Morphogenesis Changes in Protocorm Development during Symbiotic Seed Germination of Dendrobium chrysotoxum (Orchidaceae) with Its Mycobiont, Tulasnella sp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Fungal Culture
2.3. In Vitro Seed Germination
2.4. Microscopic Morphological Observations
2.5. Histological and Histochemical Studies
2.6. Fungal Infection Observation
2.7. Statistical Analysis
3. Results
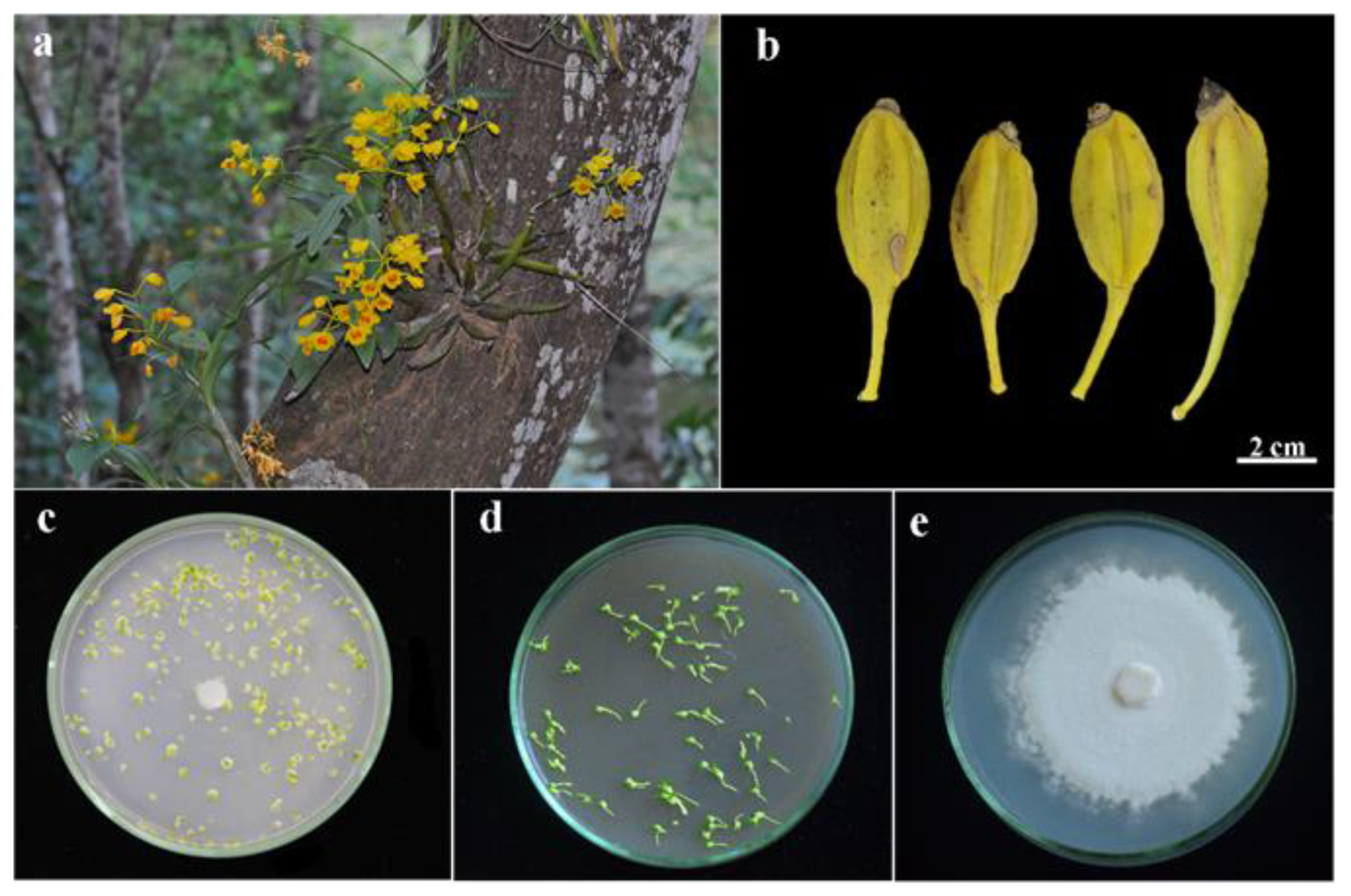
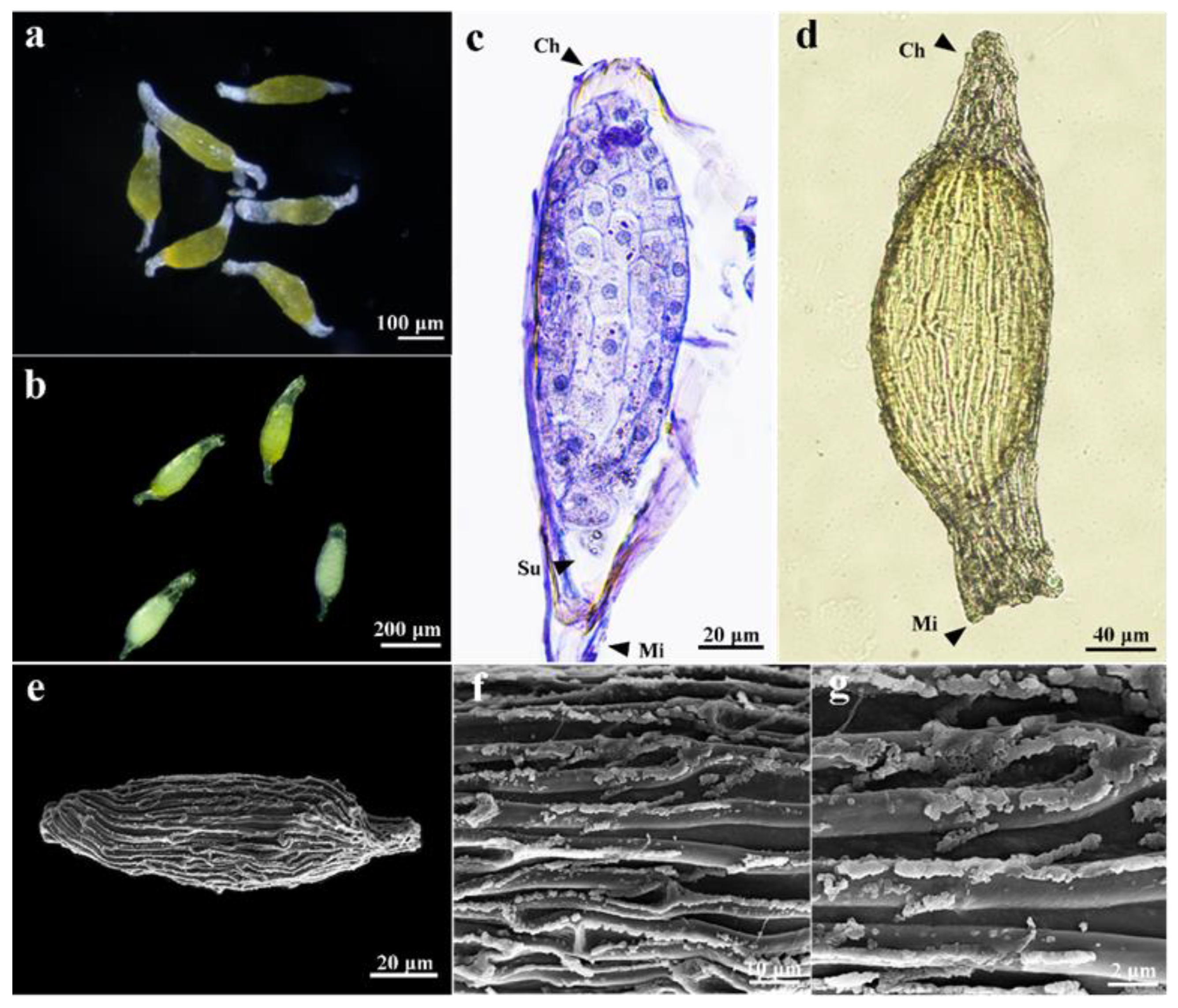
3.1. The Seed Characteristics of Dendrobium chrysotoxum
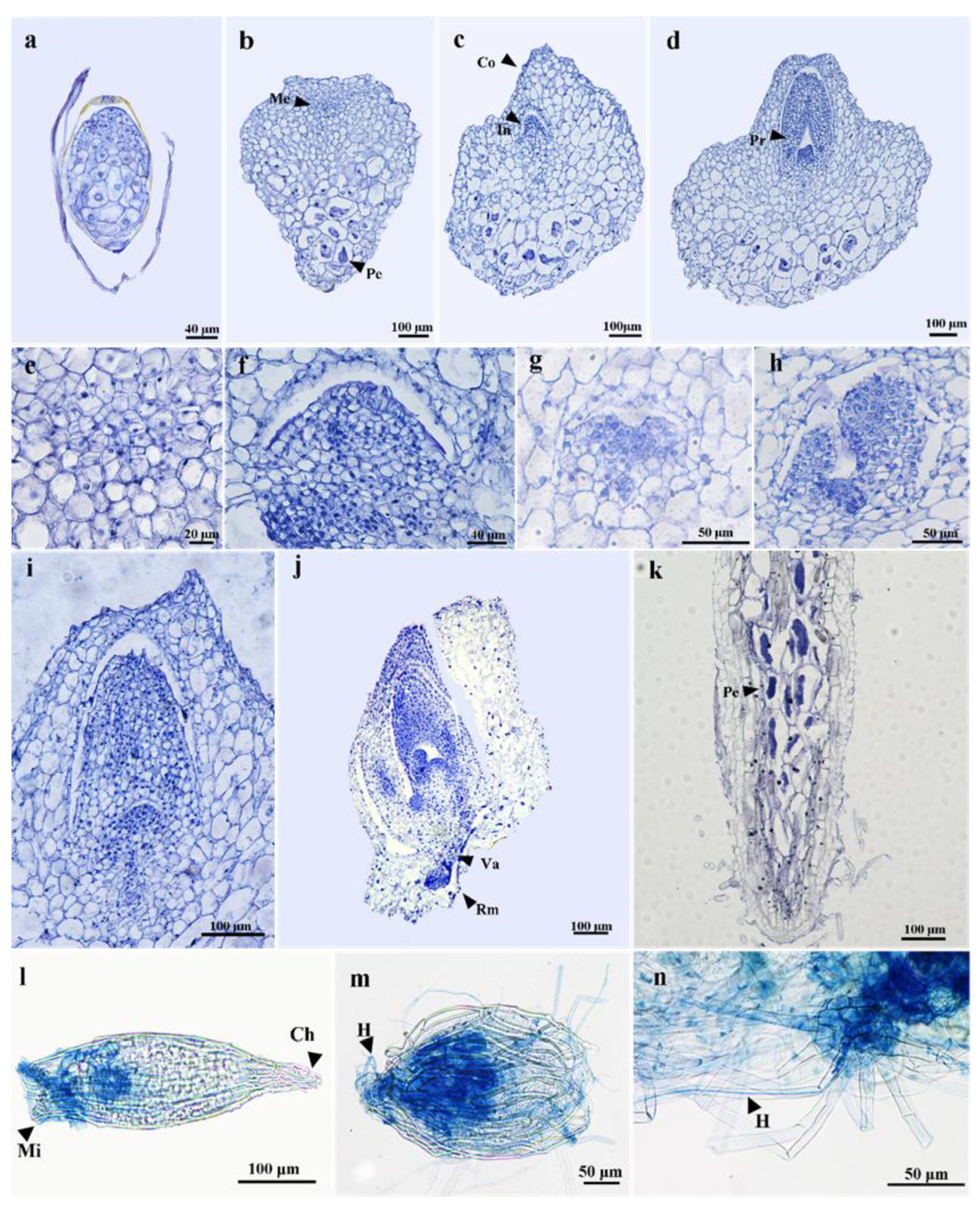
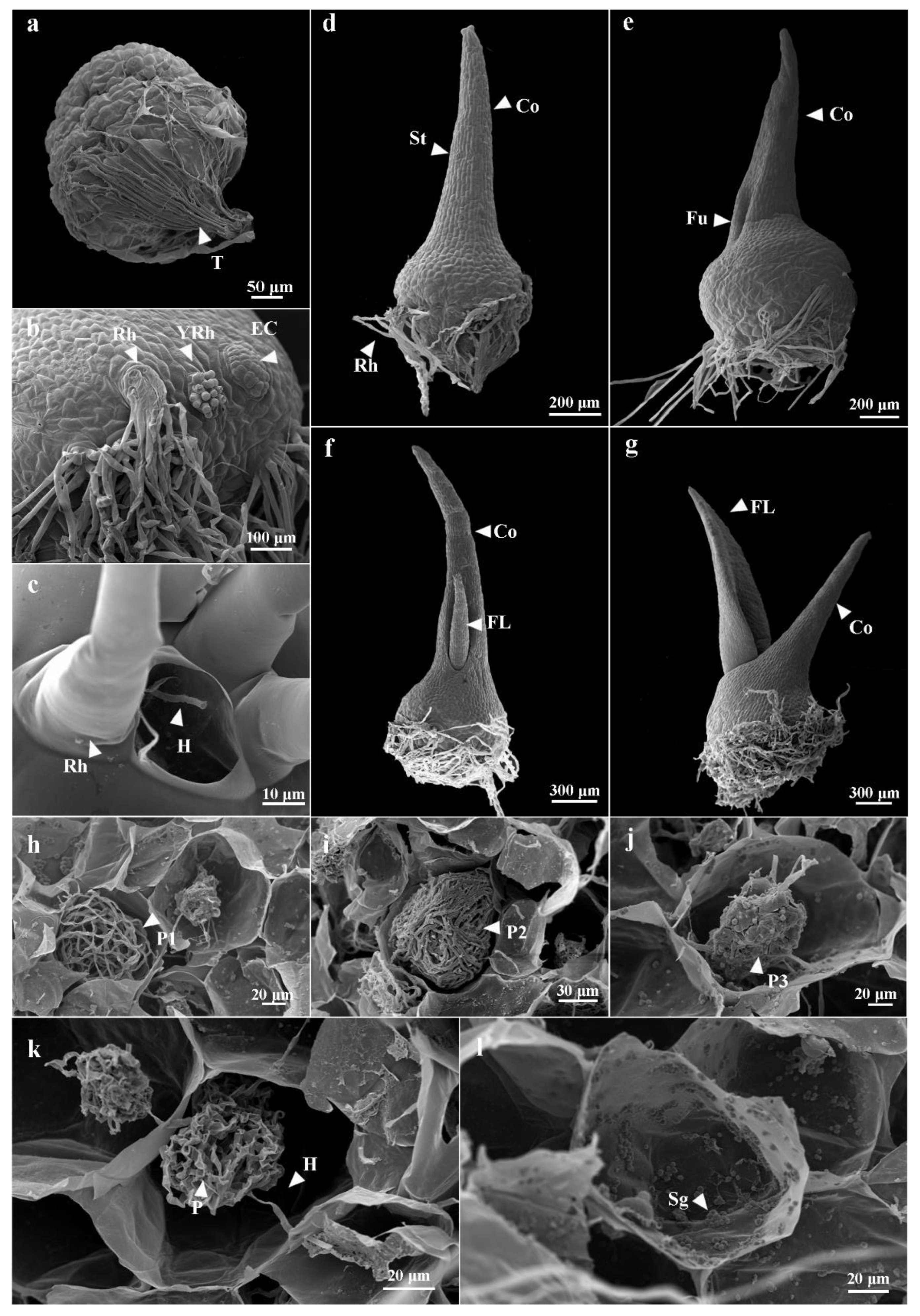
3.2. Seed Germination and Protocorm Development
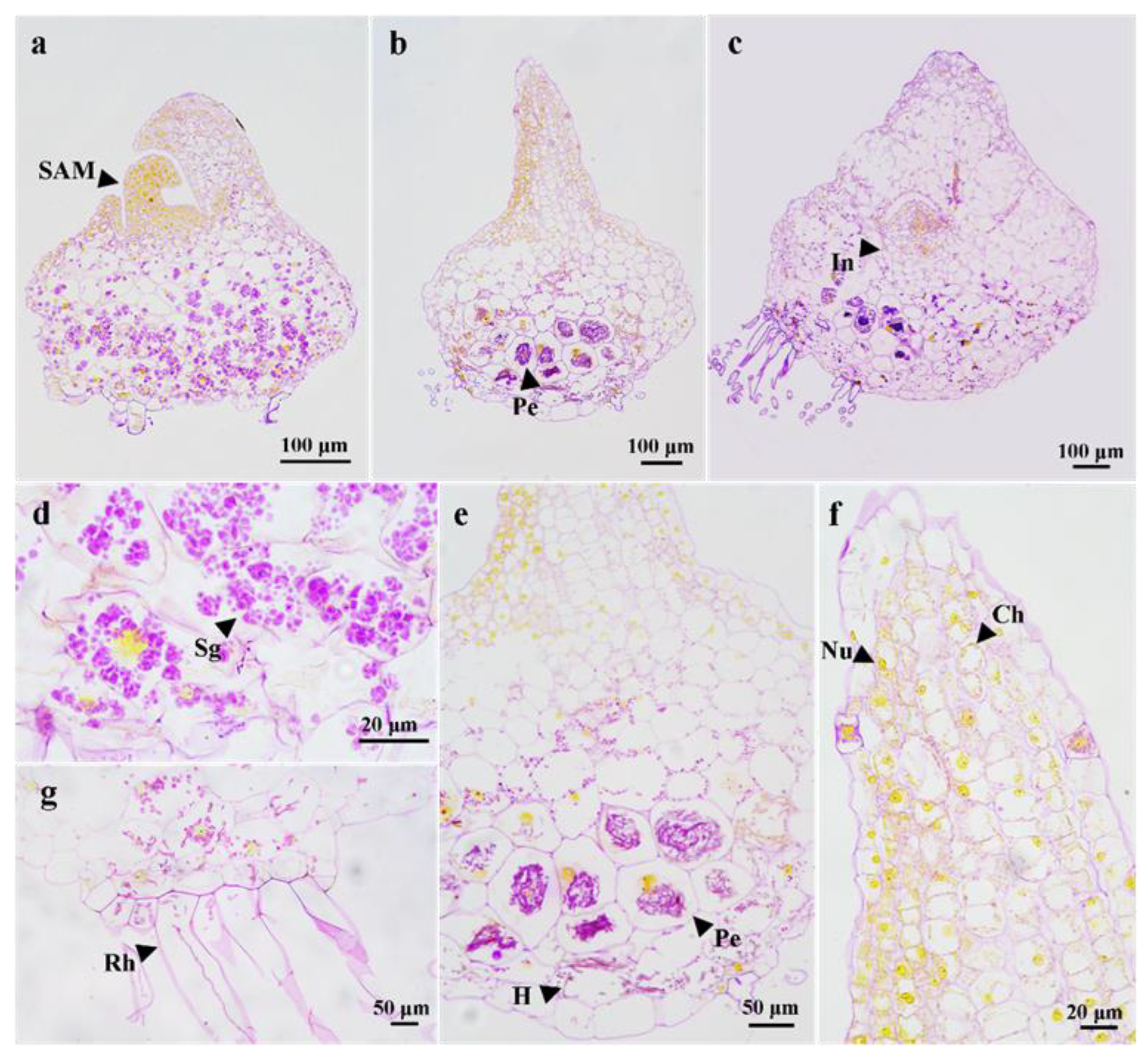
3.3. Morphological Changes of Pelotons in Symbiotically Germinated Protocorms
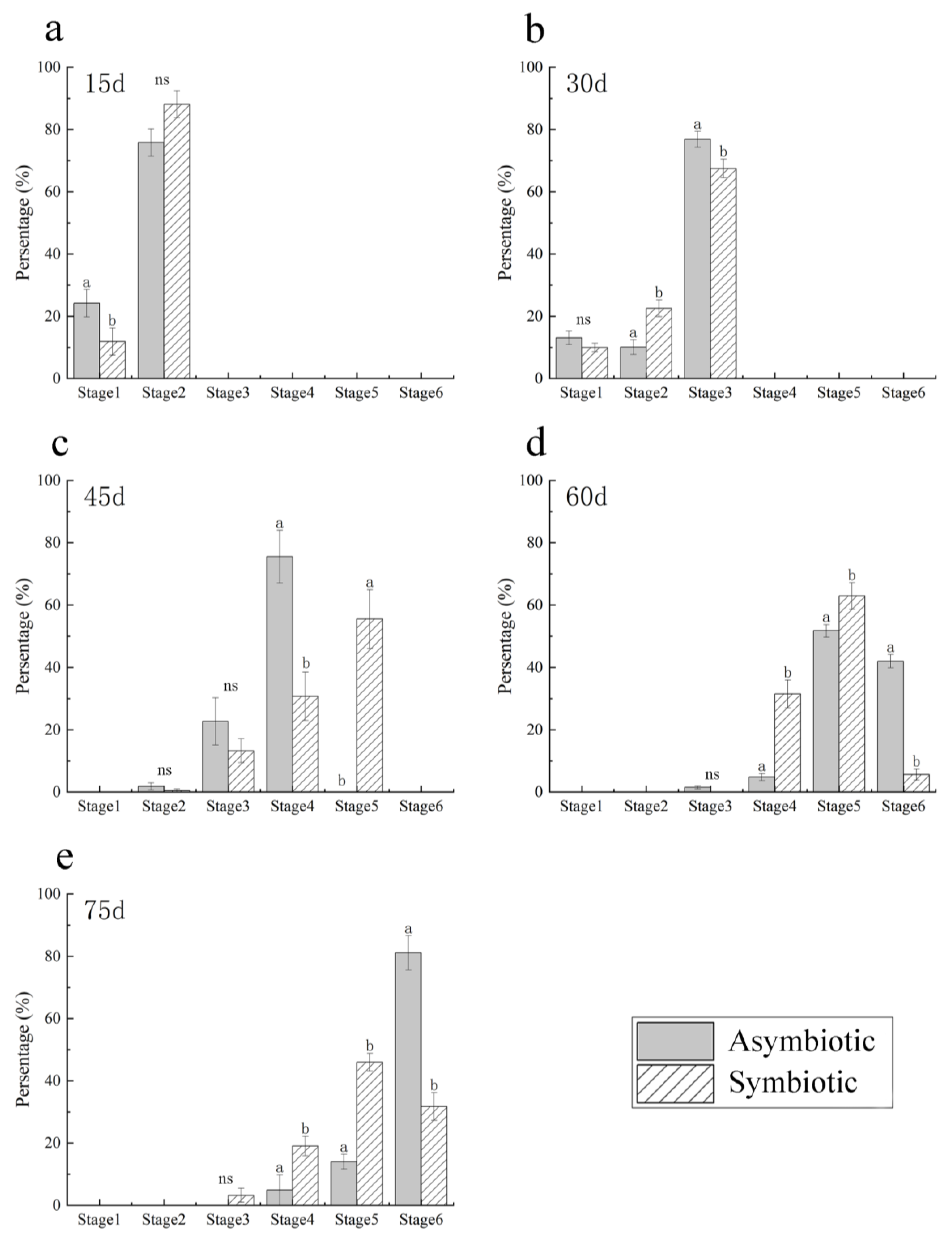
3.4. Comparative Symbiotic and Asymbiotic Seed Germination
4. Discussion
4.1. The Structure and Function of Protocorms
4.2. Associated Fungi Colonization and Digestion during Protocorm Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arditti, J. Factors affecting the germination of orchid seeds. Bot. Rev. 1967, 33, 1–97. [Google Scholar] [CrossRef]
- Arditti, J. Fundamentals of Orchid Biology; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Selosse, M.A.; Boullard, B.; Richardson, D. Noel Bernard (1874–1911): Orchids to symbiosis in a dozen years, one century ago. Symbiosis 2011, 54, 61–68. [Google Scholar] [CrossRef]
- Selosse, M.A.; Minasiewicz, J.; Boullard, B. An annotated translation of Noel Bernard’s 1899 article ‘On the germination of Neottia nidus-avis’. Mycorrhiza 2017, 27, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.N.; Dixon, K.W.; Jersakova, J.; Tesitelova, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef]
- Yeung, E.C. The orchid embryo—“An embryonic protocorm”. Botany 2022, 100, 691–706. [Google Scholar] [CrossRef]
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 58, 33. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.L.; Currah, R.S. Synthesis of mycorrhizae between protocorms of Goodyera repens (Orchidaceae) and Ceratobasidium cereal. Can. J. Bot. 1990, 68, 1117–1125. [Google Scholar] [CrossRef]
- Richardson, K.A.; Peterson, R.L.; Currah, R.S. Seed reserves and early symbiotic protocorm development of Platanthera hyperborea (Orchidaceae). Can. J. Bot. 1992, 70, 291–300. [Google Scholar] [CrossRef]
- Peterson, R.L.; Uetake, Y.; Zelmer, C. Fungal symbioses with orchid protocorms. Symbiosis 1998, 25, 29–55. [Google Scholar]
- Yagame, T.; Yamato, M. Mycoheterotrophic growth of Cephalanthera falcata (Orchidaceae) in tripartite symbioses with Thelephoraceae fungi and Quercus serrata (Fagaceae) in pot culture condition. J. Plant Res. 2013, 126, 215–222. [Google Scholar] [CrossRef]
- Yeung, E.C.; Li, Y.-Y.; Lee, Y.-I. Understanding seed and protocorm development in orchids. In Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols; Lee, Y.-I., Yeung, E.C., Eds.; Springer: New York, NY, USA, 2018; pp. 3–26. [Google Scholar]
- Leroux, G.; Barabé, D.; Vieth, J. Morphogenesis of the protocorm of Cypripedium acaule (Orchidaceae). Plant Syst. Evol. 1997, 205, 53–72. [Google Scholar] [CrossRef]
- Batygina, T.B.; Vasilyeva, V.E. Sexual reproduction in flowering plants: Zygote formation and types of karyogamy. Bot. Zh. 2000, 85, 50–66. [Google Scholar]
- Batygina, T.; Bragina, E.; Vasilyeva, V. The reproductive system and germination in orchids. Acta Biol. Cracov. Ser. Bot. 2003, 45, 21–34. [Google Scholar]
- Nishimura, G. Comparative morphology of Cattleya and Phalaenopsis (Orchidaceae) seedlings. Bot. Gaz. 1981, 142, 360–365. [Google Scholar] [CrossRef]
- Hoang, N.H.; Kane, M.E.; Radcliffe, E.N.; Zettler, L.W.; Richardson, L.W. Comparative seed germination and seedling development of the ghost orchid, Dendrophylax lindenii (Orchidaceae), and molecular identification of its mycorrhizal fungus from South Florida. Ann. Bot. 2017, 119, 379–393. [Google Scholar] [CrossRef]
- Zettler, L.W.; Hofer, C.J. Propagation of the little club-spur orchid (Platanthera clavellata) by symbiotic seed germination and its ecological implications. Environ. Exp. Bot. 1998, 39, 189–195. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, K.; Teixeira da Silva, J.A.; Zhang, J.; Chen, Z.; Xia, N.; Duan, J. Asymbiotic seed germination, seedling development and reintroduction of Paphiopedilum wardii Sumerh., an endangered terrestrial orchid. Sci. Hortic. 2012, 138, 198–209. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Y.; Xu, N.; Song, Y.; Qin, M. Observation on anatomical structures of seed germination and protocorm development of Dendrobium huoshanense. J. Plant Resour. Environ. 2018, 27, 115–117. [Google Scholar]
- Liu, X.; Fang, Y.; Yang, J.; Wan, X.; Yin, Z. Post-embryonic development and seedling morphogenesis of Dendrobium moniliforme (L.) Sw. under asymbiotic culture conditions. S. Afr. J. Bot. 2022, 149, 240–246. [Google Scholar] [CrossRef]
- Guo, S.X.; Xu, J.T. Studies on the changes of cell ultrastructure in the course of seed germination of Bletilla Striata under fungus infection conditions. Acta Bot. Sin. 1990, 32, 594–598. [Google Scholar]
- Uetake, Y.; Kobayashi, K.; Ogoshi, A. Ultrastructural changes during the symbiotic development of Spiranthes sinensis (Orchidaceae) protocorms associated with binucleate Rhizoctonia anastomosis group C. Mycol. Res. 1992, 96, 199–209. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Liu, S.S.; Li, Y.Y.; Guo, S.X. Ultrastructure of symbiotic germination of the orchid Dendrobium officinale with its mycobiont, Sebacina sp. Aust. J. Bot. 2014, 62, 229–234. [Google Scholar] [CrossRef]
- Zhu, G.H.; Ji, Z.H.; Wood, J.J.; Wood, H.P. Flora of China; Scientific Press: Beijing, China, 2009. [Google Scholar]
- Gong, Y.Q.; Fan, Y.; Wu, D.Z.; Yang, H.; Hu, Z.B.; Wang, Z.T. In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent. Eur. J. Cancer 2004, 40, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.Z.; Xu, T.T.; Wang, C.Q.; Li, Q.M.; Zha, X.Q.; Pan, L.H.; Luo, J.P. Bioactivity-guided investigation for isolation and immunoregulatory potential of polysaccharides from Dendrobium chrysotoxum stems. Process Biochem. 2021, 104, 124–131. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.Q.; Zhan, R.; Chen, Y.G. Isopentenylated bibenzyls and phenolic compounds from Dendrobium chrysotoxum Lindl. Chem. Biodivers. 2022, 19, e202200259. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Jin, Y.; Wang, W.; Xia, K.; Chen, Z. Molecular and metabolic insights into floral scent biosynthesis during flowering in Dendrobium chrysotoxum. Front. Plant Sci. 2022, 13, 1030492. [Google Scholar] [CrossRef]
- Shao, S.C.; Wang, Q.X.; Beng, K.C.; Zhao, D.K.; Jacquemyn, H. Fungi isolated from host protocorms accelerate symbiotic seed germination in an endangered orchid species (Dendrobium chrysotoxum) from southern China. Mycorrhiza 2020, 30, 529–539. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, S.; Hang, Y.; Xie, G.; Ji, N.; Zhang, M. Mycorrhizal fungus Coprinellus disseminatus influences seed germination of the terrestrial orchid Cremastra appendiculata (D. Don) Makino. Sci. Hortic. 2022, 293, 110724. [Google Scholar] [CrossRef]
- Arditti, J.; Abdul, G.A.K. Tansley Review No. 110 Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef]
- Ma, G.H.; Chen, X.G.; Selosse, M.A.; Gao, J.Y. Compatible and incompatible mycorrhizal fungi with seeds of Dendrobium Species: The colonization process and effects of coculture on germination and seedling development. Front. Plant Sci. 2022, 13, 823794. [Google Scholar] [CrossRef]
- Lee, Y.I.; Lu, C.F.; Chung, M.C. Developmental changes in endogenous abscisic acid concentrations and asymbiotic seed germination of a terrestrial orchid, Calanthe tricarinata Lindl. J. Am. Soc. Hortic. Sci. 2007, 132, 246–252. [Google Scholar] [CrossRef]
- Zettler, L.W.; Barrington, F.V.; McInnis, T.M. Developmental morphology of Spiranthes odorata seedlings in symbiotic culture. Lindleyana 1995, 10, 211–216. [Google Scholar]
- Johnson, T.R.; Kane, M.E. Asymbiotic germination of ornamental Vanda: In vitro germination and development of three hybrids. Plant Cell Tissue Organ Cult. 2007, 91, 251–261. [Google Scholar] [CrossRef]
- Johnson, T.R.; Kane, M.E.; Pérez, H.E. Examining the interaction of light, nutrients and carbohydrates on seed germination and early seedling development of Bletia purpurea (Orchidaceae). Plant Growth Regul. 2011, 63, 89–99. [Google Scholar] [CrossRef]
- Li, Y.Y.; Guo, S.X.; Lee, Y.I. Ultrastructural changes during the symbiotic seed germination of Gastrodia elata with fungi, with emphasis on the fungal colonization region. Bot. Stud. 2020, 61, 4. [Google Scholar] [CrossRef]
- Nontachaiyapoom, S.; Sasirat, S.; Manoch, L. Symbiotic seed germination of Grammatophyllum speciosum Blume and Dendrobium draconis Rchb. f., native orchids of Thailand. Sci. Hortic. 2011, 130, 303–308. [Google Scholar] [CrossRef]
- Diantina, S.; Kartikaningrum, S.; McCormick, A.C.; Millner, J.; McGill, C.; Pritchard, H.W.; Nadarajan, J. Comparative in vitro seed germination and seedling development in tropical and temperate epiphytic and temperate terrestrial orchids. Plant Cell Tissue Organ Cult. 2020, 143, 619–633. [Google Scholar] [CrossRef]
- Veyret, Y. Development of the Embryo and the Young Seedling Stages of Orchids; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Nishimura, G. Comparative morphology of cotyledonous orchid seedlings. Lindleyana 1991, 6, 140–146. [Google Scholar]
- Tillich, H.J. Seedling diversity and the homologies of seedling organs in the order Poales (Monocotyledons). Ann. Bot. 2007, 100, 1413–1429. [Google Scholar] [CrossRef]
- Gallo, F.R.; Souza, L.A.; Milaneze-Gutierre, M.A.; Almeida, O.J.G. Seed structure and in vitro seedling development of certain Laeliinae species (Orchidaceae). Rev. Mex. Biodivers. 2016, 87, 68–73. [Google Scholar] [CrossRef]
- Hew, C.S.; Khoo, S.I. Photosynthesis of young orchid seedlings. New Phytol. 1980, 86, 349–357. [Google Scholar] [CrossRef]
- Vinogradova, T.; Andronova, E. Development of Orchid seeds and Seedlings; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Guo, S.T.; Wu, J.R.; Hu, J.; Yang, H.G.; Lu, L.; Liu, F. Symbiotic seed germination of Cymbidium mastersii Griff. ex Lindl. J. Yunnan Univ. Nat. Sci. 2012, 34, 348–355. [Google Scholar]
- Zhao, J.; Li, Z.; Wang, S.; Yang, F.; Li, L.; Liu, L. Correlations between the phylogenetic relationship of 14 Tulasnella strains and their promotion effect on Dendrobium crepidatum protocorm. Horticulturae 2022, 8, 1213. [Google Scholar] [CrossRef]
- Rasmussen, H.; Andersen, T.F.; Johansen, B. Temperature sensitivity of in vitro germination and seedling development of Dactylorhiza majalis (Orchidaceae) with and without a mycorrhizal fungus. Plant Cell Environ. 1990, 13, 171–177. [Google Scholar] [CrossRef]
- Rasmussen, H. Terrestrial Orchids: From Seeds to Mycotrophic Plants; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Wright, M.; Guest, D.; Cross, R. Development of mycorrhizal associations in Caladenia tentaculata. Selbyana 2005, 26, 114–124. [Google Scholar]
- Rasmussen, H.; Rasmussen, F. Orchid mycorrhiza: Implications of mycophagous life style. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Lee, Y.I.; Yeung, E.C. The osmotic property and fluorescent tracer movement of developing orchid embryos of Phaius tankervilliae (Aiton) Bl. Sex. Plant Reprod. 2010, 23, 337–341. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Calevo, J.; Adamo, M.; Giovannini, A.; Copetta, A.; Cornara, L. Seed Micromorphology, In vitro germination, and early stage seedling morphological traits of Cattleya purpurata (Lindl. & Paxton) Van den Berg. Horticulturae 2021, 7, 480. [Google Scholar] [CrossRef]
- Xu, J.T.; Fan, L. Cytodifferentiation of the seeds (protocorm) and vegetative propagation corms colonized by mycorrhizal fungi. Acta Bot. Sinica. 2001, 43, 1003–1010. [Google Scholar]
- Rasmussen, H.N. Cell differentiation and mycorrhizal infection in Dactylorhiza majalis (Rchb. f.) Hunt & Summerh. (Orchidaceae) during germination in vitro. New Phytol. 1990, 116, 137–147. [Google Scholar]
- Hadley, G. Organization and Fine Structure of Orchid Mycorrhiza; Academic Press: London, UK, 1975. [Google Scholar]
- Li, Y.Y.; Chen, X.M.; Zhang, Y.; Cho, Y.H.; Wang, A.R.; Yeung, E.C.; Zeng, X.; Guo, S.X.; Lee, Y.I. Immunolocalization and changes of hydroxyproline-rich glycoproteins during symbiotic germination of Dendrobium officinale. Front. Plant Sci. 2018, 9, 00552. [Google Scholar] [CrossRef] [PubMed]
- Dörr, I.; Kollmann, R. Fine structure of mycorrhiza in Neottia nidus-avis (L.) L. C. Rich. (Orchidaceae). Planta 1969, 89, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Hadley, G.; Williamson, B. Analysis of the post-infection growth stimulus in orchid mycorrhiza. New Phytol. 1971, 70, 445–455. [Google Scholar] [CrossRef]
- Hijner, J.A.; Arditti, J. Orchid mycorrhiza vitamin production and requirements by symbionts. Am. J. Bot. 1973, 60, 829–835. [Google Scholar] [CrossRef]
- Ponert, J.; Šoch, J.; Vosolsobě, S.; Čiháková, K.; Lipavská, H. Integrative study supports the role of trehalose in carbon transfer from fungi to mycotrophic orchid. Front. Plant Sci. 2021, 12, 793876. [Google Scholar] [CrossRef]
- Hadley, G. Penetration and infection of orchid protocorms by Thanatephorus cucumeris and other Rhizoctonia isolates. Phytopathology 1970, 60, 1092–1096. [Google Scholar]
- Nieuwdorp, P.J. Some Observations with light and electron microscope on the endotrophic mycorrhiza of orchids. Acta Bot. Neerl. 1972, 21, 128–144. [Google Scholar] [CrossRef]
- Meng, Y.-Y.; Shao, S.-C.; Liu, S.-J.; Gao, J.-Y. Do the fungi associated with roots of adult plants support seed germination? A case study on Dendrobium exile (Orchidaceae). Glob. Ecol. Conserv. 2019, 17, e00582. [Google Scholar] [CrossRef]


| Seed Characteristics | |
|---|---|
| Seed length (μm) | 392.97 ± 41.1 |
| Seed width (μm) | 101.52 ± 10.5 |
| Seed L/W ratio | 3.89 ± 0.38 |
| Embryo length (μm) | 224.01 ± 19.83 |
| Embryo width (μm) | 99.18 ± 9.6 |
| Free air space (%) | 33.4 ± 0.07 |
| Embryo occupation (%) | 66.6 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Wang, Y.; Deng, D.; Luo, Y.; Shao, S.; Luo, Y. Morphogenesis Changes in Protocorm Development during Symbiotic Seed Germination of Dendrobium chrysotoxum (Orchidaceae) with Its Mycobiont, Tulasnella sp. Horticulturae 2023, 9, 531. https://doi.org/10.3390/horticulturae9050531
Gao X, Wang Y, Deng D, Luo Y, Shao S, Luo Y. Morphogenesis Changes in Protocorm Development during Symbiotic Seed Germination of Dendrobium chrysotoxum (Orchidaceae) with Its Mycobiont, Tulasnella sp. Horticulturae. 2023; 9(5):531. https://doi.org/10.3390/horticulturae9050531
Chicago/Turabian StyleGao, Xinzhen, Yu Wang, Die Deng, Yinling Luo, Shicheng Shao, and Yan Luo. 2023. "Morphogenesis Changes in Protocorm Development during Symbiotic Seed Germination of Dendrobium chrysotoxum (Orchidaceae) with Its Mycobiont, Tulasnella sp." Horticulturae 9, no. 5: 531. https://doi.org/10.3390/horticulturae9050531
APA StyleGao, X., Wang, Y., Deng, D., Luo, Y., Shao, S., & Luo, Y. (2023). Morphogenesis Changes in Protocorm Development during Symbiotic Seed Germination of Dendrobium chrysotoxum (Orchidaceae) with Its Mycobiont, Tulasnella sp. Horticulturae, 9(5), 531. https://doi.org/10.3390/horticulturae9050531






