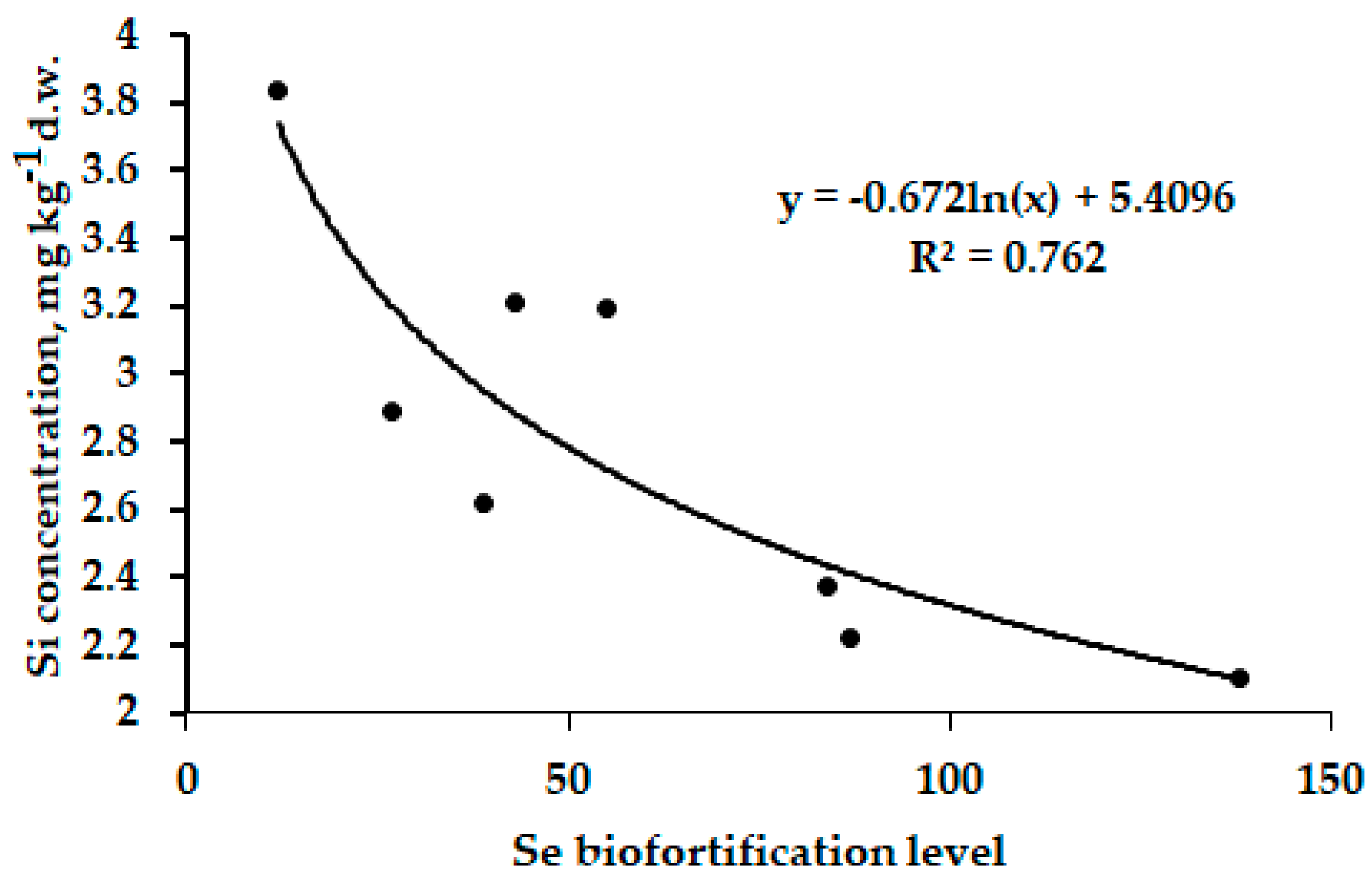
3.3.4. Cromium Relationships
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suarez, D.L.; Grieve, C.M.; Poss, J.A. Irrigation Method Affects Selenium Accumulation in Forage Brassica Species. J. Plant Nutr. 2003, 26, 191–201. [Google Scholar] [CrossRef]
- Seppänen, M.M.; Kontturi, J.; Heras, I.L.; Madrid, Y.; Cámara, C.; Hartikainen, H. Agronomic biofortification of Brassica with selenium—enrichment of SeMet and its identification in Brassica seeds and meal. Plant Soil 2010, 337, 273–283. [Google Scholar] [CrossRef]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of Selenium Enrichment and Measurement in Brassicaceous Vegetables, and Their Application to Human Health. Front. Plant Sci. 2017, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.; Matich, A.; Hunter, D.; Esfandiari, A.; Trolove, S.; Chen, R.; Lill, R. Selenium Application During Radish (Raphanus sativus) Plant Development Alters Glucosinolate Metabolic Gene Expression and Results in the Production of 4-(methylseleno)but-3-enyl glucosinolate. Plants 2019, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Bosco, A.D.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Golob, A.; Novak, T.; Marsic, N.K.; Šircelj, H.; Stibilj, V.; Jerše, A.; Kroflič, A.; Germ, M. Biofortification with selenium and iodine changes morphological properties of Brassica oleracea L. var. gongylodes) and increases their contents in tubers. Plant Physiol. Biochem. 2020, 150, 234–243. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Q.; Liao, X.; Yang, X.; Chao, W.; Cong, X.; Zhang, W.; Liao, Y.; Ye, J.; Qian, H.; et al. Exploring effects of exogenous selenium on the growth and nutritional quality of cabbage (Brassica oleracea var. capitata L.). Horticulturae 2023, 9, 330. [Google Scholar] [CrossRef]
- de Almeida, H.J.; Carmona, V.V.; Dutra, A.F.; Filho, A.B.C. Growth and physiological responses of cabbage cultivars biofortified with inorganic selenium fertilizers. Sci. Hort. 2022, 302, 111154. [Google Scholar] [CrossRef]
- Antoshkina, M.; Golubkina, N.; Poluboyarinov, P.; Skrypnik, L.; Sekara, A.; Tallarita, A.; Caruso, G. Effect of sodium selenate and selenocystine on Savoy cabbage yield, morphological and biochemical characteristics under Chlorella supply. Plants 2023, 12, 1020. [Google Scholar] [CrossRef]
- Muñoz, F.F.; Stoffel, M.M.; Céccoli, G.; Trod, B.S.; Daurelio, L.D.; Bouzo, C.A.; Guevara, M.G. Improving the foliar biofortification of broccoli with selenium without commercial quality losses. Crop. Sci. 2021, 61, 4218–4228. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, Quality and Antioxidant Properties of Indian Mustard (Brassica juncea L.) in Response to Foliar Biofortification with Selenium and Iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, C.; Zhao, X.; Tan, Q.; Sun, X.; Li, N. Impact of molybdenum on Chinese cabbage response to selenium in solution culture. Soil Sci. Plant Nutr. 2012, 58, 595–603. [Google Scholar] [CrossRef]
- Yuan, L.; Zhu, Y.; Lin, Z.Q.; Banuelos, G.; Li, W.; Yin, X. A novel selenocystine-accumulating plant in selenium-mine drainage area in Enshi, China. PLoS ONE 2013, 8, e65615. [Google Scholar] [CrossRef]
- Wu, M.; Cong, X.; Li, M.; Rao, S.; Liu, Y.; Guo, J.; Zhu, S.; Chen, S.; Xu, F.; Cheng, S.; et al. Effects of different exogenous selenium on Se accumulation, nutrition quality, elements uptake, and antioxidant response in the hyperaccumulation plant Cardamine violifolia. Ecotoxicol. Environ. Saf. 2020, 204, 111045. [Google Scholar] [CrossRef]
- Gui, J.-Y.; Rao, S.; Huang, X.; Liu, X.; Cheng, S.; Xu, F. Interaction between selenium and essential micronutrient elements in plants: A systematic review. Sci. Total Environ. 2022, 853, 158673. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; dos Reis, A.R. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; García-Caparrós, P.; Parvin, K.; Zulfiqar, F.; Ahmed, N.; Fujita, M. Selenium supplementation and crop plant tolerance to metal/metalloid toxicity. Front. Plant Sci. 2022, 12, 792770. [Google Scholar] [CrossRef] [PubMed]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium biofortification: Strategies, progress and challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium biofortification and interaction with other elements in plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Golubkina, N.; Moldovan, A.; Fedotov, M.; Kekina, H.; Kharchenko, V.; Folmanis, G.; Alpatov, A.; Caruso, G. Iodine and selenium biofortification of chervil plants treated with silicon nanoparticles. Plants 2021, 10, 2528. [Google Scholar] [CrossRef] [PubMed]
- Shiriaev, A.; Pezzarossa, B.; Rosellini, I.; Malorgio, F.; Lampis, S.; Ippolito, A.; Tonutti, P. Efficacy and comparison of different strategies for selenium biofortification of tomatoes. Horticulturae 2022, 8, 800. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Z.; Shui, Y.; Liu, X.; Chen, J.; Khan, S.; Wang, J.; Gao, Z. Methods of selenium application differentially modulate plant growth, selenium accumulation and speciation, protein, anthocyanins and concentrations of mineral elements in purple-grained wheat. Front. Plant Sci. 2020, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy, 2015. [Google Scholar]
- Kidin, V.V.; Derugin, I.P.; Konzarenko, V.I. Workshop on Agricultural Chemistry; KolosS: Moscow, Russia, 2008; p. 599. (In Russian) [Google Scholar]
- Skalny, A.V.; Lakarova, H.V.; Kuznetsov, V.V.; Skalnaya, M.G. Analytical Methods in Bioelementology; Saint Petersburg-Science: St. Petersburg, Russia, 2009. (In Russian) [Google Scholar]
- Alfthan, G.V. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 165, 187–194. [Google Scholar] [CrossRef]
- Antoshkina, M.; Golubkina, N.; Sekara, A.; Tallarita, A.; Caruso, G. Effects of selenium application on biochemical characteristics and biofortification level of kohlrabi (Brassica oleracea L. var. gongylodes) produce. Front. Biosci. 2021, 26, 533–542. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Stylianidis, G.P.; Manta, V.; Karousis, E.N.; Tzanaki, A.; Dimitriadi, D.; Bouzas, E.A.; Siyiannis, V.F.; Constantinou-Kokotou, V.; Chorianopoulou, S.N.; et al. Floret biofortification of broccoli using amino acids coupled with selenium under different surfactants: A case study of cultivating functional foods. Plants 2023, 12, 1272. [Google Scholar] [CrossRef] [PubMed]
- Antoshkina, M.S.; Golubkina, N.A.; Bondareva, L.L. Effect of foliar sodium selenate biofortification on cauliflower yield, nutritional value and antioxidant status. Veg. Crops Russ. 2020, 3, 63–68. [Google Scholar] [CrossRef]
- Utoiu, E.; Oancea, A.; Gaspar, A.; Seciu, A.M.; Stefan, L.M.; Coroiu, V.; Craciunescu, O.; Badiu, C.D.; Oancea, F. Selenium biofortification treatment of cauliflower enhances their content in chemopreventive compounds and in vitro antitumoral activity. Sci. Bull. Ser F. Biotechnol. 2017, 21, 33–40. [Google Scholar]
- Saeedi, M.; Soltani, F.; Babalar, M.; Izadpanah, F.; Wiesner-Reinhold, M.; Baldermann, S. Selenium Fortification Alters the Growth, Antioxidant Characteristics and Secondary Metabolite Profiles of Cauliflower (Brassica oleracea var. botrytis) Cultivars in Hydroponic Culture. Plants 2021, 10, 1537. [Google Scholar] [CrossRef]
- Montaner, C.; Mallor, C.; Laguna, S.; Zufiaurre, R. Bioactive compounds, antioxidant activity, and mineral content of bróquil: A traditional crop of Brassica oleracea var. italica. Front. Nutr. 2023, 9, 1006012. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Dou, J.; Lv, J.; Jin, N.; Jin, L.; Li, Z.; Zhang, B.; Tang, Z.; Yu, J. A comparative study on the nutrients, mineral elements, and antioxidant compounds in different types of Cruciferous vegetables. Agronomy 2022, 12, 3121. [Google Scholar] [CrossRef]
- Lesharadevi, K.; Parthasarathi, T.; Muneer, S. Silicon biology in crops under abiotic stress: A paradigm shift and cross-talk between genomics and proteomics. J. Biotechnol. 2021, 333, 21–38. [Google Scholar] [CrossRef]
- Wenneck, G.S.; Saath, R.; Rezende, R. Silicon accumulation in cauliflower grown in a protected environment with different water availability conditions. Pesqui. Agropecuária Bras. 2022, 57, e02392. [Google Scholar] [CrossRef]
- Longchamp, M.; Angeli, N.; Castrec-Rouelle, M. Effects on the accumulation of calcium, magnesium, iron, manganese, copper and zinc of adding the two inorganic forms of selenium to solution cultures of Zea mays. Plant Physiol. Biochem. 2016, 98, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-W.; Dong, Y.-Y.; Feng, L.-Y.; Deng, Z.-L.; Xu, Q.; Tao, Q.; Wang, C.-Q.; Chen, Y.-E.; Yuan, M.; Yuan, S. Selenium Enhances Cadmium Accumulation Capability in Two Mustard Family Species—Brassica napus and B. juncea. Plants 2020, 9, 904. [Google Scholar] [CrossRef]
- Golubkina, N.; Mironov, J. Element composition of mushrooms in contrasting anthropogenic loading. Geochem. Internat. 2018, 56, 1263–1275. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Morel, M.; Crouzet, J.; Gravot, A.; Auroy, P.; Leonhardt, N.; Vavasseur, A.; Richaud, P. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 2009, 149, 894–904. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Qin, H.; Lin, X.; Tang, D.; Wu, Z. A rice chloroplast-localized ABC transporter ARG1 modulates cobalt and nickel homeostasis and contributes to photosynthetic capacity. New Phytol. 2020, 228, 163–178. [Google Scholar] [CrossRef]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An essential micronutrient for plant growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef]
- Patra, A.; Pradhan, S.N.; Dutta, A.; Mohapatra, K.K. Nickel the ultra-micronutrient: Significant for plant growth and metabolism. Food Sci. Rep. 2020, 1, 35–37. [Google Scholar]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of nickel in plants: A role in plant stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Interactions of zinc with other nutrients in soils and plants—A review. Ind. J. Fertil. 2016, 12, 16–26. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Ammar, A.; Bilal, S.; Ali, U.; Huma, N.; Adnan, M. Mitigating zinc deficiency in plants and soils through agronomic techniques: A review. J. Environ. Agric. Sci. 2021, 23, 1–10. [Google Scholar]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of silicon with essential and beneficial elements in plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Kharchenko, V.A.; Golubkina, N.A.; Moldovan, A.I.; Caruso, G. Biofortification of chervil with selenium. Veg. Crops Russ. 2021, 1, 79–86. (In Russian) [Google Scholar] [CrossRef]
- Golubkina, N.; Logvinenko, L.; Konovalov, D.; Garsiya, E.; Fedotov, M.; Alpatov, A.; Shevchuk, O.; Skrypnik, L.; Sekara, A.; Caruso, G. Foliar application of selenium under nano silicon on Artemisia annua: Effects on yield, antioxidant status, essential oil, artemisinin content and mineral composition. Horticulturae 2022, 8, 597. [Google Scholar] [CrossRef]
- Ahire, M.L.; Mundada, P.S.; Nikam, T.D.; Bapat, V.A.; Penna, S. Multifaceted roles of silicon in mitigating environmental stresses in plants. Plant Physiol. Biochem. 2021, 169, 291–310. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.; Zhao, J.; Wang, F.; Du, Y.; Zou, S.; Li, H.; Wen, D.; Huang, Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exper. Bot. 2017, 133, 1–11. [Google Scholar] [CrossRef]
- Orabi, A.A.; Ismail, A.S.; Mashadi, H. Zinc-phosphorus relationship in the nutrition of tomato plants as affected both by the soil and by the rate of applied zinc. Plant Soil 1982, 69, 67–72. [Google Scholar] [CrossRef]
- Pongrac, P.; McNicol, J.W.; Lilly, A.; Thompson, J.A.; Wright, G.; Hillier, S.; White, P.J. Mineral element composition of cabbage as affected by soil type and phosphorus and zinc fertilization. Plant Soil 2018, 434, 151–165. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Deng, Y.; Cen, R.-Y.; Cui, Z.-L.; Chen, X.-P.; Yost, R.; Zhang, F.-S.; Zou, C.-Q. The reduction in zinc concentration of wheat grain upon increased phosphorus fertilization and its mitigation by foliar zinc application. Plant Soil 2012, 361, 143–152. [Google Scholar] [CrossRef]
- Yawco, N.J.; Saito, M.A. Competitive inhibition of Co uptake by Zn and Mn in a pacific Prochlotococcus strain: Insights into metal homeostasis in a streamlines oligotrophic cyanobacterium. Limnol. Oceanogr. 2018, 63, 222902249. [Google Scholar] [CrossRef]
- Akoumianaki-Ioannidou, A.; Barouchas, P.E.; Ilia, E.; Kyramariou, A.; Moustakas, N.K. Effect of vanadium on dry matter and nutrient concentration in sweet basil (Ocimum basilicum L.). Aust. J. Crop Sci. 2016, 10, 199–206. [Google Scholar]
- Hanus-Fajerska, E.; Wiszniewska, A.; Kamińska, I. A dual role of vanadium in environmental systems—Beneficial and detrimental effects on terrestrial plants and humans. Plants 2021, 10, 1110. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wang, X.; Qing, X.; Wang, P.; Zhang, Y.; Zhang, X.; Zhao, X. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019, 173, 314–321. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Huang, H.; Ali, S.; Mwamba, T.M.; Ali, B.; Huang, Q.; Hamid, Y.; Khan, A.R.; Wang, J.; et al. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Biochem. 2019, 145, 142–152. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Protective role of selenium against chromium stress involving metabolites and essential elements in Brassica juncea L. seedlings. 3 Biotech 2018, 8, 66. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An Overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Fu, X.; Mehmood, S.; Ahmed, W.; Ou, W.; Suo, P.; Zhang, Q.; Fu, X.; Sun, Z.; Li, W. Reducing chromium toxicity in Chinese cabbage through synergistic effects of silicon and selenium: A study of plant growth, chromium content, and biochemical parameters. Sustainability 2023, 15, 5361. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Ibrahim, M.; Rizwan, M.; Hannan, F.; Adrees, M.; Khan, M.D. Silicon and Chromium Toxicity in Plants: An Overview. In Silicon in Plants, Advances and Future Prospects; Chapter 12 in book; Tripathi, D.K., Singh, V.P., Ahmad, P., Chauhan, D.K., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Zia ur Rehman, M.; Hameed, A.; Hafeez, F.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L. Role of Zinc–Lysine on Growth and Chromium Uptake in Rice Plants under Cr Stress. J. Plant Growth Regul. 2018, 37, 1413–1422. [Google Scholar] [CrossRef]
- Hassan, M.U.; Nawaz, M.; Mahmood, A.; Shah, A.A.; Shah, A.N.; Muhammad, F.; Batool, M.; Rasheed, A.; Jaremko, M.; Abdelsalam, N.R.; et al. The role of zinc to mitigate heavy metals toxicity in crops. Front. Environ. Sci. 2022, 10, 90223. [Google Scholar] [CrossRef]
- Li, L.; Zhang, K.; Gill, R.A.; Islam, F.; Farooq, M.A.; Wang, J.; Zhou, W. Ecotoxicological and interactive effects of copper and chromium on physiochemical, ultrastructural, and molecular profiling in Brassica napus L. Biomed Res. Int. 2018, 2018, 9248123. [Google Scholar] [CrossRef]
- Owolabi, I.A.; Mandiwana, K.L.; Panichev, N. Speciation of chromium and vanadium in medicinal plants. S. Afr. J. Chem. 2016, 69, 67–71. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, R.; Chen, Y.; Jiang, L.; Lei, L.; Xu, H.; Feng, H. Effects of secondary release of chromium and vanadium on soil properties, nutrient cycling and bacterial communities in contaminated acidic paddy soil. J. Environ. Manag. 2023, 326, 116725. [Google Scholar] [CrossRef]
| 2021 | 2022 | |||
|---|---|---|---|---|
| Month | Mean Temperature (°C) | Rainfall (mm) | Mean Temperature (°C) | Rainfall (mm) |
| May | 13.8 | 81 | 10.0 | 55.5 |
| June | 21.8 | 20 | 18.6 | 24.6 |
| July | 22.0 | 38 | 20.2 | 66.1 |
| August | 19.4 | 36 | 22.3 | 13.7 |
| September | 9.1 | 58 | 9.6 | 125.7 |
| Species | Cultivar | Plant Phenological Phase | Date of Foliar Sodium Selenate Treatment | Harvesting Date |
|---|---|---|---|---|
| Kohlrabi (Brassica oleracea var. gongylodes) | Dobrynya F1 | Stem formation |
| 24/07 |
| Broccoli (Brassica oleracea var. italica) | Tonus | Florets formation | ||
| White cabbage (Brassica oleracea L. var. capitata) | Early ripe F1 Aurora | Head formation |
| 29/07 |
| Medium-ripe Slava 1355 |
| 22/08 | ||
| Red cabbage (Brassica oleracea var. capitata f. rubra) | Kamennaya Golovka 447 |
| 22/08 | |
| Savoy cabbage (Brassica oleracea L. var. sabauda) | Vertu 1340 | |||
| Melissa F1 | ||||
| Cauliflower (Brassica oleracea var. botrytis) | Freedom F1 |
| 12/08 |
| Early Ripe White Cabbage | Medium- Ripe White Cabbage | Cauliflower | Broccoli | Red Cabbage | Savoy Cabbage * | Savoy Cabbage ** | Kohlrabi | M ± SD | |
|---|---|---|---|---|---|---|---|---|---|
| Ca | 5400 a | 2708 | 3031 bc | 4535 a | 2998 bc | 2031 d | 1456 e | 3620 b | 3222 ± 1283 |
| K | 26,411 bc | 24,088 c | 32,400 ab | 29,099 b | 27,086 bc | 29,896 b | 32,605 ab | 39,640 a | 30,153 ± 4817 |
| Mg | 1482 bc | 997 bd | 1573 b | 2201 a | 1261 c | 1256 c | 1342 c | 1102 d | 1402 ± 373 |
| Na | 1997 a | 856 b | 845 b | 558 c | 408 d | 230 e | 441 d | 839 b | 772 ± 548 |
| P | 4117 b | 2889 c | 4611 b | 7746 a | 2979 c | 4596 b | 4802 b | 6599 a | 4792 ± 1665 |
| Al | 6.27 d | 5.40 d | 3.85 e | 3.60 e | 15.31 b | 4.23 e | 5.44 d | 10.69 c | 6.85 ± 4.09 |
| As | 0.03 b | 0.018 c | 0.01 d | 0.02 c | 0.04 a | 0.01 d | 0.01 d | 0.01 d | 0.0185 ± 0.011 |
| Cd | 0.04 b | 0.021 c | 0.05 a | 0.04 b | 0.01 d | 0.02 c | 0.02 c | 0.04 b | 0.030 ± 0.014 |
| Cr | 0.31 c | 0.97 a | 0.28 c | 0.17 d | 0.72 b | 0.31 c | 0.33 c | 0.20 d | 0.411 ± 0.281 |
| Ni | 1.24 d | 2.82 b | 1.35 d | 0.44 e | 2.97 b | 5.78 a | 3.08 b | 1.88 c | 2.45 ± 1.64 |
| Pb | 0.09 e | 0.34 a | 0.19 c | 0.07 | 0.17 c | 0.23 b | 0.13 d | 0.19 c | 0.176 ± 0.085 |
| Sn | 0.1 b | 0.035 c | 0.02 d | 0.008 e | 0.008 e | 0.04 c | 0.02 d | 0.053 a | 0.036 ± 0.030 |
| Sr | 22.07 b | 13.1 d | 12.89 d | 37.05 a | 11.51 d | 6.87 e | 3.67 f | 15.97 c | 15.39 ± 10.36 |
| V | 0.05 c | 0.12 b | 0.03 e | 0.04 d | 0.20 a | 0.04 d | 0.03 e | 0.04 d | 0.071 ± 0.065 |
| B | 4.8 c | 19.5 a | 2.18 e | 10.02 b | 3.91 d | 3.69 d | 5.38 c | 8.93 b | 7.30 ± 5.60 |
| Co | 0.13 de | 0.18 b | 0.09 f | 0.09 f | 0.17 bc | 0.19 b | 0.15 cd | 0.11 e | 0.139 ± 0.040 |
| Cu | 2.18 e | 3.64 d | 3.51 d | 5.41 b | 2.73 e | 4.25 cd | 4.28 c | 4.38 c | 3.80 ± 1.02 |
| Fe | 48.72 d | 72.9 b | 59.44 bc | 110 a | 118 a | 52.16 cd | 63.1 b | 43.15 d | 70.93 ± 28.17 |
| I | 0.95 a | 0.24 e | 0.11 f | 0.31 cd | 0.34 c | 0.40 b | 0.27 de | 0.23 e | 0.356 ± 0.255 |
| Li | 0.07 c | 0.092 b | 0.04 d | 0.04 d | 0.22 a | 0.10 b | 0.09 b | 0.02 e | 0.084 ± 0.062 |
| Mn | 16.39 d | 24.00 bc | 17.96 cd | 26.45 b | 23.48 bc | 34.99 a | 21.73 c | 9.33 e | 21.79 ± 7.58 |
| Mo | 0.83 a | 0.46 c | 0.63 b | 0.86 a | 1.05 a | 0.59 b | 1.07 a | 0.6 b | 0.76 ± 0.23 |
| Se | 0.11 c | 0.12 bc | 0.13 ab | 0.09 c | 0.07 | 0.09 c | 0.06 d | 0.15 a | 0.103 ± 0.030 |
| Si | 2.57 b | 3.63 a | 2.14 c | 2.63 b | 3.67 a | 2.62 b | 3.58 a | 2.7 b | 2.94 ± 0.59 |
| Zn | 25.9 d | 13.1 e | 52.55 b | 80.3 a | 26.84 d | 31.73 c | 33.95 c | 29.64 c | 36.75 ± 20.72 |
| Early Ripe White Cabbage | Medium- Ripe White Cabbage | Cauliflower | Broccoli | Red Cabbage | Savoy Cabbage * | Savoy Cabbage ** | Kohlrabi | M ± SD | |
|---|---|---|---|---|---|---|---|---|---|
| Ca | 5026 a | 4981 a | 2597 c | 6169 a | 2840 c | 3128 c | 2699 c | 3951 b | 3924 ± 1333 |
| K | 23,729 c | 26,651 bc | 36,654 a | 35,849 a | 24,576 bc | 25,781 bc | 30,792 b | 38,889 a | 30,365 ± 6034 |
| Mg | 1298 c | 1089 c | 1914 b | 2555 a | 1117 | 1281 c | 1261 c | 1316 c | 1479 ± 504 |
| Na | 1637 a | 775 c | 1564 a | 470 e | 481 e | 609 d | 336 f | 1111 b | 873 ± 507 |
| P | 3737 b | 3133 cd | 4582 b | 6831 a | 2624 d | 3905 b | 4113 b | 6086 a | 4376 ± 1430 |
| Al | 5.53 c | 14.6 b | 4.28 d | 3.1 e | 3.99 d | 18.75 a | 3.74 d | 13.53 b | 8.44 ± 6.17 |
| As | 0.04 a | 0.0088 c | 0.01 b | 0.01 b | 0.01 b | 0.04 a | 0.01 b | 0.01 b | 0.017 ± 0.014 |
| Cd | 0.07 a | 0.024 d | 0.04 b | 0.04 b | 0.007 e | 0.03 c | 0.02 d | 0.03 c | 0.033 ± 0.019 |
| Cr | 0.74 a | 0.25 c | 0.38 b | 0.17 d | 0.38 b | 0.36 b | 0.12 e | 0.21 c | 0.326 ± 0.194 |
| Ni | 1.11 d | 2.4 c | 1.28 d | 0.54 f | 2.90 b | 4.13 a | 2.94 b | 0.87 e | 2.02 ± 1.26 |
| Pb | 0.13 d | 0.24 b | 0.21 b | 0.09 e | 0.12 d | 0.50 a | 0.19 c | 0.09 e | 0.196 ± 0.135 |
| Sn | 0.06 b | 0.021 d | 0.006 | 0.02 d | 0.04 c | 0.01 e | 0.01 e | 0.042 a | 0.026 ± 0.019 |
| Sr | 19.54 c | 25.4 b | 10.18 e | 46.49 a | 10.34 e | 13.14 d | 7.01 f | 16.46 c | 18.57 ± 12.72 |
| V | 0.04 c | 0.019 e | 0.04 c | 0.03 d | 0.04 c | 0.19 a | 0.03 d | 0.07 b | 0.057 ± 0.056 |
| B | 3.5 de | 21.9 a | 1.76 f | 11.19 b | 3.12 e | 4.09 d | 3.9 d | 7.5 c | 7.12 ± 6.68 |
| Co | 0.10 de | 0.15 c | 0.11 d | 0.06 f | 0.19 b | 0.19 b | 0.27 a | 0.08 e | 0.144 ± 0.070 |
| Cu | 1.98 e | 3.54 bc | 3.53 bc | 4.69 a | 2.82 d | 2.94 d | 3.24 c | 4.13 ab | 3.36 ± 0.83 |
| Fe | 48.01 de | 40.2 e | 65.9 c | 98.19 b | 49.24 d | 121 a | 40.14 e | 60.25 c | 65.37 ± 29.35 |
| I | 0.97 a | 0.32 c | 0.48 b | 0.23 e | 0.25 de | 0.29 cd | 0.45 b | 0.08 f | 0.384 ± 0.268 |
| Li | 0.05 d | 0.054 d | 0.05 d | 0.04 e | 0.26 a | 0.17 b | 0.11 c | 0.03 f | 0.096 ± 0.081 |
| Mn | 16.34 f | 17.5 ef | 21.75 d | 28.21 c | 18.59 de | 37.94 b | 46.39 a | 9.09 g | 24.48 ± 12.36 |
| Mo | 0.62 b | 0.37 c | 0.66 b | 0.61 b | 0.58 b | 0.64 b | 0.86 a | 0.55 b | 0.611 ± 0.135 |
| Se | 4.29 d | 1.45 g | 17.91 a | 7.56 c | 3.0 e | 2.44 f | 3.31 e | 13.05 b | 6.62 ± 5.89 |
| Si | 2.62 cd | 3.83 a | 2.10 c | 2.37 c | 3.21 ab | 2.89 bc | 3.19 b | 2.22 d | 2.80 ± 0.59 |
| Zn | 48.2 b | 11.4 f | 35.28 c | 74.72 a | 22.92 e | 21.42 e | 25.14 de | 28.16 d | 33.40 ± 19.87 |
| Early Ripe White Cabbage | Medium-Ripe White Cabbage | Cauliflower | Broccoli | Red Cabbage | Savoy Cabbage * | Savoy Cabbage ** | Kohlrabi | |
|---|---|---|---|---|---|---|---|---|
| Macro-elements and Si | ||||||||
| Mg | Relatively high stability levels | |||||||
| P | ||||||||
| Si | ||||||||
| Ca | 1.84 | 1.36 | 1.54 | 1.85 | ||||
| K | 1.23 | |||||||
| Na | 1.85 | 2.65 | 0.76 | 1.32 | ||||
| Micro-elements | ||||||||
| B | 0.73 | 0.81 | 0.8 | 0.72 | ||||
| Co | 0.77 | 0.83 | 1.22 | 0.67 | 1.80 | 0.73 | ||
| Cu | 0.69 | 0.76 | ||||||
| Fe | 0.42 | 2.32 | 0.64 | 1.40 | ||||
| I | 1.33 | 4.36 | 0.74 | 0.74 | 0.73 | 1.67 | 0.06 | |
| Li | 0.71 | 0.59 | 1.70 | 1.50 | ||||
| Mn | 0.73 | 0.79 | 2.13 | |||||
| Mo | 0.75 | 0.80 | 0.71 | 0.55 | ||||
| Zn | 1.86 | 0.67 | 0.68 | 0.74 | ||||
| As, heavy metals and Al | ||||||||
| Al | 2.70 | 0.26 | 4.43 | 0.69 | 1.27 | |||
| As | 1.33 | 0.49 | 0.50 | 0.25 | 4.00 | |||
| Cd | 1.75 | 0.70 | 1.50 | 0.75 | ||||
| Cr | 2.39 | 0.26 | 1.36 | 0.53 | 0.36 | |||
| Pb | 1.44 | 0.71 | 0.71 | 2.17 | 1.46 | 0.47 | ||
| Ni | 0.71 | 0.46 | ||||||
| Sr | 1.94 | 1.25 | 1.91 | 1.91 | ||||
| V | 0.80 | 0.16 | 1.33 | 0.75 | 0.20 | 4.75 | 1.75 | |
| Elements | Ca | K | Mg | Na | P | Al | As | Cd | Cr | Ni | Pb | Sn | |
| Ca | 1 | −0.148 | 0.475 | 0.746 g | 0.318 | 0.028 | 0.454 | 0.585 | −0.272 | −0.723 g | −0.509 | 0.523 | |
| K | 0.015 | 1 | −0.066 | −0.196 | 0.593 | 0.111 | −0.596 | 0.392 | −0.651 k | −0.079 | −0.176 | −0.032 | |
| Mg | 0.434 | 0.514 | 1 | 0.025 | 0.641 | −0.416 | 0.073 | 0.484 | −0.558 | −0.575 | −0.737 g | −0.256 | |
| Na | 0.046 | −0.155 | 0.022 | 1 | −0.117 | −0.082 | 0.281 | 0.551 | −0.074 | −0.530 | −0.241 | 0.837 c | |
| P | 0.439 | −0.093 | 0.761 f | 0.029 | 1 | −0.289 | −0.409 | 0.565 | −0.794 e | −0.430 | −0.547 | −0.430 | |
| Al | −0.011 | −0.493 | −0.444 | −0.036 | −0.112 | 1 | 0.615 | −0.437 | 0.323 | 0.055 | 0.017 | −0.064 | |
| As | 0.061 | −0.075 | −0.221 | 0.305 | −0.227 | 0.355 | 1 | −0.344 | 0.419 | −0.196 | −0,248 | 0.078 | |
| Cd | 0.486 | 0.072 | 0.317 | 0.735 g | 0.277 | −0.124 | 0.580 | 1 | −0.634 | −0.700 1 | −0.350 | 0.301 | |
| Cr | 0.065 | −0.033 | −0.222 | 0.691 j | −0.410 | −0.069 | 0.710 1 | 0.666 k | 1 | 0.263 | 0.699 d | −0.159 | |
| Ni | −0.573 | 0.089 | −0.596 | −0.518 | −0.668 k | 0.435 | 0.288 | −0.552 | −0.088 | 1 | 0.511 | −0.071 | |
| Pb | −0.348 | 0.170 | −0.268 | −0.156 | −0.341 | 0.676 k | 0.536 | −0.115 | 0.035 | 0.769 f | 1 | −0.086 | |
| Sn | 0.351 | −0.582 | −0.301 | 0.384 | −0.668 k | −0.103 | 0.287 | 0.387 | 0.611 | −0.376 | −0.532 | 1 | |
| Sr | 0.922 b | 0.211 | 0.679k | −0.146 | 0.582 | −0.092 | −0.114 | 0.301 | −0.169 | −0.538 | −0.298 | 0.071 | |
| V | −0.291 | −0.215 | −0.178 | −0.106 | −0.027 | 0.696 h | 0.644 | −0.033 | 0.090 | 0.570 | 0.812 e | −0.216 | |
| B | 0.619 | −0.035 | −0.016 | −0.226 | 0.041 | 0.367 | −0.332 | −0.141 | −0.357 | −0.110 | −0.040 | −0.071 | |
| Co | −0.655 k | 0.171 | −0.576 | −0.550 | −0.624 | 0.035 | 0.010 | −0.581 | −0.230 | 0.840 c | 0.425 | −0.369 | |
| Cu | 0.325 | 0.024 | 0.638 | −0.313 | 0.778 f | −0.004 | −0.667 h | −0.257 | −0.790 e | −0.428 | −0.265 | −0.411 | |
| Fe | 0.184 | 0.112 | 0.472 | −0.078 | 0.394 | 0.392 | 0.417 | 0.158 | 0.013 | 0.086 | 0.506 | −0.262 | |
| I | 0.094 | 0.281 | −0.109 | 0.579 | −0.339 | −0.337 | 0.565 | 0.737 g | 0.789 e | −0.130 | −0.041 | 0.359 | |
| Li | −0.549 | 0.054 | −0.426 | −0.511 | −0.621 | −0.001 | 0.116 | −0.618 | 0.059 | 0.744 g | 0.306 | −0.024 | |
| Mn | −0.312 | 0.535 | 0.097 | −0.583 | −0.046 | −0.083 | 0.139 | −0.223 | −0.366 | 0.572 | 0.500 | −0.665 k | |
| Mo | −0.482 | 0.301 | 0.155 | −0.164 | 0.127 | −0.471 | 0.107 | 0.029 | −0.109 | 0.167 | 0.045 | −0.293 | |
| Se | −0.171 | −0.049 | 0.489 | 0.572 | 0.555 | −0.219 | −0.328 | 0.230 | −0.064 | −0.616 | −0.301 | −0.142 | |
| Si | −0.028 | 0.082 | −0.622 | −0.516 | −0.715 g | 0.251 | −0.072 | −0.497 | −0.139 | 0.632 | 0.267 | −0.075 | |
| Zn | 0.616 | 0.333 | 0.856 b | 0.121 | 0.689 d | −0.550 | 0.057 | 0.573 | 0.103 | −0.688 h | −0.456 | 0.158 | |
| Sr | V | B | Co | Cu | Fe | I | Li | Mn | Mo | Se | Si | Zn | |
| Ca | 0.824 c | −0.066 | 0.038 | −0.544 | −0.24 | 0.074 | 0.609 | −0.304 | −0.396 | −0.005 | 0.371 | −0.400 | 0.308 |
| K | −0.139 | −0.519 | −0.301 | −0.469 | 0.424 | −0.440 | −0.395 | −0.507 | −0.522 | −0.058 | 0.378 | −0.476 | 0.497 |
| Mg | 0.764 f | −0.349 | −0.22 | −0.661 k | 0.397 | 0.430 | 0.082 | −0.307 | 0.167 | 0.336 | −0.214 | −0.458 | 0.952 a |
| Na | 0.331 | −0.158 | 0.044 | −0.296 | −0.600 | −0.393 | 0.729 g | −0.314 | −0.560 | −0.112 | 0.437 | −0.198 | −0.205 |
| P | 0.612 | −0.633 | −0.043 | −0.703 h | 0.768 f | −0.01− | −0.161 | −0.681 k | −0.161 | 0.024 | 0.186 | −0.579 | 0.925 b |
| Al | −0.185 | 0.736 g | −0.125 | 0.223 | −0.408 | 0.334 | 0.013 | 0.633 | −0.033 | 0.357 | −0.082 | 0.462 | −0.389 |
| As | 0.265 | 0.772 f | −0.061 | 0.193 | −0.618 | 0.619 | 0.498 | 0.700 h | 0.012 | 0.485 | −0.336 | 0.527 | −0.219 |
| Cd | 0.531 | −0.663 k | −0.128 | −0.884 b | 0.134 | −0.373 | 0.043 | −0.837 c | −0.528 | −0.316 | 0.671 j | −0.834 c | 0.590 |
| Cr | −0.311 | 0.798 e | 0.563 | 0.659 k | −0.406 | 0.331 | −0.114 | 0.618 | 0.200 | −0.149 | −0.115 | 0.731 g | −0.707 h |
| Ni | −0.738 g | 0.153 | −0.127 | 0.847 b | −0.009 | −0.202 | −0.083 | 0.449 | 0.620 | −0.147 | −0.341 | 0.246 | −0.533 |
| Pb | −0.513 | 0.319 | 0.536 | 0.575 | −0.074 | −0.202 | −0.421 | 0.147 | 0.179 | −0.705 h | 0.332 | 0.242 | −0.606 |
| Sn | 0.061 | −0.270 | −0.023 | 0.024 | −0.508 | 0.679 k | 0.796 e | −0.294 | −0.385 | −0.258 | 0.418 | −0.182 | −0.428 |
| Sr | 1 | −0.135 | 0.232 | −0.623 | 0.273 | 0.380 | 0.242 | −0.383 | −0.104 | 0.016 | 0.194 | −0.340 | 0.672 k |
| V | −0.239 | 1 | 0.202 | 0.509 | −0.467 | 0.641 | −0.024 | 0.860 b | 0.134 | 0.220 | −0.276 | 0.670 k | −0.463 |
| B | 0.574 | −0.293 | 1 | 0.181 | 0.251 | 0.111 | −0.191 | −0.163 | −0.003 | −0.466 | 0.279 | 0.389 | −0.207 |
| Co | −0.643 | 0.164 | −0.203 | 1 | −0.277 | 0.019 | 0.122 | 0.677 k | 0.561 | −0.049 | −0.420 | 0.646 | −0.805 e |
| Cu | 0.570 | −0.172 | 0.420 | −0.404 | 1 | 0.116 | −0.564 | −0.473 | 0.257 | −0.123 | −0.031 | −0.392 | 0.665 k |
| Fe | 0.376 | 0.727 g | −0.091 | −0.359 | 0.265 | 1 | −0.195 | 0.567 | 0.353 | 0.497 | −0.533 | 0.367 | 0.360 |
| I | −0.146 | −0.201 | −0.296 | 0.006 | −0.731 g | −0.296 | 1 | 0.087 | −0.045 | 0.212 | −0.112 | 0.330 | −0.266 |
| Li | −0.455 | 0.332 | −0.365 | 0.628 | −0.422 | 0.063 | −0.184 | 1 | 0.393 | 0.476 | −0.642 | 0.674 j | −0.513 |
| Mn | −0.158 | 0.301 | −0.238 | 0.691 1,j | −0.035 | 0.131 | 0.021 | 0.276 | 1 | 0.012 | −0.600 | 0.096 | 0.065 |
| Mo | −0.393 | 0.096 | −0.735 g | 0.479 | −0.154 | −0.095 | 0.244 | 0.157 | 0.721 g | 1 | −0.779 f | 0.415 | 0.180 |
| Se | −0.055 | −0.132 | −0.306 | −0.546 | 0.424 | 0.112 | −0.126 | −0.484 | −0.350 | 0.099 | 1 | −0.524 | −0.015 |
| Si | −0.122 | −0.112 | 0.525 | 0.634 | −0.295 | −0.398 | −0.030 | 0.415 | 0.213 | −0.289 | −0.826 d | 1 | −0.663 k |
| Zn | 0.712 h | −0.236 | −0.127 | −0.621 | 0.334 | 0.345 | 0.200 | −0.402 | −0.010 | 0.198 | 0.269 | −0.609 | 1 |
| Ca | K | Mg | Na | P | Al | As | Cd | Cr | Ni | Pb | Sn | Sr | V | B | Co | Cu | Li | Mn | Mo | Se | Si | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | − | |||||||||||||||||||||
| P | + | |||||||||||||||||||||
| Cd | + | |||||||||||||||||||||
| Cr | − | + | − | + | ||||||||||||||||||
| Ni | − | + | − | |||||||||||||||||||
| Pb | − | + | − | + | ||||||||||||||||||
| Sn | − | + | ||||||||||||||||||||
| Sr | + | − | ||||||||||||||||||||
| V | − | − | − | + | ||||||||||||||||||
| Co | + | − | − | − | − | |||||||||||||||||
| Cu | + | |||||||||||||||||||||
| Fe | − | + | ||||||||||||||||||||
| I | − | + | + | − | + | |||||||||||||||||
| Li | − | − | − | + | − | |||||||||||||||||
| Mn | + | + | ||||||||||||||||||||
| Mo | − | + | + | |||||||||||||||||||
| Se | − | − | ||||||||||||||||||||
| Si | + | − | − | − | − | − | + | |||||||||||||||
| Zn | − | − | + | + | − | − | − |
 significant ‘r’ decrease (p < 0.05);
significant ‘r’ decrease (p < 0.05);  significant ‘r’ increase (p < 0.05)
significant ‘r’ increase (p < 0.05)Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Antoshkina, M.; Bondareva, L.; Sekara, A.; Campagna, E.; Caruso, G. Effect of Foliar Application of Sodium Selenate on Mineral Relationships in Brassicaceae Crops. Horticulturae 2023, 9, 535. https://doi.org/10.3390/horticulturae9050535
Golubkina N, Antoshkina M, Bondareva L, Sekara A, Campagna E, Caruso G. Effect of Foliar Application of Sodium Selenate on Mineral Relationships in Brassicaceae Crops. Horticulturae. 2023; 9(5):535. https://doi.org/10.3390/horticulturae9050535
Chicago/Turabian StyleGolubkina, Nadezhda, Marina Antoshkina, Ludmila Bondareva, Agnieszka Sekara, Erica Campagna, and Gianluca Caruso. 2023. "Effect of Foliar Application of Sodium Selenate on Mineral Relationships in Brassicaceae Crops" Horticulturae 9, no. 5: 535. https://doi.org/10.3390/horticulturae9050535
APA StyleGolubkina, N., Antoshkina, M., Bondareva, L., Sekara, A., Campagna, E., & Caruso, G. (2023). Effect of Foliar Application of Sodium Selenate on Mineral Relationships in Brassicaceae Crops. Horticulturae, 9(5), 535. https://doi.org/10.3390/horticulturae9050535









