Nano-Biochar Suspension Mediated Alterations in Yield and Juice Quality of Kinnow (Citrus reticulata L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.2.1. Nano-Biochar Suspension Characteristics
2.2.2. NBS Treatment and Experimental Design
2.3. Fruit Drop and Yield
2.4. Juice Quality and Chemical Analysis
2.5. Statistical Analysis
3. Results
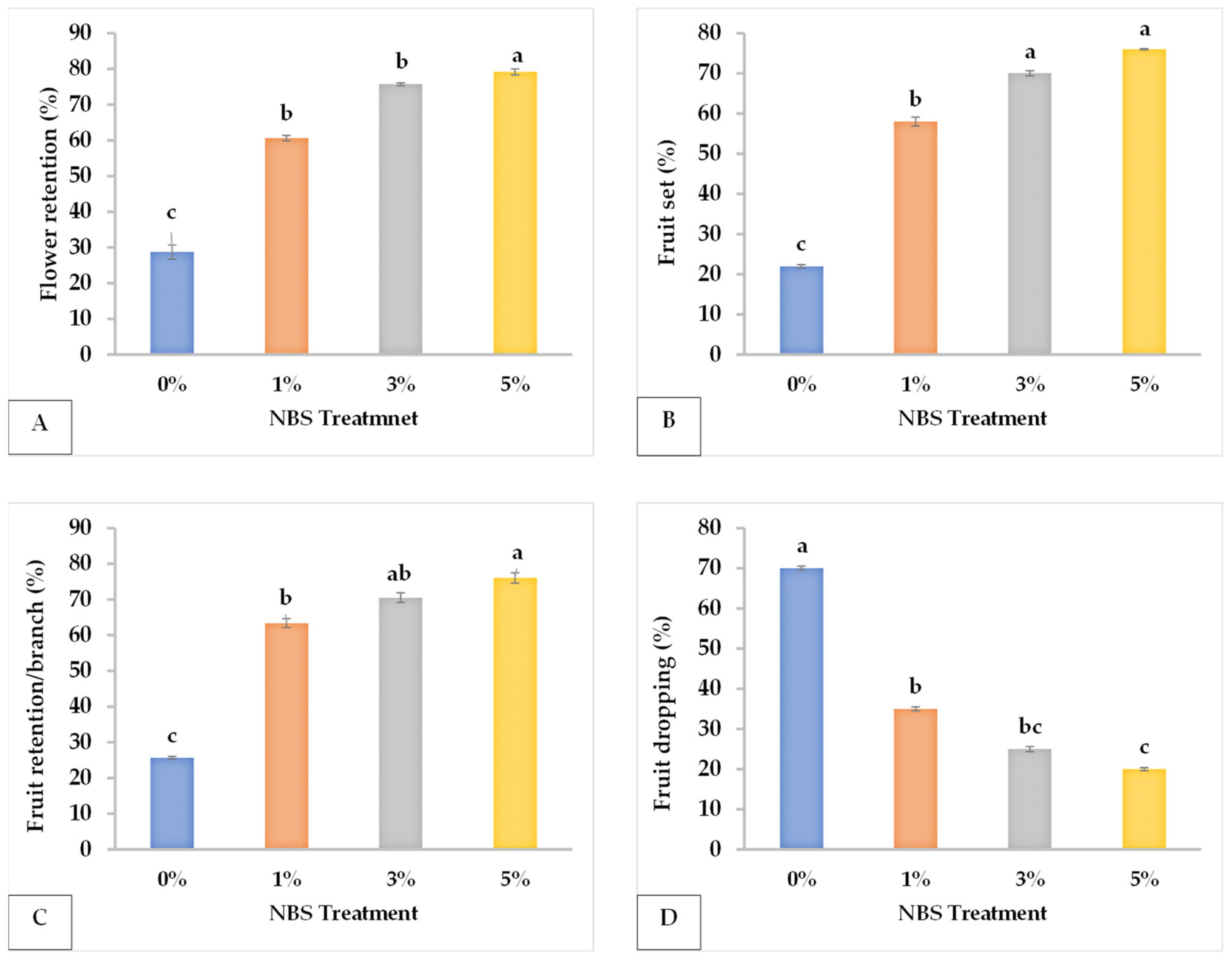
3.1. Fruit Yield and Yield Components
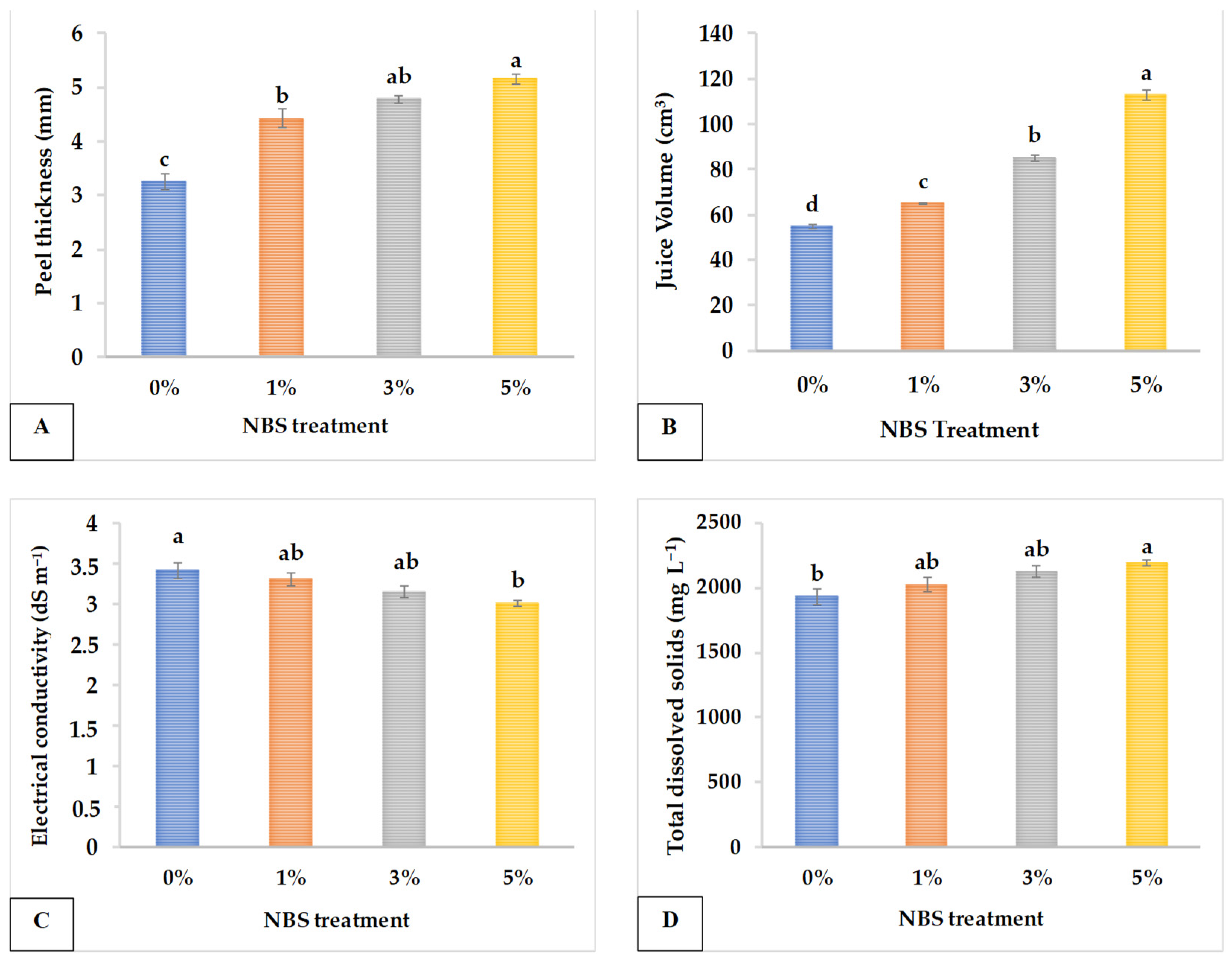
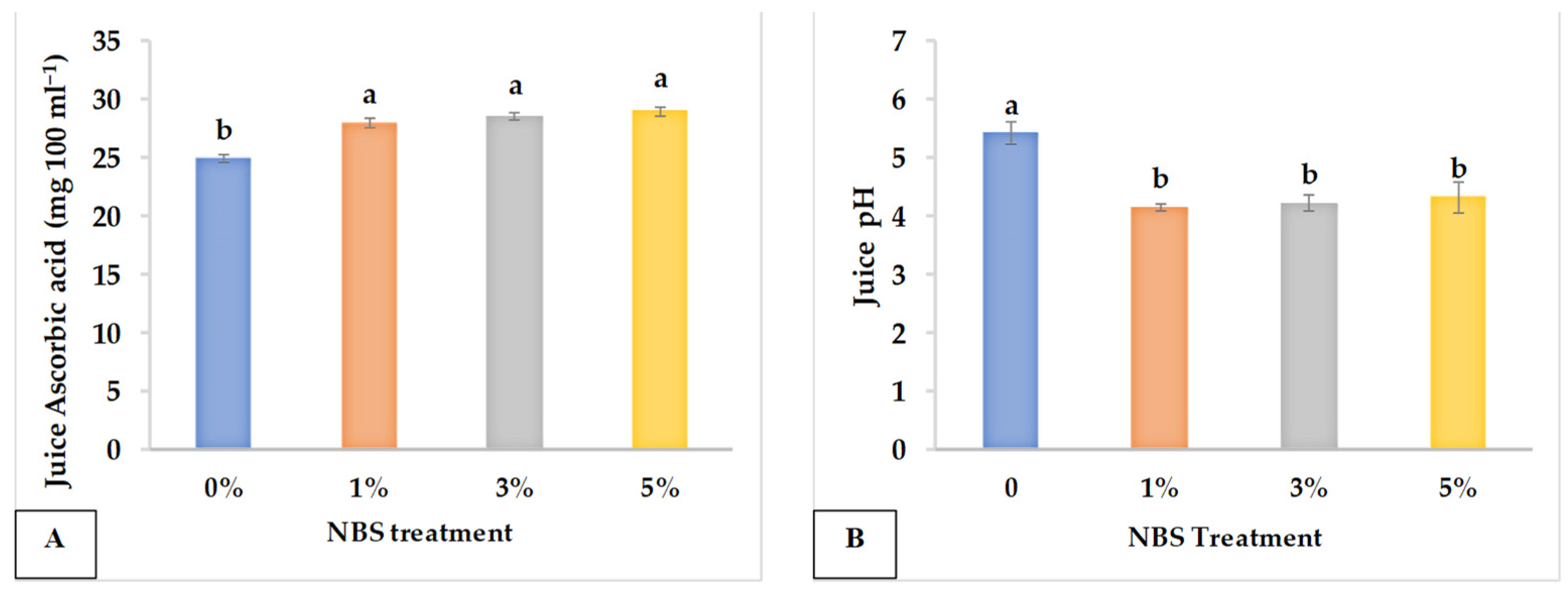
3.2. Juice Quality
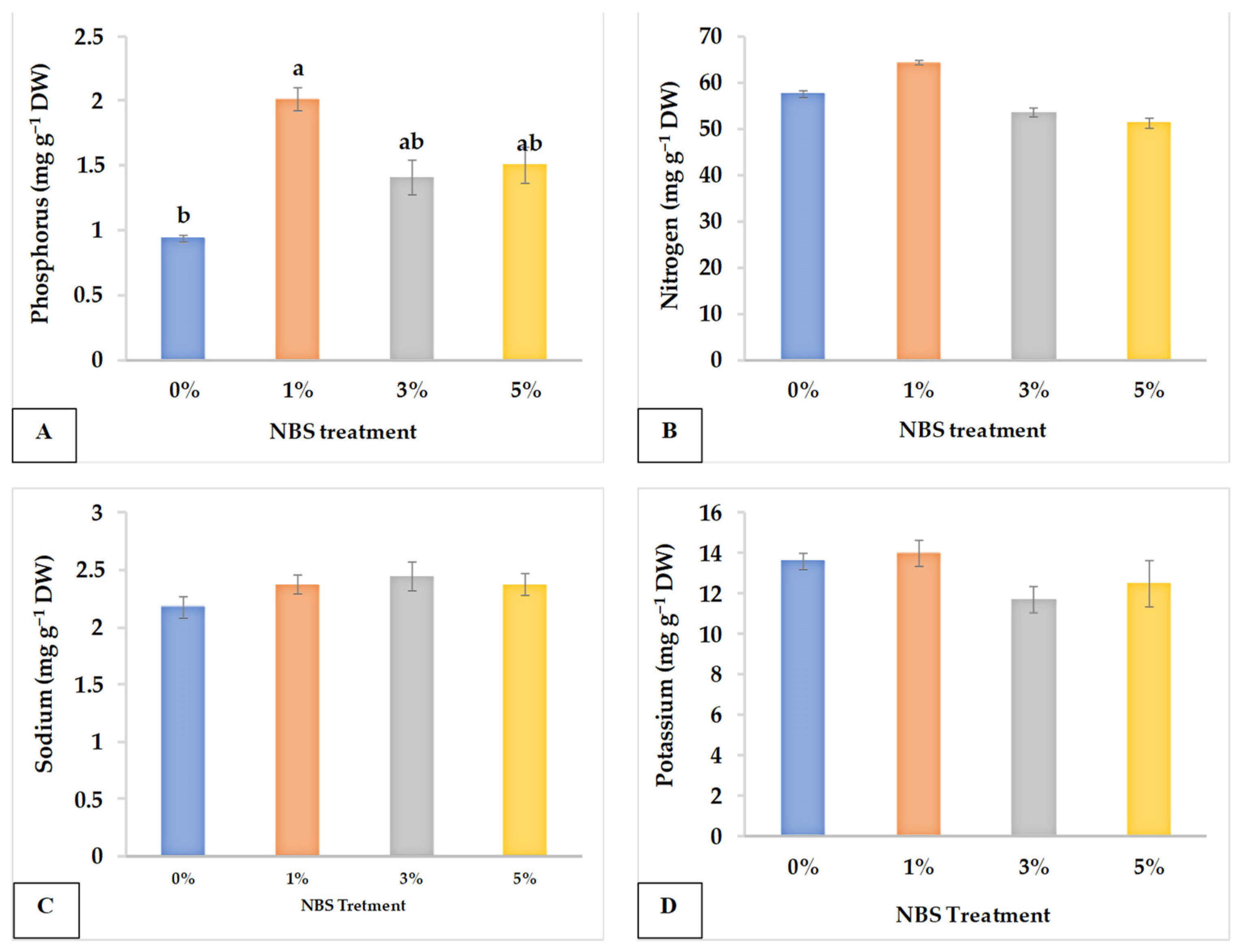
3.3. Leaf Chemical Analysis
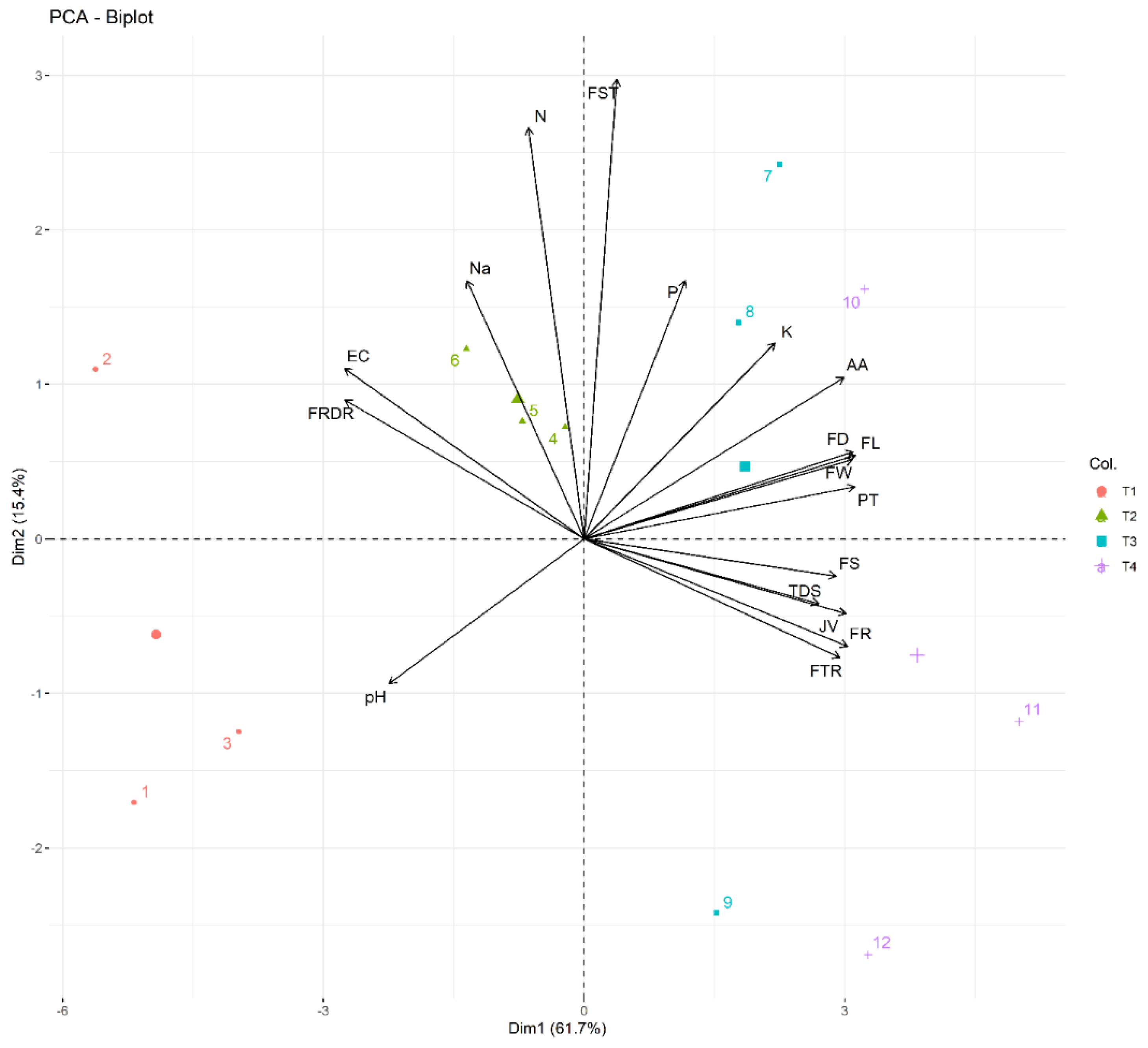
3.4. Principal Component Analysis and Correlation Revealed a Strong Relation among Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barry, G.H.; Caruso, M.; Gmitter, F.G., Jr. Commercial scion varieties. In The Genus Citrus, 1st ed.; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 83–104. [Google Scholar] [CrossRef]
- Luckstead, J.; Devadoss, S. The importance of h-2a guest workers in agriculture. Choices 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Neves, M.F.; Trombin, V.G.; Marques, V.N.; Martinez, L.F. Global orange juice market: A 16-year summary and opportunities for creating value. Trop. Plant Pathol. 2020, 45, 166–174. [Google Scholar] [CrossRef]
- Urbaneja, A.; Grout, T.G.; Gravena, S.; Wu, F.; Cen, Y.; Stansly, P.A. Citrus pests in a global world. In The Genus Citrus, 1st ed.; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 333–348. [Google Scholar] [CrossRef]
- Cheema, I.A.; Jamali, H.K. Growth of Citrus Fruits in Pakistan. Amazon. Investig. 2020, 9, 74–81. [Google Scholar] [CrossRef]
- Ahmad, B.; Mehdi, M.; Ghafoor, A.; Anwar, H. Value chain assessment and measuring export determinants of citrus fruits in Pakistan: An analysis of primary data. Pak. J. Agric. Sci. 2018, 55, 677–693. [Google Scholar] [CrossRef]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Zacarias, L.; Cronje, P.J.; Palou, L. Postharvest technology of citrus fruits. In The Genus Citrus, 1st ed.; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 421–446. [Google Scholar] [CrossRef]
- Donkersley, P.; Silva, F.W.; Carvalho, C.M.; Al-Sadi, A.M.; Elliot, S.L. Biological, environmental and socioeconomic threats to citrus lime production. J. Plant. Dis. Prot. 2018, 125, 339–356. [Google Scholar] [CrossRef]
- Anwar, S.; Ashraf, M.Y.; Saleem, M.; Shafiq, F.; Khan, N.; Khan, R.A.; Farid, G.; Ashraf, M. Integrated hormonal and nutrient management promote fruit retention and quality traits of Citrus reticulata. J. Plant Nutr. 2022, 46, 83–100. [Google Scholar] [CrossRef]
- Nawaz, R.; Abbasi, N.A.; Hafiz, I.A.; Khalid, A.; Ahmad, T. Economic analysis of citrus (Kinnow mandarin) during on-year and off-year in the Punjab province, Pakistan. J. Hortic. 2018, 5, 2376–3354. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Hussain, F.; Ashraf, M.; Akhter, J.; Ebert, G. Modulation in yield and juice quality characteristics of citrus fruit from trees supplied with zinc and potassium foliarly. J. Plant Nutr. 2013, 36, 1996–2012. [Google Scholar] [CrossRef]
- Abu-Alrub, I.; Saleh, S.; Awaga, A.A. Effect of different rates of nitrogen and phosphorus fertilizers on yield and quality of greenhouse tomato under the UAE condition. EC Agri. 2019, 5, 139–146. [Google Scholar]
- Elanchezhian, R.; Kumar, D.; Ramesh, K.; Biswas, A.K.; Guhey, A.; Patra, A.K. Morpho-physiological and biochemical response of maize (Zea mays L.) plants fertilized with nano-iron (Fe3O4) micronutrient. J. Plant Nutr. 2017, 40, 1969–1977. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant growth under drought stress: Significance of mineral nutrients. In Water Stress and Crop Plants: A Sustainable Approach, 1st ed.; Ahmad, P., Ed.; John Wiley & Sons: Chichester, UK, 2016; pp. 649–668. [Google Scholar] [CrossRef]
- Fatima, S.; Riaz, M.; Al-Wabel, M.I.; Arif, M.S.; Yasmeen, T.; Hussain, Q.; Roohi, M.; Fahad, S.; Ali, K.; Arif, M. Higher biochar rate strongly reduced decomposition of soil organic matter to enhance C and N sequestration in nutrient-poor alkaline calcareous soil. J. Soil Sediments. 2021, 21, 148–162. [Google Scholar] [CrossRef]
- Zhao, D.; Yan, B.; Liu, C.; Yao, B.; Luo, L.; Yang, Y.; Zhou, Y. Mitigation of acidogenic product inhibition and elevated mass transfer by biochar during anaerobic digestion of food waste. Bioresour. Technol. 2021, 338, 125531–125547. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Khan, Z.; Xianting, F.; Khan, M.N.; Khan, M.A.; Zhang, K.; Fu, Y.; Shen, H. The toxicity of heavy metals and plant signaling facilitated by biochar application: Implications for stress mitigation and crop production. Chemosphere 2022, 308, 136466. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Vithanage, M.; Alessi, D.S.; Liu, W.J.; Jayasundera, A.C.; Ok, Y.S. Nanobiochar: Production, properties, and multifunctional applications. Environ. Sci. Nano 2020, 7, 3279–3302. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of carbon nanomaterials in the plant system: A perspective view on the pros and cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Gul, A.; Ashraf, M.; Hussain, F.; Ebert, G. Improvement in yield and quality of Kinnow (Citrus deliciosa × Citrus nobilis) by potassium fertilization. J. Plant Nutr. 2010, 33, 1625–1637. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Yaqub, M.; Akhtar, J.; Khan, M.A.; Ali-Khan, M.; Ebert, G. Control of excessive fruit drop and improvement in yield and juice quality of Kinnow (Citrus deliciosa × Citrus nobilis) through nutrient management. Pak. J. Bot. 2012, 44, 259–265. [Google Scholar]
- Yaseen, M.; Ahmad, M. Nutrition management in citrus: Effect of multinutrients foliar feeding on the yield of Kinnow at different locations. Pak. J. Bot. 2010, 42, 1863–1870. [Google Scholar]
- Soil Survey Staff. Key to Soil Taxonomy, 12th ed.; USDA-Natural Resources, Conservation Service: Washington, DC, USA, 2014; p. 379.
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall: Hoboken, NJ, USA, 1962; 498p. [Google Scholar]
- Khaliq, H.; Anwar, S.; Shafiq, F.; Ashraf, M.; Zhang, L.; Haider, I.; Khan, S. Interactive Effects of Soil and Foliar-Applied Nanobiochar on Growth, Metabolites, and Nutrient Composition in Daucus carota. J. Plant Growth Regul. 2022, 15, 1–15. [Google Scholar] [CrossRef]
- Bremner, J.M.; Edwards, A.P. Determination and isotope-ratio analysis of different forms of nitrogen in soils: I. Apparatus and procedure for distillation and determination of ammonium. Soil Sci. Soc. Am. J. 1965, 29, 504–507. [Google Scholar] [CrossRef]
- Ruck, J.A. Chemical Method for Fruit and Vegetable Products; Research Station, Summerland, Department of Agriculture: Ottawa, ON, Canada, 1961. [Google Scholar]
- Graber, E.R.; Frenkel, O.; Jaiswal, A.K.; Elad, Y. How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag. 2014, 5, 169–183. [Google Scholar] [CrossRef]
- Lal, G.; Dayal, H. Effect of integrated nutrient management on yield and quality of acid lime (Citrus aurantifolia Swingle). Afr. J. Agric. Res. 2014, 9, 2985–2991. [Google Scholar] [CrossRef]
- Clarke, A.R.; Powell, K.S.; Weldon, C.W.; Taylor, P.W. The ecology of Bactrocera tryoni (Diptera: Tephritidae): What do we know to assist pest management? Ann. Appl. Biol. 2011, 158, 26–54. [Google Scholar] [CrossRef]
- Gregory, M.M.; Leslie, T.W.; Drinkwater, L.E. Agroecological and social characteristics of New York city community gardens: Contributions to urban food security, ecosystem services, and environmental education. Urban Ecosyst. 2016, 19, 763–794. [Google Scholar] [CrossRef]
- Hasan, M.; Rahim, M.A.; Islam, A.K.M.S.; Rahman, M.M.; Rahman, M.A.; Naher, N. Effect of management practices on the growth and yield of lime and lemon. Int. J. Biosci. 2016, 8, 22–33. [Google Scholar] [CrossRef]
- Khalid, S.; Malik, A.U.; Saleem, B.A.; Khan, A.S.; Khalid, M.S.; Amin, M. Tree age and canopy position affect rind quality, fruit quality and rind nutrient content of ‘Kinnow’mandarin (Citrus nobilis Lour × Citrus deliciosa Tenora). Sci. Hortic. 2012, 135, 137–144. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Haider, M.S.; Shafqat, W.; Asif, M.; Mehmood, A. Influence of different rootstocks on physico-chemical quality attributes of Kinnow mandarin. Pak. J. Agric. Sci. 2021, 58, 929–935. [Google Scholar]
- Chakraborty, R.; Mukhopadhyay, A.; Paul, S.; Sarkar, S.; Mukhopadhyay, R. Nanocomposite-based smart fertilizers: A boon to agricultural and environmental sustainability. Sci. Total Environ. 2023, 863, 160859. [Google Scholar] [CrossRef]
- Saleem, B.A.; Malik, A.U.; Maqbool, M.; Islam-ud-din, M.F.; Rajwana, I.A. Early winter spray of low biuret urea improves marketable yield and fruit quality of sweet oranges. Pak. J. Bot. 2008, 40, 1455–1465. [Google Scholar]
- Palou, L.; Valencia-Chamorro, S.A.; Pérez-Gago, M.B. Anti fungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Song, J.K.; Bae, J.M. Citrus fruit intake and breast cancer risk: A quantitative systematic review. J. Breast Cancer 2013, 16, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Canamas, T.P.; Viñas, I.; Usall, J.; Torres, R.; Anguera, M.; Teixidó, N. Control of postharvest diseases on citrus fruit by preharvest applications of biocontrol agent Pantoea agglomerans CPA-2: Part II. Effectiveness of different cell formulations. Postharvest Biol. Technol. 2008, 49, 96–106. [Google Scholar] [CrossRef]
- Nicosia, M.G.L.D.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Schena, L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]

| Soil Characteristics | Kot Addu |
|---|---|
| Soil texture | Clay loam |
| EC (dS m−1) | 3.5 |
| pH | 7.3 |
| Organic matter (%) | 0.8 |
| P (mg kg−1) | 9 |
| K (mg kg−1) Total Nitrogen (%) | 175 0.75 |
| NBS Concentration (%) | FR | FTR | FL (cm) | FW (g) | FD (cm) | FS (cm3) | PT (mm) | FRDR | FST |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 28.77 c | 25.65 c | 7.073 c | 99.533 b | 6.061 c | 358.867 b | 3.266 c | 70 a | 22 c |
| 1 | 60.59 b | 63.35 b | 8.113 b | 143.800 b | 6.891 b | 390.800 ab | 4.438 b | 35 b | 58 b |
| 3 | 75.72 a | 70.5 ab | 9.040 a | 199.933 a | 7.791 a | 438.333 ab | 4.796 ab | 25 bc | 70 a |
| 5 | 79.15 a | 76 a | 9.167 a | 204.400 a | 7.845 a | 463.000 a | 5.172 a | 20 c | 76 a |
| Significance | *** | **** | *** | *** | *** | * | **** | *** | *** |
| NBS Concentration (%) | EC (ds m−1) | TDS (mg L−1) | AA (mg 100 mL−1) | pH | JV (mL) | N (mg g−1 DW) | P (mg g−1 DW) | Na+ (mg g−1 DW) | K+ (mg g−1 DW) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3.428 a | 1936.640 b | 25.033 b | 5.439 a | 55.067 d | 5.787 a | 0.941 b | 2.229 a | 11.673 a |
| 1 | 3.321 ab | 2028.373 ab | 28.107 a | 4.164 b | 65.267 c | 6.440 a | 2.382 a | 2.026 a | 12.346 a |
| 3 | 3.169 ab | 2125.653 ab | 28.667 a | 4.240 b | 85.333 b | 5.367 a | 2.079 ab | 2.099 a | 13.278 a |
| 5 | 3.026 b | 2193.920 a | 29.060 a | 4.345 b | 113.133 a | 5.133 a | 1.510 ab | 2.073 a | 14.271 a |
| Significance | * | * | *** | * | **** | ns | * | ns | ns |
| FR | FTR | FL | FW | FD | FS | PT | FRDR | EC | TDS | AA | pH | JV | FST | N | P | Na+ | K+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR | ||||||||||||||||||
| FTR | 0.958 ** | |||||||||||||||||
| FL | 0.821 ** | 0.779 ** | ||||||||||||||||
| FW | 0.813 ** | 0.765 ** | 0.992 ** | |||||||||||||||
| FD | 0.810 ** | 0.756 ** | 0.995 ** | 0.997 ** | ||||||||||||||
| FS | 0.792 ** | 0.741 ** | 0.876 ** | 0.887 ** | 0.879 ** | |||||||||||||
| PT | 0.840 ** | 0.848 ** | 0.942 ** | 0.911 ** | 0.927 ** | 0.769 ** | ||||||||||||
| FRDR | −0.797 ** | −0.790 ** | −0.687 * | −0.701 ** | −0.693 ** | −0.795 ** | −0.705 ** | |||||||||||
| EC | −0.792 ** | −0.823 ** | −0.712 ** | −0.702 ** | −0.694 ** | −0.791 ** | −0.752 ** | 0.921 ** | ||||||||||
| TDS | 0.843 ** | 0.735 ** | 0.781 ** | 0.780 ** | 0.791 ** | 0.705 ** | 0.765 ** | −0.654 * | −0.580 * | |||||||||
| AA | 0.779 ** | 0.748 ** | 0.924 ** | 0.892 ** | 0.904 ** | 0.723 ** | 0.931 ** | −0.647 * | −0.633 * | 0.742 ** | ||||||||
| pH | −0.556 * | −0.589 * | −0.676 * | −0.610 * | −0.634 * | −0.424 | −0.808 ** | 0.447 | 0.549 * | −0.362 | −0.802 * | |||||||
| JV | 0.941 ** | 0.947 ** | 0.853 ** | 0.861 ** | 0.847 ** | 0.820 ** | 0.841 ** | −0.776 ** | −0.803 ** | 0.808 ** | 0.756 ** | −0.462 | ||||||
| FST | −0.152 | −0.154 | 0.252 | 0.235 | 0.258 | 0.011 | 0.236 | 0.122 | 0.128 | 0.010 | 0.387 | −0.351 | −0.039 | |||||
| N | −0.262 | −0.329 | −0.102 | −0.120 | −0.107 | −0.167 | −0.194 | 0.364 | 0.455 | −0.219 | 0.088 | 0.020 | −0.267 | 0.642 * | ||||
| P | 0.151 | 0.173 | 0.408 | 0.402 | 0.404 | 0.324 | 0.405 | −0.260 | −0.165 | −0.016 | 0.509 * | −0.642 * | 0.077 | 0.374 | 0.229 | |||
| Na+ | −0.436 | −0.386 | −0.270 | −0.207 | −0.228 | −0.420 | −0.352 | 0.540 * | 0.576 * | −0.382 | −0.322 | 0.369 | −0.235 | 0.395 | 0.359 | −0.091 | ||
| K+ | 0.621 * | 0.553 * | 0.617 * | 0.640 * | 0.637 * | 0.559 * | 0.556 * | −0.614 | −0.468 | 0.613 * | 0.683 * | −0.306 | 0.671 * | 0.447 | 0.361 | 0.178 | 0.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shani, M.Y.; Ahmed, S.R.; Ashraf, M.Y.; Khan, Z.; Cocozza, C.; De Mastro, F.; Gul, N.; Pervaiz, S.; Abbas, S.; Nawaz, H.; et al. Nano-Biochar Suspension Mediated Alterations in Yield and Juice Quality of Kinnow (Citrus reticulata L.). Horticulturae 2023, 9, 521. https://doi.org/10.3390/horticulturae9050521
Shani MY, Ahmed SR, Ashraf MY, Khan Z, Cocozza C, De Mastro F, Gul N, Pervaiz S, Abbas S, Nawaz H, et al. Nano-Biochar Suspension Mediated Alterations in Yield and Juice Quality of Kinnow (Citrus reticulata L.). Horticulturae. 2023; 9(5):521. https://doi.org/10.3390/horticulturae9050521
Chicago/Turabian StyleShani, Muhammad Yousaf, Syed Riaz Ahmed, Muhammad Yasin Ashraf, Zafran Khan, Claudio Cocozza, Francesco De Mastro, Nimra Gul, Soha Pervaiz, Shahid Abbas, Hummera Nawaz, and et al. 2023. "Nano-Biochar Suspension Mediated Alterations in Yield and Juice Quality of Kinnow (Citrus reticulata L.)" Horticulturae 9, no. 5: 521. https://doi.org/10.3390/horticulturae9050521
APA StyleShani, M. Y., Ahmed, S. R., Ashraf, M. Y., Khan, Z., Cocozza, C., De Mastro, F., Gul, N., Pervaiz, S., Abbas, S., Nawaz, H., & Brunetti, G. (2023). Nano-Biochar Suspension Mediated Alterations in Yield and Juice Quality of Kinnow (Citrus reticulata L.). Horticulturae, 9(5), 521. https://doi.org/10.3390/horticulturae9050521










