Flowering Response of Cannabis sativa L. ‘Suver Haze’ under Varying Daylength-Extension Light Intensities and Durations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Plant Growing Conditions
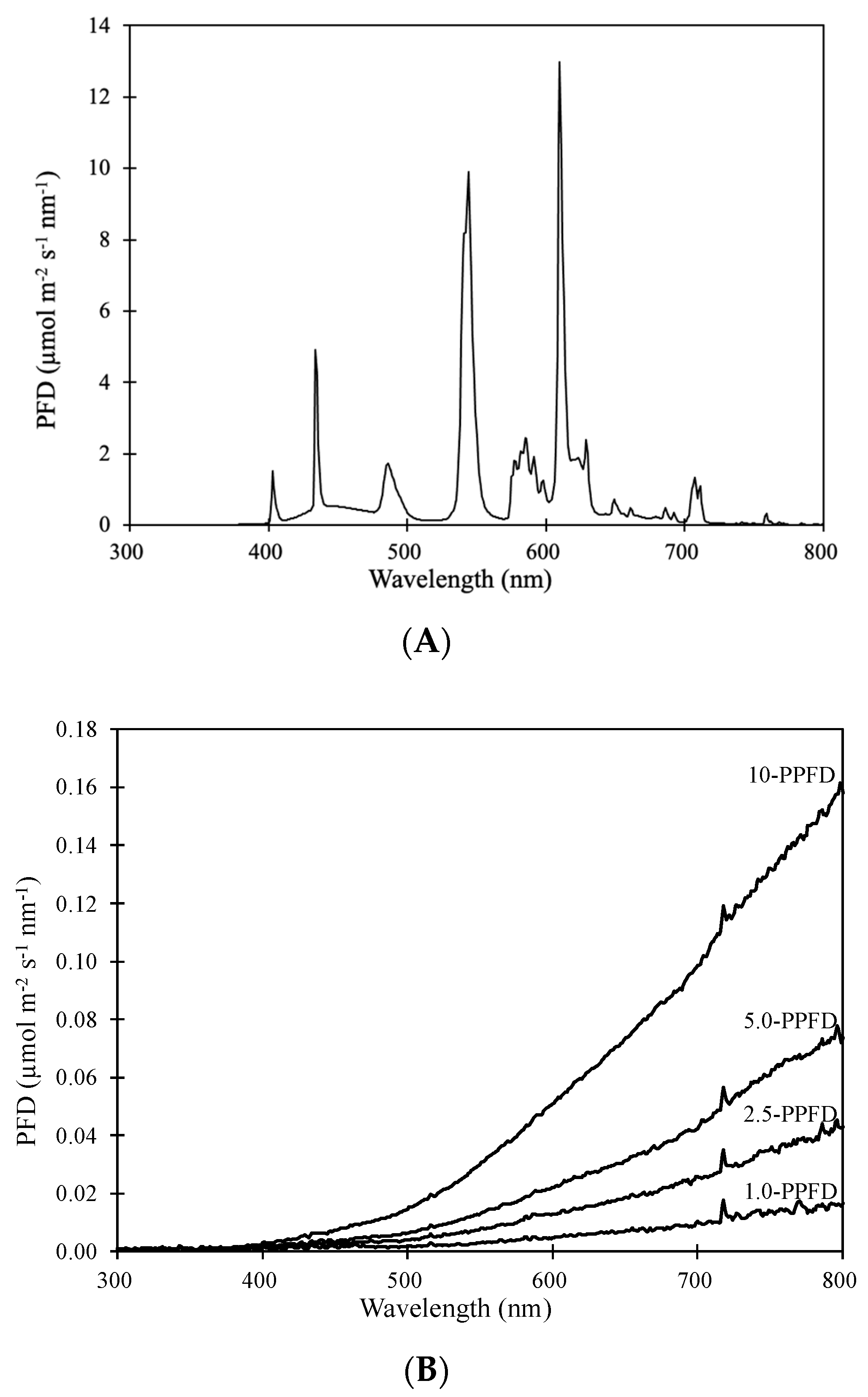
2.3. Lighting
2.4. Treatments
2.5. Measurement of Plant Growth Parameters
2.6. Statistical Analysis
3. Results and Discussion
3.1. Experiment 1: Daylength-Extension Experiment
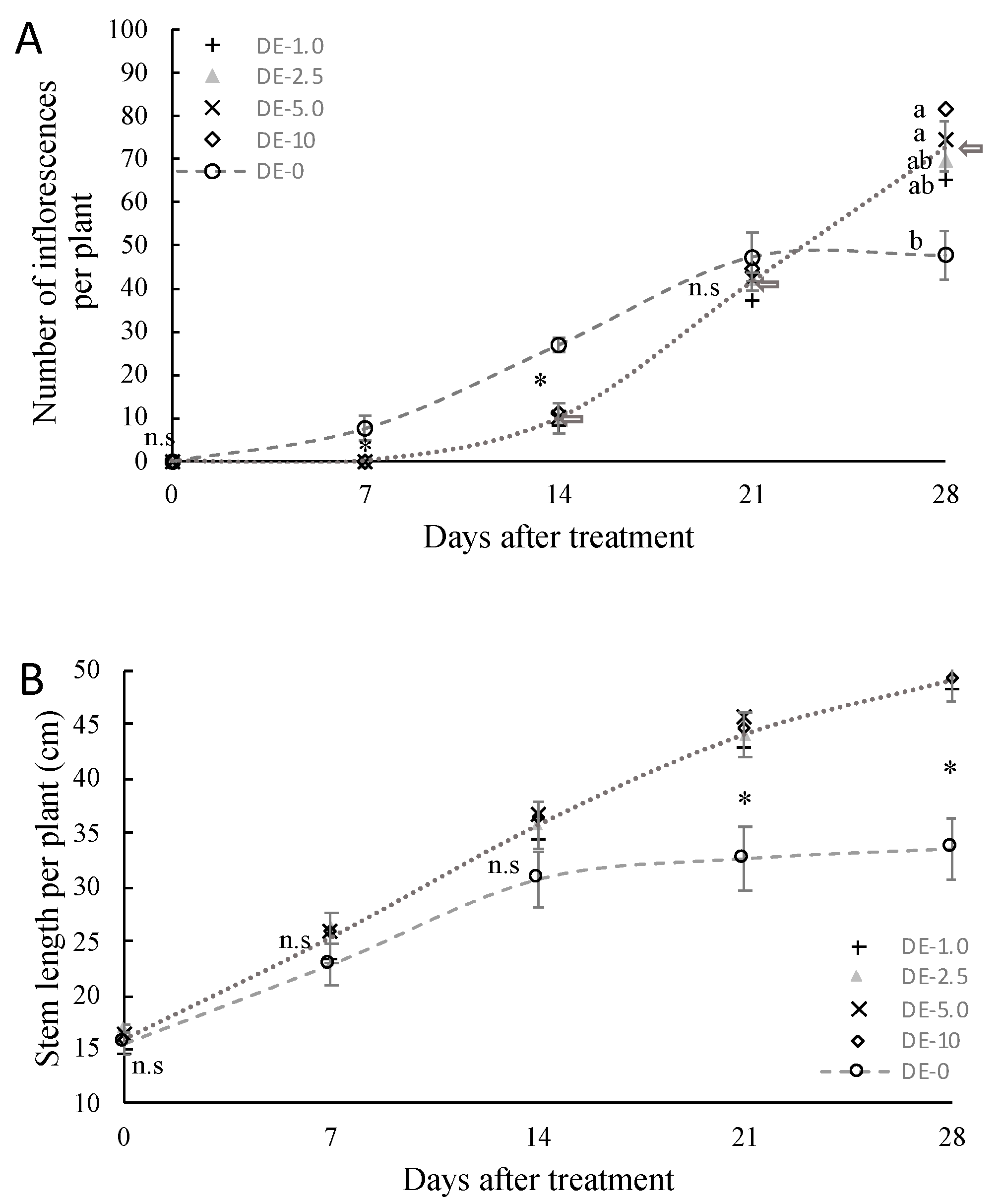
3.1.1. Effect of Light Intensity on Flower Transition
3.1.2. Incomplete Inflorescent Development
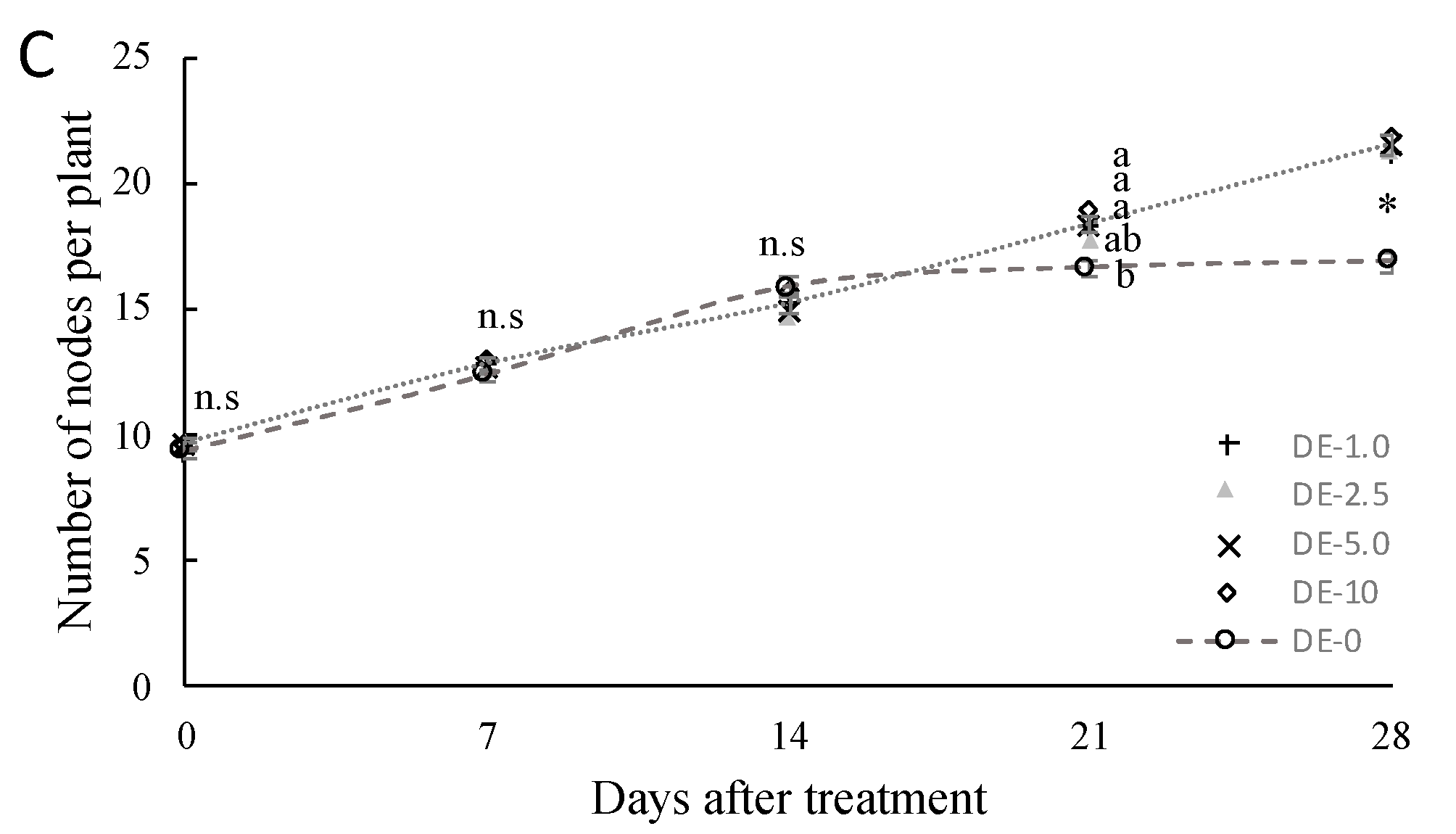
3.1.3. Growth and Morphology
3.2. Experiment 2: Photoperiod
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherney, J.H.; Small, E. Industrial hemp in North America: Production, politics, and potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef]
- Karche, T.; Singh, M.R. The application of hemp (Cannabis sativa L.) for a green economy: A review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New insight into ornamental applications of cannabis: Perspectives and challenges. Plants 2022, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; van Eeden, T.; Beswa, D. Cannabis sativa cannabinoids as functional ingredients in snack foods—Historical and developmental aspects. Plants 2022, 11, 3330. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Pepe, M.; Baiton, A.; Maxwell, A.; Jones, P. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol. Adv. 2023, 62, 108074. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Pellino, M.; Rigault, P.; van Velzen, R.; Ebersbach, J.; Ashnest, J.R.; Mau, M.; Schranz, M.E.; Alcorn, J.; Laprairie, R.B.; et al. The genomics of cannabis and its close relatives. Annu. Rev. Plant Biol. 2020, 71, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Pepe, M.; Alizadeh, M.; Rakei, A.; Baiton, A.; Maxwell, A.; Jones, P. Recent advances in cannabis biotechnology. Ind. Crops Prod. 2020, 158, 113026. [Google Scholar] [CrossRef]
- USDA. Establishment of a Domestic Hemp Production Program. Available online: https://www.ams.usda.gov/rules-regulations/establishment-domestic-hemp-production-program (accessed on 1 April 2023).
- NCSL. State Medical Cannabis Laws. Available online: https://www.ncsl.org/health/state-medical-cannabis-laws (accessed on 1 April 2023).
- Stack, G.M.; Toth, J.A.; Carlson, C.H.; Cala, A.R.; Marrero-González, M.I.; Wilk, R.L.; Gentner, D.R.; Crawford, J.L.; Philippe, G.; Rose, J.K.C. Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy 2021, 13, 546–561. [Google Scholar] [CrossRef]
- Toth, J.A.; Stack, G.M.; Cala, A.R.; Carlson, C.H.; Wilk, R.L.; Crawford, J.L.; Viands, D.R.; Philippe, G.; Smart, C.D.; Rose, J.K.C. Development and validation of genetic markers for sex and cannabinoid chemotype in Cannabis sativa L. GCB Bioenergy 2020, 12, 213–222. [Google Scholar] [CrossRef]
- Dang, M.; Arachchige, N.M.; Campbell, G. Optimizing photoperiod switch to maximize floral biomass and cannabinoid yield in Cannabis sativa L.: A meta-analytic quantile regression approach. Front. Plant Sci. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Whipker, B.E.; Cockson, P.; Smith, J.T. Night interruption lighting equally effective as daylength extension in retaining the vegetative state of Cannabis mother plants. Crop Forage Turfgrass Manag. 2020, 6, e20001. [Google Scholar] [CrossRef]
- Justice, D.; Justice, A.; Justice Schell, A.; Justice, M. Superior Hemp Genetics: 2022 Catalog; The Hemp Mine: Fair Play, CA, USA, 2022; pp. 1–29. [Google Scholar]
- Lopez, R.; Runkle, E.; Lopez, R.; Fisher, P. Photoperiodic control of flowering. In Light Management in Controlled Environments; Lopez, R., Runkle, E., Eds.; Meister Media Worldwide: Willoughby, OH, USA, 2017; pp. 49–61. [Google Scholar]
- Whitman, C.M.; Heins, R.D.; Cameron, A.C.; Carlson, W.H. Lamp type and irradiance level for daylength extensions influence flowering of Campanula carpatica `blue clips’, Coreopsis grandiflora `early sunrise’, and Coreopsis verticillata ‘moonbeam’. JASHS 1998, 123, 802–807. [Google Scholar] [CrossRef]
- Craig, D.S.; Runkle, E.S. A Moderate to High Red to Far-red Light Ratio from Light-emitting Diodes Controls Flowering of Short-day Plants. J. Amer. Soc. Hort. Sci. 2013, 138, 167–172. [Google Scholar] [CrossRef]
- Amaducci, S.; Colauzzi, M.; Zatta, A.; Venturi, G. Flowering dynamics in monoecious and dioecious hemp genotypes. J. Ind. Hemp 2008, 13, 5–19. [Google Scholar] [CrossRef]
- Amaducci, S.; Colauzzi, M.; Bellocchi, G.; Cosentino, S.L.; Pahkala, K.; Stomph, T.J.; Westerhuis, W.; Zatta, A.; Venturi, G. Evaluation of a phenological model for strategic decisions for hemp (Cannabis sativa L.) biomass production across European sites. Ind. Crops Prod. 2012, 37, 100–110. [Google Scholar] [CrossRef]
- Petit, J.; Salentijn, E.M.J.; Paulo, M.J.; Denneboom, C.; Trindade, L.M. Genetic architecture of flowering time and sex determination in hemp (Cannabis sativa L.): A genome-wide association study. Front. Plant Sci. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Petit, J.; Trindade, L.M. The complex interactions between flowering behavior and fiber quality in hemp. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Cho, L.H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef]
- Moher, M.; Jones, M.; Zheng, Y. Photoperiodic response of in vitro cannabis sativa plants. HortScience Horts 2021, 56, 108–113. [Google Scholar] [CrossRef]
- Zhang, M.; Anderson, S.L.; Brym, Z.T.; Pearson, B.J. Photoperiodic flowering response of essential oil, grain, and fiber hemp (Cannabis sativa L.) cultivars. Front. Plant Sci. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Wallace, D.H.; Yourstone, K.S.; Masaya, P.N.; Zobel, R.W. Photoperiod gene control over partitioning between reproductive and vegetative growth. Theor. Appl. Genet. 1993, 86, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Copani, V. Sowing time and prediction of flowering of different hemp (Cannabis sativa L.) genotypes in southern Europe. Ind. Crops Prod. 2012, 37, 108–113. [Google Scholar] [CrossRef]
- Sengloung, T.; Kaveeta, L.; Nanakorn, W. Effect of sowing date on growth and development of thai hemp (Cannabis sativa L.). Kasetsart J. (Nat. Sci.) 2009, 43, 423–431. [Google Scholar]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Ind. Crops Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. Review of flowering control in industrial hemp. J. Nat. Fibers 2012, 9, 23–36. [Google Scholar] [CrossRef]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. The effects of photoperiod on phenological development and yields of industrial hemp. J. Nat. Fibers 2014, 11, 87–106. [Google Scholar] [CrossRef]





| Experiment 1 a | DE-0 | DE-1.0 | DE-2.5 | DE-5.0 | DE-10 |
|---|---|---|---|---|---|
| DE-PPFD (400–700 nm: µmol·m−2·s−1) | 0 | 1.0 ± 0.3 | 2.5 ± 0.5 | 5.8 ± 1.2 | 10.3 ± 1.7 |
| DE-FR (700–800 nm: µmol·m−2·s−1) | 0 | 1.2 ± 0.3 | 3.0 ± 0.5 | 7.1 ± 1.2 | 12.0 ± 1.7 |
| DE-PF (300–800 nm: µmol·m−2·s−1) | 0 | 2.2 ± 0.6 | 5.5 ± 1.1 | 12.9 ± 2.8 | 22.2 ± 3.6 |
| DE-R:FR | 0 | 0.524 | 0.540 | 0.524 | 0.564 |
| PPFD (µmol·m−2·s−1) | 400.6 ± 2.6 | ||||
| DE-Photoperiod (h) | 0 | 6 | 6 | 6 | 6 |
| PPFD-Photoperiod (h) | 9 | ||||
| Photoperiod (h) | 9 | 15 | 15 | 15 | 15 |
| Daily light integral (mol·m−2·d−1) | 13.1 ± 0.1 | ||||
| Temperature (°C) | 24.5 ± 0.3 | ||||
| Relative humidity (%) | 69.8 ± 6.9 | ||||
| Experiment 2 a | 15 h | 18 h | 21 h | ||
| Photoperiod (h) | 15 | 18 | 21 | ||
| DE-Photoperiod (h) | 6 | 9 | 12 | ||
| PPFD-Photoperiod (h) | 9 | ||||
| DE-PPFD (400–700 nm: µmol·m−2·s−1) | 9.0 ± 0.2 | ||||
| DE-FR (700–800 nm: µmol·m−2·s−1) | 10.5 ± 0.2 | ||||
| PPFDy (µmol·m−2·s−1) | 463.0 ± 5.0 | ||||
| Daily temp (°C) | 25.5 ± 0.3 | ||||
| Relative humidity (%) | 63.6 ± 2.2 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Collado, C.E.; Lam, V.P.; Hernández, R. Flowering Response of Cannabis sativa L. ‘Suver Haze’ under Varying Daylength-Extension Light Intensities and Durations. Horticulturae 2023, 9, 526. https://doi.org/10.3390/horticulturae9050526
Park J, Collado CE, Lam VP, Hernández R. Flowering Response of Cannabis sativa L. ‘Suver Haze’ under Varying Daylength-Extension Light Intensities and Durations. Horticulturae. 2023; 9(5):526. https://doi.org/10.3390/horticulturae9050526
Chicago/Turabian StylePark, Jongseok, Cristian E. Collado, Vu Phong Lam, and Ricardo Hernández. 2023. "Flowering Response of Cannabis sativa L. ‘Suver Haze’ under Varying Daylength-Extension Light Intensities and Durations" Horticulturae 9, no. 5: 526. https://doi.org/10.3390/horticulturae9050526
APA StylePark, J., Collado, C. E., Lam, V. P., & Hernández, R. (2023). Flowering Response of Cannabis sativa L. ‘Suver Haze’ under Varying Daylength-Extension Light Intensities and Durations. Horticulturae, 9(5), 526. https://doi.org/10.3390/horticulturae9050526





