Preharvest Multiple Applications of GABA Improve Quality Traits and Antioxidant Compounds of Pomegranate Fruit during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and GABA Treatments
2.2. Fruit Quality Parameter Measures
2.3. Total Phenolic, Total Anthocyanin and Individual Anthocyanin Measures
2.4. Statistical Analysis
3. Results
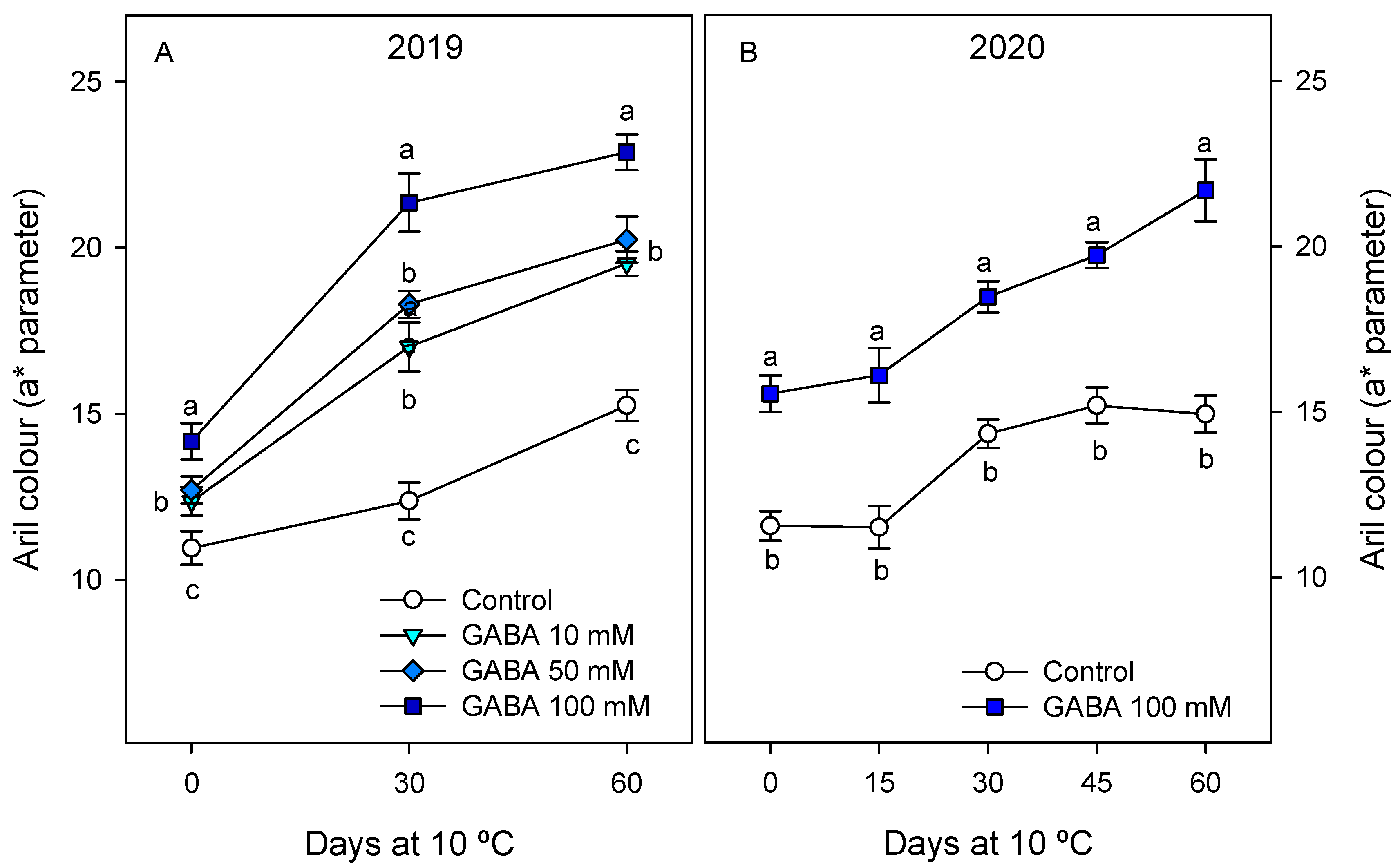
3.1. The Influence of GABA Treatments on the Physicochemical Quality Parameters of Fruit
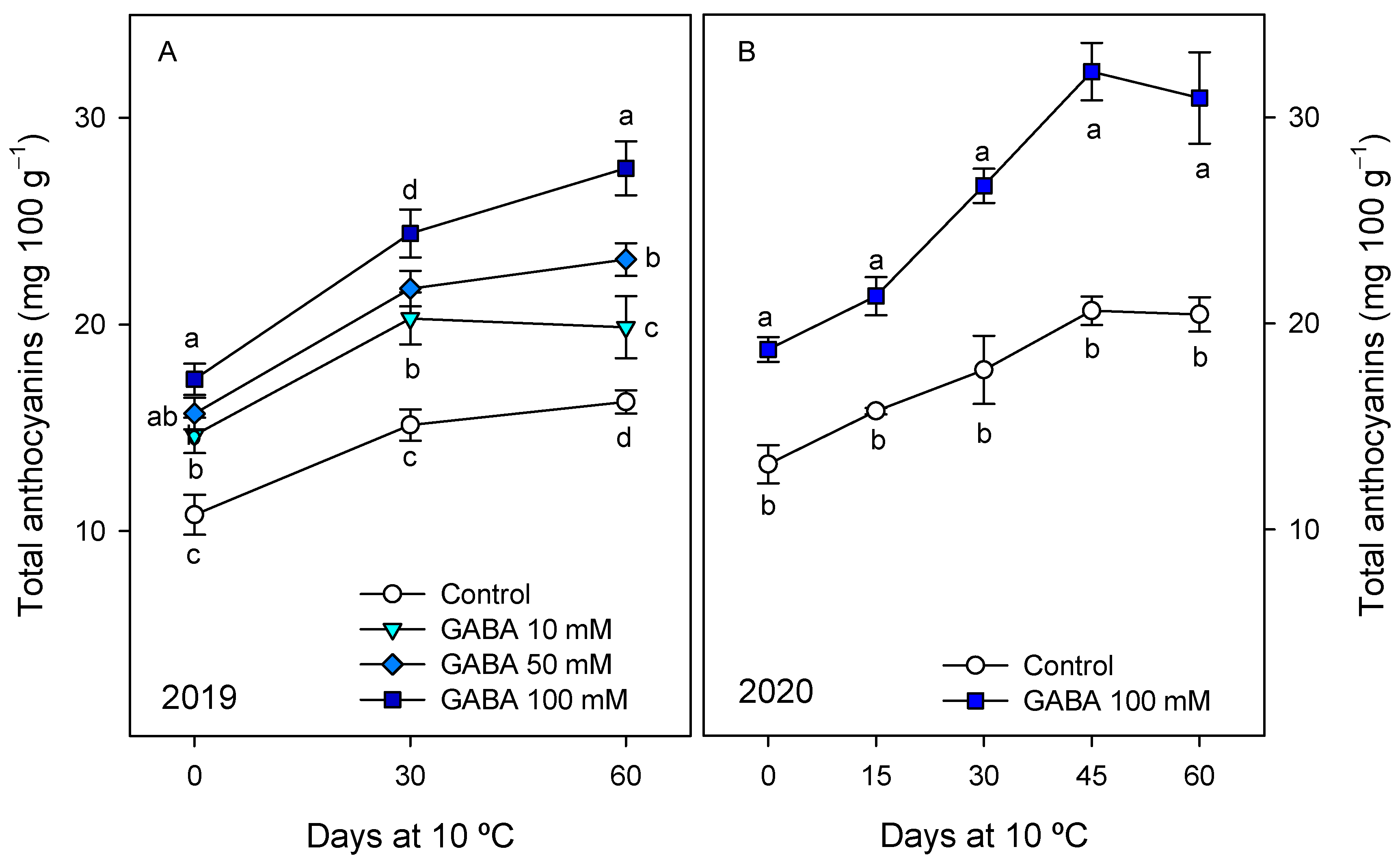
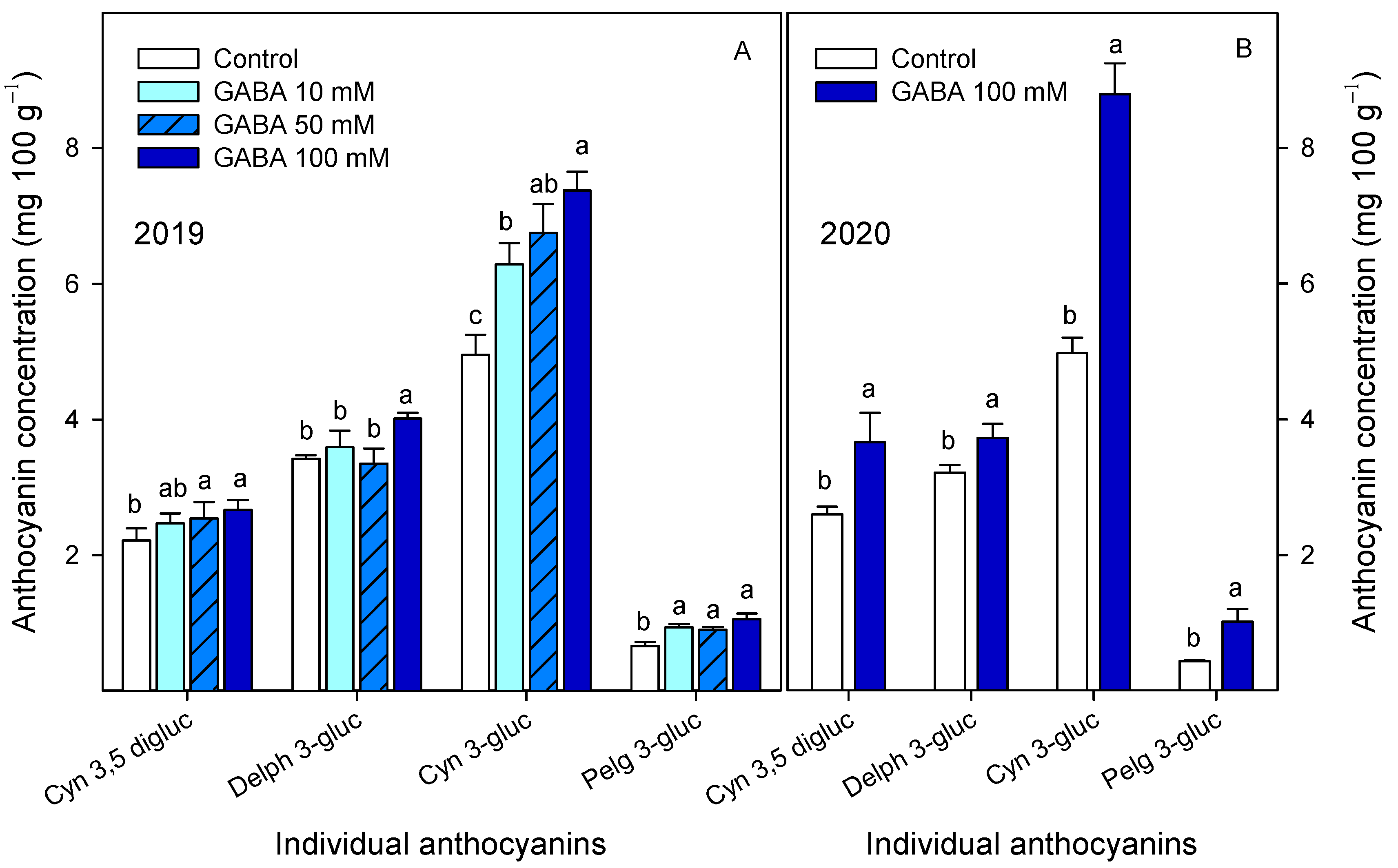
3.2. Effects of GABA Treatments on Bioactive Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015, 95, 2360–2369. [Google Scholar] [CrossRef] [PubMed]
- García-Pastor, M.E.; Giménez, M.J.; Valverde, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest application of oxalic acid improved pomegranate fruit yield, quality, and bioactive compounds at harvest in a concentration dependent manner. Agronomy 2020, 10, 1522. [Google Scholar] [CrossRef]
- Czieczor, L.; Bentkamp, C.; Damerow, L.; Blanke, M. Non-invasive determination of the quality of pomegranate fruit. Postharvest Biol. Technol. 2018, 136, 74–79. [Google Scholar] [CrossRef]
- Asgary, S.; Keshvari, M.; Sahebkar, A.; Sarrafzadegan, N. Pomegranate consumption and blood pressure: A review. Curr. Pharm. Des. 2017, 23, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Manandhar, B.; Paudel, K.R. Anticancer activity of Punica granatum (pomegranate): A review. Phytother. Res. 2017, 31, 568–578. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.A.; Espín, J.C.; González-Sarrías, A. Evidence for health properties of pomegranate juices and extracts beyond nutrition: A critical systematic review of human studies. Trends Food Sci. Technol. 2021, 114, 410–423. [Google Scholar] [CrossRef]
- Melgarejo-Sánchez, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 2. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Calín-Sánchez, A.; Carbonell-Barrachina, A.; Hernández, F. Changes in quality parameters, proline, antioxidant activity and color of pomegranate (Punica granatum L.) as affected by fruit position within tree, cultivar and ripening stage. Sci. Hortic. 2014, 165, 181–189. [Google Scholar] [CrossRef]
- Li, X.; Wasila, H.; Liu, L.; Yuan, T.; Gao, Z.; Zhao, B.; Ahmad, I. Physicochemical characteristics, polyphenol compositions and antioxidant potential of pomegranate juices from 10 Chinese cultivars and the environmental factors analysis. Food Chem. 2015, 175, 575–584. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Galindo, A.; Collado-González, J.; Rodríguez, P.; Cruz, Z.N.; Legua, P.; Burló, F.; Morales, D.; Carbonell-Barrachina, A.; Hernández, F. Influence of deficit irrigation and crop load on the yield and fruit quality in Wonderful and Mollar de Elche pomegranates. J. Sci. Food Agric. 2018, 98, 3098–3108. [Google Scholar] [CrossRef]
- Tozzi, F.; Legua, P.; Martínez-Nicolás, J.J.; Núñez-Gómez, D.; Giordani, E.; Melgarejo, P. Morphological and nutraceutical characterization of six pomegranate cultivars of global commercial interest. Sci. Hortic. 2020, 272, 109557. [Google Scholar] [CrossRef]
- Bartual, J.; Pérez-Gago, M.B.; Pomares, F.; Palou, L.; Intrigliolo, D.S. Nutrient status and irrigation management affect an-thocyanins in ‘Mollar de Elche’ pomegranate. Acta Hortic. 2015, 1106, 85–92. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Guillén, F.; Valero, D.; Serrano, M. The effects of salicylic acid and its derivatives on increasing pomegranate fruit quality and bioactive compounds at harvest and during storage. Front. Plant Sci. 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Giménez, M.J.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Preharvest application of methyl jasmonate increases crop yield, fruit quality and bioactive compounds in pomegranate ‘Mollar de Elche’ at harvest and during postharvest storage. J. Sci. Food Agric. 2020, 100, 145–153. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C. The bioactivity of pomegranate: Impact on health and disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Mirdehghan, S.H.; Sayyari, M.; Serrano, M. Vapor treatments, chilling, storage, and antioxidants in pomegranates. In Processing and Impact on Active Components in Food; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 189–196. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; Zarei, A. γ-Aminobutyrate (GABA): A metabolite and signal with practical significance. Botany 2017, 95, 1015–1032. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 862565. [Google Scholar] [CrossRef]
- Asgarian, Z.S.; Karimi, R.; Ghabooli, M.; Maleki, M. Biochemical changes and quality characterization of cold-stored ‘Sahebi’ grape in response to postharvest application of GABA. Food Chem. 2022, 373, 131401. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Zhang, X.; Liu, Y.; Liu, C.; Wang, H.; Ren, S.; Ma, F.; Liang, W.; Li, C. Exogenous GABA prevents Marssonina apple blotch damage in ‘Royal Gala’ apple seedling. Sci Hortic. 2022, 299, 111005. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Kakavand, F.; Rabiei, V.; Zaare-Nahandi, F.; Razavi, F. γ-Aminobutyric acid and nitric oxide treatments preserve sensory and nutritional quality of cornelian cherry fruits during postharvest cold storage by delaying softening and enhancing phenols accumulation. Sci. Hortic. 2019, 246, 812–817. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Syed, U.J.; Priyanka, C.; Himanshu, C.; Antonio, F.; Nafees, A.K.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Zhu, J.; Li, C.; Fan, Y.; Qu, L.; Huang, R.; Liu, J.; Zhang, C.; Ge, Y. γ-Aminobutyric acid regulates mitochondrial energy metabolism and organic acids metabolism in apples during postharvest ripening. Postharvest Biol. Technol. 2022, 186, 111846. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effect of γ-aminobutyric acid on the antioxidant system and biochemical changes of mango fruit during storage. J. Food Meas. Charact. 2020, 14, 778–789. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Serrano, M.; Valero, D. Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 2020, 306, 125634. [Google Scholar] [CrossRef]
- Nazoori, F.; Zamanibahramabadi, E.; Mirdehghan, S.H.; Rafie, A. Extending the shelf life of pomegranate (Punica granatum L.) by GABA coating application. J. Food Meas. Charact. 2020, 14, 2760–2772. [Google Scholar] [CrossRef]
- Nazoori, F.; Zamanibahramabadi, E.; Rafie, A.; Mirdehghan, S.H. Combined application of gamma-aminobutyric acid and carnauba wax as edible coating on pomegranates in cold storage. J. Agric. Sci. Technol. 2022, 24, 591–602. [Google Scholar]
- Lorente-Mento, J.M.; Guillén, F.; Martínez-Romero, D.; Carrión-Antoli, A.; Valero, D.; Serrano, M. γ-Aminobutyric acid treatments of pomegranate trees increase crop yield and fruit quality at harvest. Sci. Hortic. 2023, 309, 111633. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Serrano, M.; Valero, D. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharvest Biol. Technol. 2009, 53, 152–154. [Google Scholar] [CrossRef]
- Sayyari, M.; Aghdam, M.S.; Salehi, F.; Ghanbari, F. Salicyloyl chitosan alleviates chilling injury and maintains antioxidant capacity of pomegranate fruits during cold storage. Sci. Hortic. 2016, 211, 110–117. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J.M.; García-Pastor, E.; Zapata, P.J.; Valero, D.; Guillén, F. Melatonin treatment of pomegranate trees increases crop yield and quality parameters at harvest and during storage. Agronomy 2021, 11, 861. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Aradhya, S.M. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005, 93, 319–324. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Ejaz, S.; Anwar, R.; Khalik, G.; Hussain, S.; Ullah, S.; Hussain, R.; Saleem, M.S.; et al. Postharvest γ-aminobutyric acid application mitigates chilling injury of aonla (Emblica officinalis Gaertn.) fruit during low temperature storage. Postharvest Biol. Technol. 2022, 185, 111803. [Google Scholar] [CrossRef]
- Ngaffo Mekontso, F.; Duan, W.; Cisse, E.H.M.; Chen, T.; Xu, X. Alleviation of postharvest chilling injury of carambola fruit by γ-aminobutyric acid: Physiological, biochemical, and structural characterization. Front. Nutr. 2021, 8, 752583. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Duan, B.; Li, C.; Tang, Q.; Li, X.; Wei, M.; Chen, Y.; Li, J. γ-Aminobutyric acid delays senescence of blueberry fruit by regulation of reactive oxygen species metabolism and phenylpropanoid pathway. Sci. Hortic. 2018, 240, 303–309. [Google Scholar] [CrossRef]
- Radunić, M.; Jukić Špika, M.; Goreta Ban, S.; Gadzĕ, J.; Díaz-Pérez, J.C.; Maclean, D. Physical and chemical properties of pomegranate fruit accessions from Croatia. Food Chem. 2015, 177, 53–60. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Stander, M.A.; Fawole, O.A.; Opara, U.L. Effect of fruit maturity and growing location on the postharvest contents of flavonoids, phenolic acids, vitamin C and antioxidant activity of pomegranate juice (cv. Wonderful). Sci. Hortic. 2014, 179, 36–45. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Stander, M.A.; Opara, U.L. Preharvest and postharvest factors influencing bioactive compounds in pomegranate (Punica granatum L.)—A review. Sci. Hortic. 2014, 178, 114–123. [Google Scholar] [CrossRef]
- Boussaa, F.; Zaouay, F.; Burlo-Carbonell, F.; Nuncio-Jáuregui, N.; Gmati, M.; El Arbi, B.; Melgarejo, P.; Hernandez, F.; Mars, M. Combined effects of cropping system and harvest date determine quality and nutritional value of pomegranate fruits (Punica granatum L. cv. Gabsi). Sci. Hortic. 2019, 249, 419–431. [Google Scholar] [CrossRef]
- Mena, P.; Gironés-Vilaplana, A.; Martí, N.; García-Viguera, C. Pomegranate varietal wines: Phytochemical composition and quality parameters. Food Chem. 2012, 133, 108–115. [Google Scholar] [CrossRef]
- Laribi, A.I.; Palou, L.; Intrigliolo, D.S.; Nortes, P.A.; Rojas-Argudo, C.; Taberner, V.; Bartual, J.; Pérez-Gago, M.B. Effect of sustained and regulated deficit irrigation on fruit quality of pomegranate cv. ‘Mollar de Elche’ at harvest and during cold storage. Agric. Water Manag. 2013, 125, 61–70. [Google Scholar] [CrossRef]
- Gil, M.I.; García-Viguera, C.; Artés, F.; Tomás-Barberán, F.A. Changes in pomegranate juice pigmentation during ripening. J. Sci. Food Agric. 1995, 68, 77–81. [Google Scholar] [CrossRef]
- Mousavinejad, G.; Emam-Djomeh, Z.; Rezaei, K.; Khodaparast, M.H. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009, 115, 1274–1278. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Mars, M.; Ghaffari, S.; Trifi, M.; Melgarejo, P.; Hernandez, F. Seed and juice characterization of pomegranate fruits grown in Tunisia: Comparison between sour and sweet cultivars revealed interesting properties for prospective industrial applications. Ind. Crops Prod. 2011, 33, 374–381. [Google Scholar] [CrossRef]
- Doostkam, A.; Bassiri-Jahromi, S.; Iravani, K. Punica granatum with multiple effects in chronic diseases. Int. J. Fruit Sci. 2020, 20, 471–494. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Hou, H.; Liu, Z.; Wang, L.; Rasekhmagham, R.; HamedKord-Varkaneh, H.; Santos, H.O.; Yao, G. The effects of pomegranate supplementation on biomarkers of inflammation and endothelial dysfunction: A meta-analysis and systematic review. Complement. Ther. Med. 2020, 49, 102358. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Lopéz-Cortés, I.; Salazar, D.; González-Álvarez, J.; Ramalhosa, E. Physicochemical composition and antioxidant activity of several pomegranate (Punica granatum L.) cultivars grown in Spain. Eur. Food Res. Technol. 2017, 243, 1799–1814. [Google Scholar] [CrossRef]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Crit. Rev. Food Sci. Nutrit. 2021, 61, 982–999. [Google Scholar] [CrossRef]



| 2019 Experiment | ||||
|---|---|---|---|---|
| Colour a* | Control | GABA 10 mM | GABA 50 mM | GABA 100 mM |
| Day 0 | 14.84 ± 0.44 cB | 16.99 ± 0.65 bB | 17.84 ± 0.67 bB | 20.38 ± 0.77 aB |
| Day 60 | 17.95 ± 0.51 cA | 21.05 ± 0.25 bA | 21.95 ± 0.47 bA | 24.38 ± 0.56 aA |
| 2020 Experiment | ||||
| Colour a* | Control | GABA 100 mM | ||
| Day 0 | 11.51 ± 0.77 bB | 15.69 ± 0.51 aB | ||
| Day 60 | 15.44 ± 0.69 bA | 19.92 ± 0.81 aA | ||
| 2019 Experiment | ||||
| TSS (g 100 g−1) | Control | GABA 10 mM | GABA 50 mM | GABA 100 mM |
| Day 0 | 15.03 ± 0.04 aA | 14.98 ± 0.19 aA | 15.01 ± 0.08 aA | 15.26 ± 0.12 aA |
| Day 60 | 15.17 ± 0.32 aA | 15.19 ± 0.08 aA | 14.72 ± 0.17 aA | 14.90 ± 0.14 aA |
| 2020 Experiment | ||||
| TSS (g 100 g−1) | Control | GABA 100 mM | ||
| Day 0 | 15.58 ± 0.15 aA | 15.33 ± 0.18 aA | ||
| Day 60 | 15.72 ± 0.10 aA | 15.43 ± 0.11 aA | ||
| 2019 Experiment | ||||
| TA (g 100 g−1) | Control | GABA 10 mM | GABA 50 mM | GABA 100 mM |
| Day 0 | 0.31 ± 0.01 aA | 0.32 ± 0.01 aA | 0.32 ± 0.01 aA | 0.33 ± 0.01 aA |
| Day 60 | 0.23 ± 0.02 bB | 0.24 ± 0.01 bB | 0.27 ± 0.01 abB | 0.31 ± 0.01 aA |
| 2020 Experiment | ||||
| TA (g 100 g−1) | Control | GABA 100 mM | ||
| Day 0 | 0.34 ± 0.02 aA | 0.35 ± 0.01 aA | ||
| Day 60 | 0.26 ± 0.01 bB | 0.32 ± 0.02 aA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente-Mento, J.M.; Valero, D.; Martínez-Romero, D.; Badiche, F.; Serrano, M.; Guillén, F. Preharvest Multiple Applications of GABA Improve Quality Traits and Antioxidant Compounds of Pomegranate Fruit during Storage. Horticulturae 2023, 9, 534. https://doi.org/10.3390/horticulturae9050534
Lorente-Mento JM, Valero D, Martínez-Romero D, Badiche F, Serrano M, Guillén F. Preharvest Multiple Applications of GABA Improve Quality Traits and Antioxidant Compounds of Pomegranate Fruit during Storage. Horticulturae. 2023; 9(5):534. https://doi.org/10.3390/horticulturae9050534
Chicago/Turabian StyleLorente-Mento, José Manuel, Daniel Valero, Domingo Martínez-Romero, Fátima Badiche, María Serrano, and Fabián Guillén. 2023. "Preharvest Multiple Applications of GABA Improve Quality Traits and Antioxidant Compounds of Pomegranate Fruit during Storage" Horticulturae 9, no. 5: 534. https://doi.org/10.3390/horticulturae9050534
APA StyleLorente-Mento, J. M., Valero, D., Martínez-Romero, D., Badiche, F., Serrano, M., & Guillén, F. (2023). Preharvest Multiple Applications of GABA Improve Quality Traits and Antioxidant Compounds of Pomegranate Fruit during Storage. Horticulturae, 9(5), 534. https://doi.org/10.3390/horticulturae9050534









