Evaluation of Removed and Recycled Mineral Nutrients in Italian Commercial Persimmon Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Orchards Investigated
2.2. Plant Organ Sampling and Analysis
2.3. Statistical Analysis
3. Results
3.1. Tree Biomass
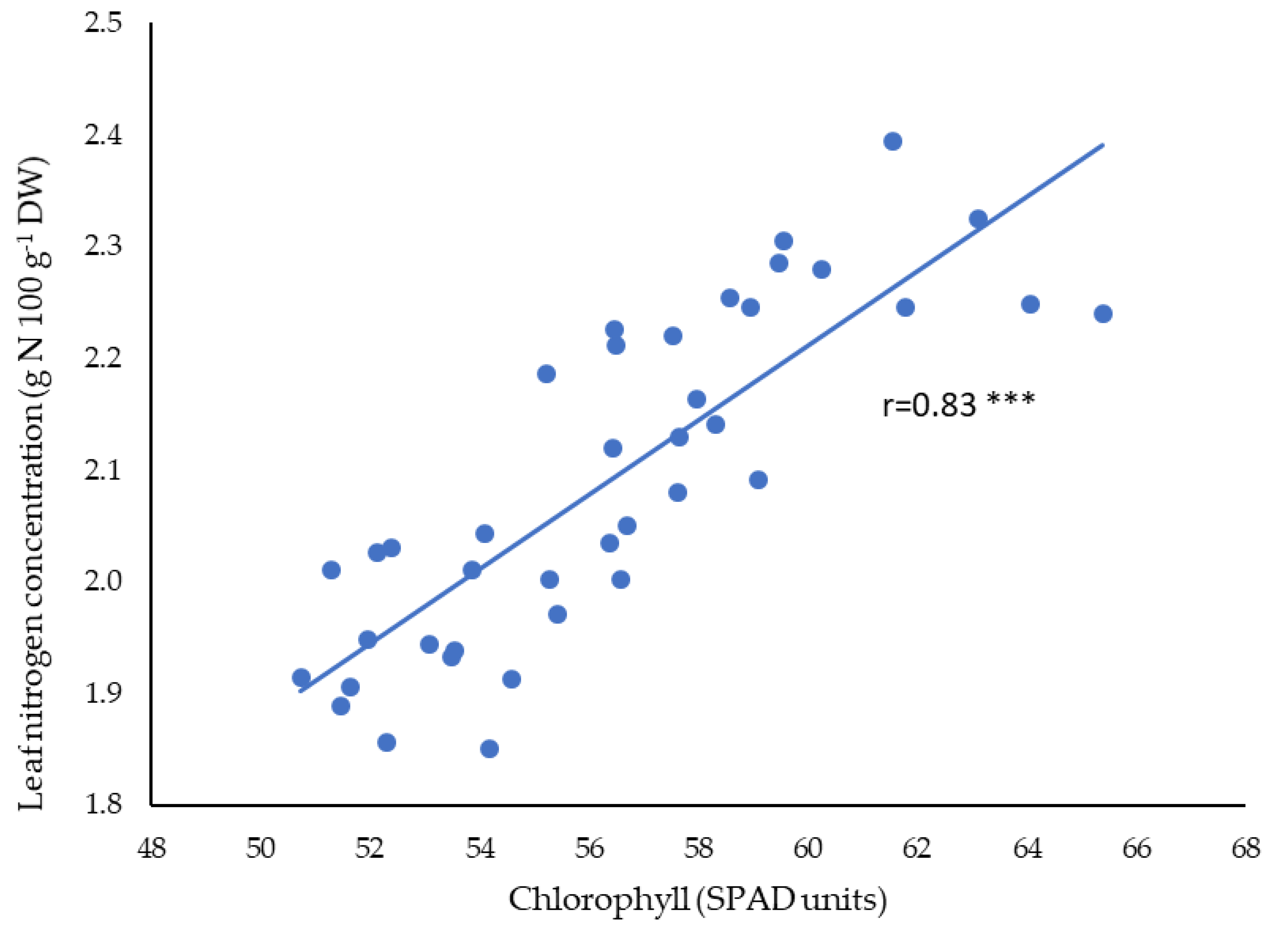
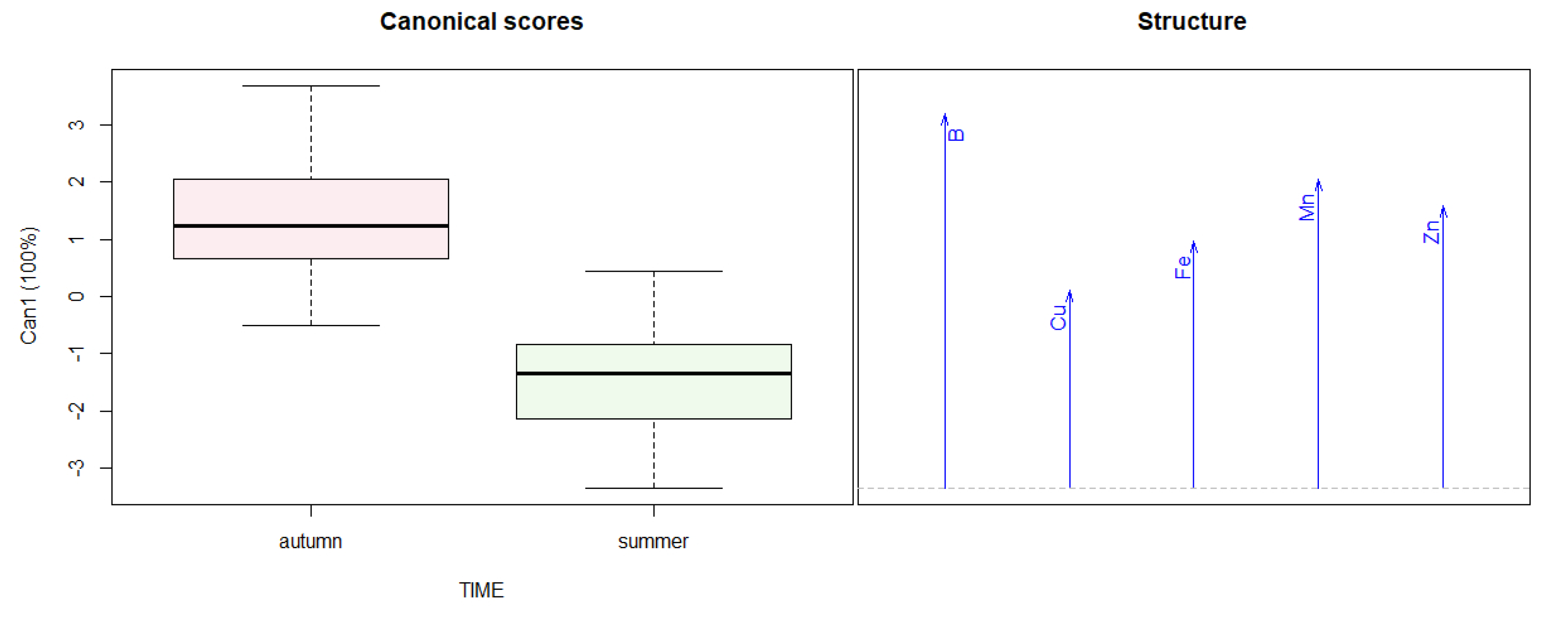
3.2. Leaf Chlorophyll and Mineral Concentration
3.3. Tree Nutrients Content
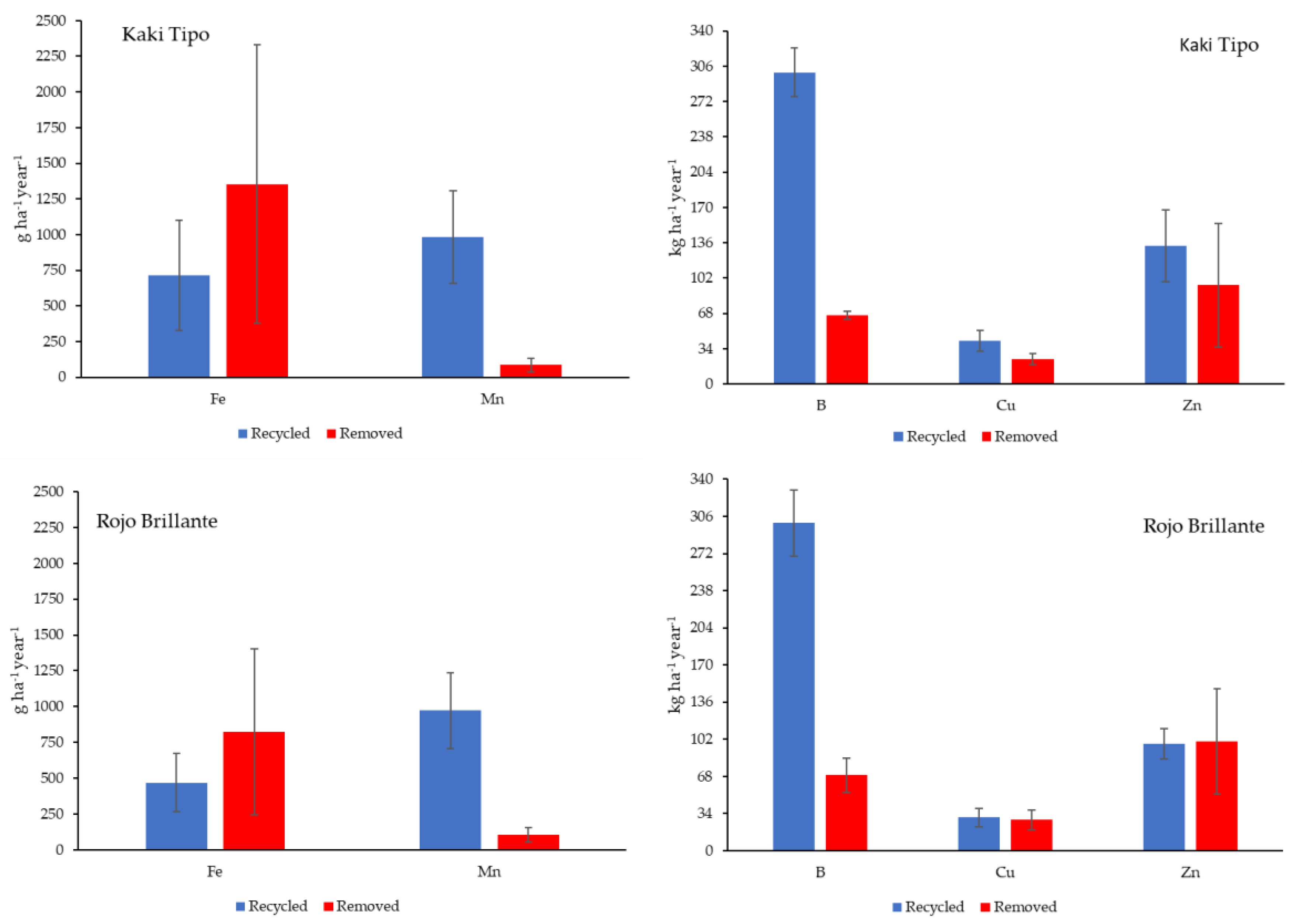
3.4. Amounts of Nutrient Removed and Recycled in the Orchard
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Istat. Available online: http://dati.istat.it/viewhtml.aspx?il=blank&vh=0000&vf=0&vcq=1100&graph=0&view-metadata=1&lang=it&QueryId=33654 (accessed on 9 May 2022).
- Baldi, E.; Quartieri, M.; Sorrenti, G.; Toselli, M. Evaluation of nutrients removed and recycled in a commercial peach orchard over a 14-years-production cycle. Italus Hortus 2021, 28, 1–12. [Google Scholar] [CrossRef]
- Malaguti, D.; Rombola, A.D.; Quartieri, M.; Lucchi, A.; Inderst, C.; Marangoni, B.; Tagliavini, M. Effects of the rate of nutrients by fertigation and broadcast application in ‘Gala’ and ‘Fuji’ apple. Acta Hortic. 2006, 721, 165–172. [Google Scholar] [CrossRef]
- Toselli, M.; Mazzanti, F.; Marangoni, B.; Tagliavini, M.; Scudellari, D. Determination of leaf standards for mineral diagnosis in pear orchards in the Po Valley, Italy. Acta Hortic. 2002, 596, 665–669. [Google Scholar] [CrossRef]
- Choi, S.T.; Kim, S.C.; Park, D.S.; Kang, S.M. Tree growth and physiological fruit drop of “Hachiya” persimmon on D. kaki and D. lotus rootstocks. Acta Hortic. 2008, 772, 345–349. [Google Scholar] [CrossRef]
- Badal, E.; El-Mageed, T.A.; Buesa, I.; Guerra, D.; Bonet, L.; Intrigliolo, D.S. Moderate plant water stress reduces fruit drop of “Rojo Brillante” persimmon (Dyospiros kaki) in a Mediterranean climate. Agric. Water Manag. 2013, 119, 154–160. [Google Scholar] [CrossRef]
- Ventura, M.; Scandellari, F.; Ventura, F.; Guzzon, B.; Rossi Pisa, P.; Tagliavini, M. Nitrogen balance and losses through drainage waters in an agricultural watershed of the Po Valley (Italy). Eur. J. Agron. 2008, 29, 108–115. [Google Scholar] [CrossRef]
- Volk, M.; Liersch, S.; Schmidt, G. Towards the implementation of the European Water Framework Directive? Lessons learned from water quality simulations in an agricultural water-shed. Land Use Policy 2009, 26, 580–588. [Google Scholar] [CrossRef]
- Ahmed, Z.F.; Kaur, N.; Hassan, F.E. ornamental date palm and sidr trees: Fruit elements composition and concerns regarding consumption. Int. J. Fruit Sci. 2022, 22, 17–34. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S. Seasonal changes in the mineral nutrient content of persimmon leaves. Sci. Hortic. 1990, 42, 85–97. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S. Seasonal changes in the composition, distribution and accumulation of mineral nutrients in persimmon fruit. Sci. Hortic. 1990, 42, 99–111. [Google Scholar] [CrossRef]
- Mir–Marqués, A.; Domingo, A.; Cervera, M.L.; De la Guardia, M. Mineral profile of kaki fruits (Diospyros kaki L.). Food Chem. 2015, 172, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, E.; Kaplankiran, M. Seasonal Variations in Mineral Nutrients in Leaves and Shoots of Some Persimmons Cultivars grown in Turkey. Acta Hortic. 2013, 996, 351–357. [Google Scholar] [CrossRef]
- Llácer, G.; Martínez-Calvo, J.; Naval, M.; Badenes, M.L. From germplasm to fruit export: The case of ‘Rojo Brillante’ persimmon. Adv. Hort. Sci. 2008, 22, 281–285. Available online: https://www.jstor.org/stable/42883470 (accessed on 9 May 2022).
- Emilia-Romagna. 2022. Available online: https://agricoltura.regione.emilia-romagna.it/produzioniagroalimentari/temi/bio-agro-climambiente/agricoltura-integrata/disciplinari-produzione-integratavegetale# (accessed on 27 January 2023).
- Scandellari, F.; Ventura, M.; Malaguti, D.; Ceccon, C.; Menarbin, G.; Tagliavini, M. Net primary productivity and partitioning of absorbed nutrients in field-grown apple trees. Acta Hortic. 2010, 868, 115–122. [Google Scholar] [CrossRef]
- Tagliavini, M.; Quartieri, M. I fabbisogni nutrizionali del pero per la definizione del piano di fertilizzazione. Italus Hortus 2008, 15, 131–137. [Google Scholar]
- Choi, S.T.; Park, D.S. Use of a chlorophyll meter to diagnose nitrogen status of ‘Fuyu’ persimmon leaves. HortScience 2011, 46, 821–824. [Google Scholar] [CrossRef]
- Neilsen, D.; Hogue, E.J.; Neilsen, G.H.; Parchomchuk, P. Using SPAD-502 Values to assess the nitrogen status of apple trees. HortScience 1995, 30, 508–512. [Google Scholar] [CrossRef]
- Porro, D.; Bertoldi, D.; Bottura, M.; Pedò, S. Five-year period of evaluation of leaf mineral concentrations in resistant varieties in Trentino (Northeastern Italy). BIO Web Conf. 2022, 44, 01002. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Alnuaimi, A.K.H.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae 2021, 7, 292. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar] [CrossRef]
- Natali, S.; Bignami, C. Tecnica colturale del terreno: Lavorazione, concimazione ed irrigazione del kaki. In Il Kaki, Aggiornamenti Della Coltura; Ismea: Roma, Italy, 1988; pp. 63–80. [Google Scholar]
- George, A.; Nissen, B.; Broadley, R. Persimmon nutrition. In Sweet Persimmon Grower’s Handbook, 1st ed.; Grower Guide Series QI05102; Belisle, J., George, A., Broadley, R., Nissen, B., Eds.; Department of Primary Industries and Fisheries: Brisbane, Australia, 2005; Chapter 4; pp. 1–64. ISSN 0727-6273. [Google Scholar]
- Millard, P. Ecophysiology of the internal cycling of nitrogen for tree growth. J. Plant Nutr. Soil Sci. 1996, 159, 1–10. [Google Scholar] [CrossRef]
- Millard, P.; Grelet, G.A. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, N.Q.; Quiñones, A.; Rodríguez, I.; Gil, R.; Fernández-Serrano, P.; Salvador, A. Leaf and fruit nutrient concentration in Rojo Brillante persimmon grown under conventional and organic management, and its correlation with fruit quality parameters. Agronomy 2022, 12, 237. [Google Scholar] [CrossRef]
- Quartieri, M.; Toselli, M. Ottimizzare la concimazione del kaki valutando le asportazioni minerali. Riv. Fruttic. 2021, 2, 52–56. [Google Scholar]
- Tagliavini, M.; Tonon, G.; Scandellari, F.; Quinones, A.; Palmieri, S.; Menarbin, G.; Gioacchini, P.; Masia, A. Nutrient recycling during the decomposition of apple leaves (Malus domestica) and mowed grasses in an orchard. Agric. Ecosyst. Environ. 2007, 118, 191–200. [Google Scholar] [CrossRef]
- Choi, S.T.; Park, D.S.; Yoon, T.H.; Jae, H.J.; Lee, Y.H. Response of “Fuyu” Persimmon to K Forms and Fertigation Rates on Tree Growth and Fruit Quality. Acta Hortic. 2013, 996, 293–297. [Google Scholar] [CrossRef]
- Baldi, E.; Marcolini, G.; Quartieri, M.; Sorrenti, G.; Toselli, M. Effect of organic fertilization on nutrient concentration and accumulation in nectarine (Prunus persica var. nucipersica) trees: The effect of rate application. Sci. Hortic. 2014, 179, 174–179. [Google Scholar] [CrossRef]
- Stassen, P.J.C.; North, M.S. Nutrient distribution and requirement of “Forelle” pear trees on two rootstocks. Acta Hortic. 2005, 671, 493–500. [Google Scholar] [CrossRef]
- Marcolini, G.; Toselli, M.; Quartieri, M.; Gioacchini, P.; Baldi, E.; Sorrenti, G.; Mariani, S. Nitrogen and carbon mineralisation of different Meliaceae derivatives. Plant Soil Environ. 2016, 62, 121–127. [Google Scholar] [CrossRef]
- Tagliavini, M.; Zavalloni, C.; Rombolà, A.D.; Quartieri, M.; Malaguti, D.; Mazzanti, F.; Millard, P.; Marangoni, B. Mineral nutrient partitioning to fruits of deciduous trees. Acta Hortic. 2000, 512, 131–140. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zachwieja, Z.; Folta, M.; Barton, H.; Piotrowicz, J.; Zemser, M.; Wisz, M.; Trakhtenberg, S.; Martìn-Belloso, O. Comparative contents of dietary fiber, total phenolics and mineral in persimmons and apples. J. Agric. Food Chem. 2001, 49, 952–957. [Google Scholar] [CrossRef]



| Cultivar | Chlorophyll | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|---|
| Kaki Tipo | 59.1 ± 2.84 | 2.21 ± 0.10 | 0.12 ± 0.01 | 1.76 ± 0.35 | 2.52 ± 0.43 | 0.59 ± 0.11 | 0.20 ± 0.03 |
| Rojo Brillante | 53.7 ± 2.19 | 1.97 ± 0.07 | 0.12 ± 0.01 | 1.86 ± 0.36 | 2.59 ± 0.51 | 0.54 ± 0.07 | 0.17 ± 0.02 |
| Cultivar | B | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|
| Kaki Tipo | 65.9 ± 7.78 | 4.86 ± 7.78 | 61.8 ± 6.42 | 153 ± 67.1 | 11.9 ± 1.98 |
| Rojo Brillante | 64.0 ± 8.32 | 3.86 ± 0.57 | 53.4 ± 5.56 | 134 ± 44.9 | 11.3 ± 1.56 |
| Tree Organs | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| Kaki Tipo | ||||||
| Fruits | 53.9 ± 7.95 | 9.44 ± 0.86 | 103 ± 19.4 | 5.33 ± 1.31 | 4.16 ± 0.56 | 5.52 ± 0.84 |
| Abscissed leaves | 54.9 ± 15.8 | 3.31 ± 0.98 | 30.6 ± 15.3 | 151 ± 58.7 | 25.1 ± 13.2 | 5.64 ± 1.67 |
| Pruning wood | 45.6 ± 21.8 | 6.12 ± 3.05 | 24.5 ± 12.3 | 53.7 ± 25.5 | 7.74 ± 3.74 | 4.88 ± 2.48 |
| Skeleton | 5.18 ± 0.43 | 0.26 ± 0.06 | 1.56 ± 0.51 | 13.3 ± 2.04 | 1.31 ± 0.32 | 0.51 ± 0.07 |
| Roots | 1.30 ± 0.52 | 0.13 ± 0.04 | 0.74 ± 0.46 | 3.75 ± 1.21 | 0.58 ± 0.35 | 0.14 ± 0.03 |
| TOTAL | 161 ± 37.7 | 19.3 ± 4.04 | 160 ± 37.3 | 227 ± 74.4 | 38.8 ± 15.7 | 16.7 ± 3.91 |
| Rojo Brillante | ||||||
| Fruits | 46.8 ± 12.1 | 8.99 ± 2.45 | 85.6 ± 29.1 | 5.38 ± 1.87 | 3.71 ± 1.25 | 4.56 ± 1.33 |
| Abscissed leaves | 55.7 ± 20.9 | 3.38 ± 0.91 | 36.1 ± 16.4 | 201 ± 29.8 | 29.4 ± 6.64 | 6.05 ± 1.45 |
| Pruning wood | 25.9 ± 11.2 | 3.41 ± 1.85 | 13.8 ± 6.01 | 32.5 ± 11.1 | 4.61 ± 2.01 | 3.01 ± 1.15 |
| Skeleton | 12.4 ± 5.01 | 1.54 ± 0.24 | 5.23 ± 0.99 | 22.6 ± 10.2 | 3.62 ± 0.85 | 1.28 ± 0.46 |
| Roots | 7.76 ± 4.41 | 0.37 ± 0.23 | 1.36 ± 0.45 | 4.28 ± 1.57 | 0.71 ± 0.26 | 0.37 ± 0.14 |
| TOTAL | 149 ± 31.6 | 17.7 ± 3.23 | 142 ± 30.1 | 266 ± 30.6 | 42.1 ± 7.81 | 15.3 ± 2.39 |
| Tree Organs | B | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|
| Kaki Tipo | |||||
| Fruits | 78.7 ± 9.95 | 16.6 ± 10.9 | 71.1 ± 16.5 | 33.2 ± 14.2 | 43.7 ± 13.6 |
| Abscissed leaves | 493 ± 80.4 | 29.4 ± 5.70 | 1116 ± 769 | 1478 ± 613 | 77.6 ± 15.1 |
| Pruning wood | 23.5 ± 11.7 | 42.6 ± 17.1 | 155 ± 76.3 | 213 ± 84.2 | 153 ± 71.0 |
| Skeleton | 23.1 ± 7.32 | 15.1 ± 4.15 | 165 ± 19.2 | 36.1 ± 8.92 | 97.6 ± 83.4 |
| Roots | 10.7 ± 3.36 | 8.64 ± 7.10 | 1811 ± 1464 | 63.9 ± 51.0 | 7.46 ± 4.25 |
| TOTAL | 629 ± 86.7 | 112 ± 24.8 | 3318 ± 805 | 1824 ± 611 | 379 ± 83.5 |
| Rojo Brillante | |||||
| Fruits | 88.9 ± 30.0 | 12.4 ± 5.97 | 66.7 ± 15.8 | 31.1 ± 12.1 | 35.8 ± 15.7 |
| Abscissed leaves | 484 ± 77.6 | 25.3 ± 6.63 | 626 ± 347 | 1476 ± 420 | 82.6 ± 20.8 |
| Pruning wood | 13.7 ± 5.94 | 24.8 ± 12.6 | 160 ± 168 | 125 ± 61.4 | 78.9 ± 26.2 |
| Skeleton | 19.9 ± 7.84 | 29.1 ± 12.7 | 952 ± 835 | 112 ± 66.0 | 113 ± 69.4 |
| Roots | 11.6 ± 4.49 | 3.24 ± 0.69 | 79.1 ± 33.9 | 13.5 ± 3.97 | 5.52 ± 1.94 |
| TOTAL | 618 ± 83.7 | 94.8 ± 14.1 | 1884 ± 394 | 1758 ± 441 | 316 ± 37.9 |
| Total Demand | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| Kaki Tipo | 91.4 ± 3.78 | 10.9 ± 0.52 | 90.7 ± 1.61 | 132 ± 4.26 | 22.3 ± 1.03 | 9.4 ± 0.1 |
| Rojo Brillante | 88.9 ± 1.42 | 10.3 ± 0.52 | 79.2 ± 8.10 | 162 ± 23.9 | 25.5 ± 6.23 | 9.1 ± 1.2 |
| Total Demand | B | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|
| Kaki Tipo | 366 ± 32 | 99 ± 3.5 | 2067 ± 975 | 1066 ± 276 | 228 ± 57 |
| Rojo Brillante | 369 ± 48 | 59 ± 8.2 | 1223 ± 540 | 1295 ± 106 | 198 ± 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartieri, M.; Polidori, G.; Baldi, E.; Toselli, M. Evaluation of Removed and Recycled Mineral Nutrients in Italian Commercial Persimmon Orchards. Horticulturae 2023, 9, 374. https://doi.org/10.3390/horticulturae9030374
Quartieri M, Polidori G, Baldi E, Toselli M. Evaluation of Removed and Recycled Mineral Nutrients in Italian Commercial Persimmon Orchards. Horticulturae. 2023; 9(3):374. https://doi.org/10.3390/horticulturae9030374
Chicago/Turabian StyleQuartieri, Maurizio, Greta Polidori, Elena Baldi, and Moreno Toselli. 2023. "Evaluation of Removed and Recycled Mineral Nutrients in Italian Commercial Persimmon Orchards" Horticulturae 9, no. 3: 374. https://doi.org/10.3390/horticulturae9030374
APA StyleQuartieri, M., Polidori, G., Baldi, E., & Toselli, M. (2023). Evaluation of Removed and Recycled Mineral Nutrients in Italian Commercial Persimmon Orchards. Horticulturae, 9(3), 374. https://doi.org/10.3390/horticulturae9030374








