13 Cycles of Consecutive Tomato Monoculture Cropping Alter Soil Chemical Properties and Soil Fungal Community in Solar Greenhouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Measure of Tomato Fruit Fresh per Plant
2.3. Soil Sampling
2.4. Analysis of Soil Chemical Properties
2.5. Soil DNA Extraction, PCR and Sequencing Analysis of Soil Fungal Community
2.6. Statistical Analysis
3. Results
3.1. Tomato Fruit Fresh per Plant and Soil Chemical Properties
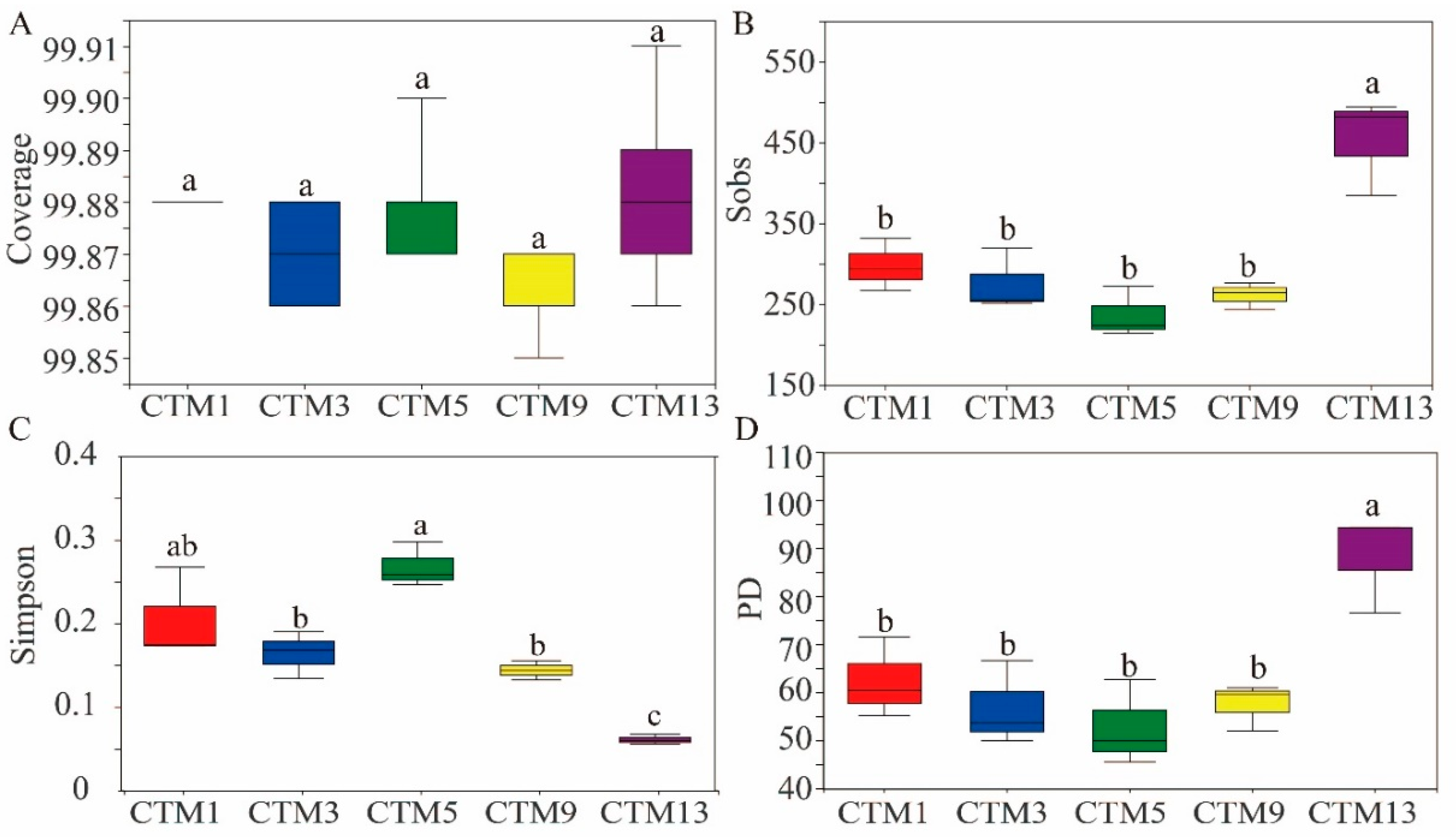
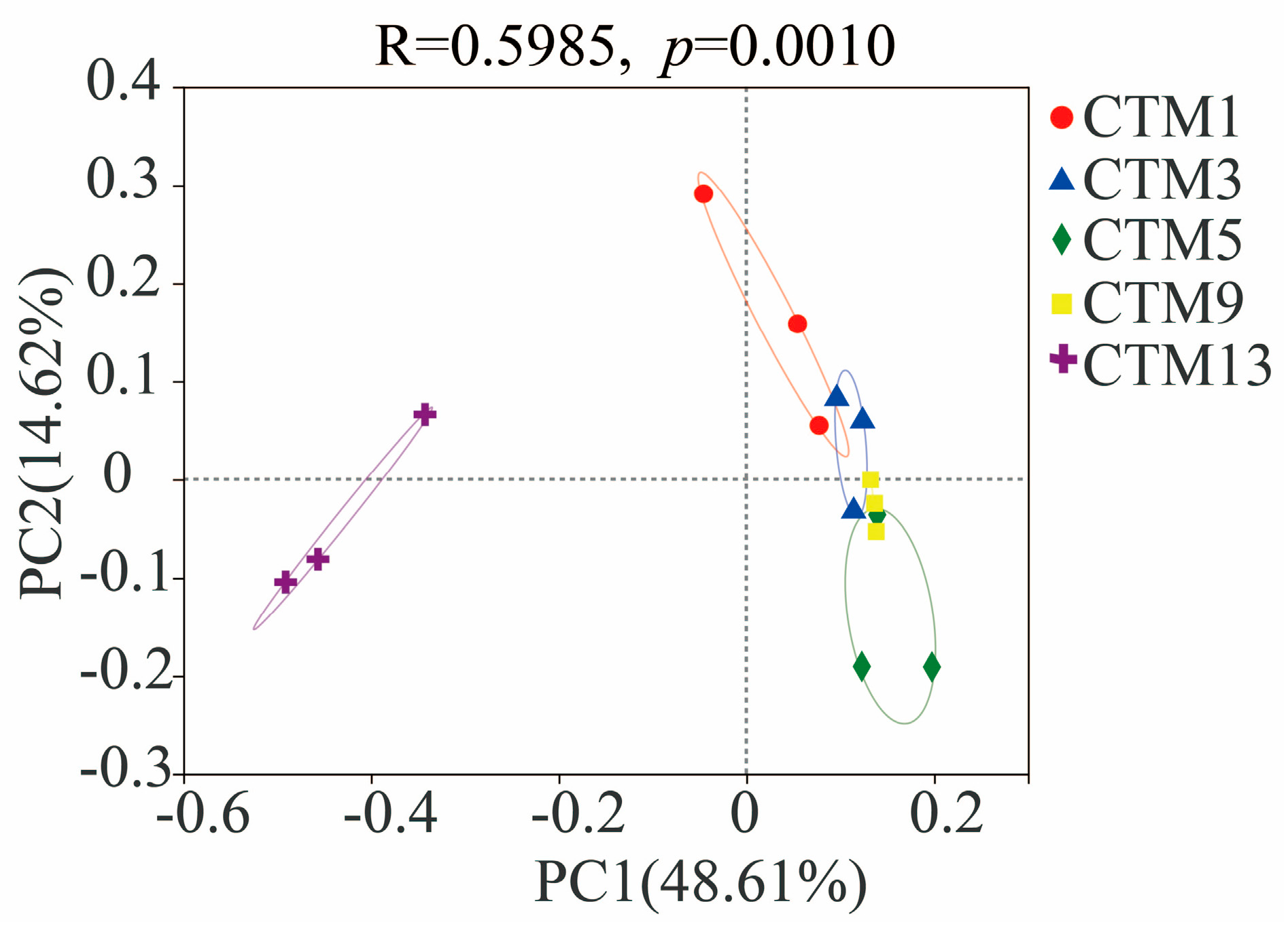
3.2. Soil Fungal Community Diversity
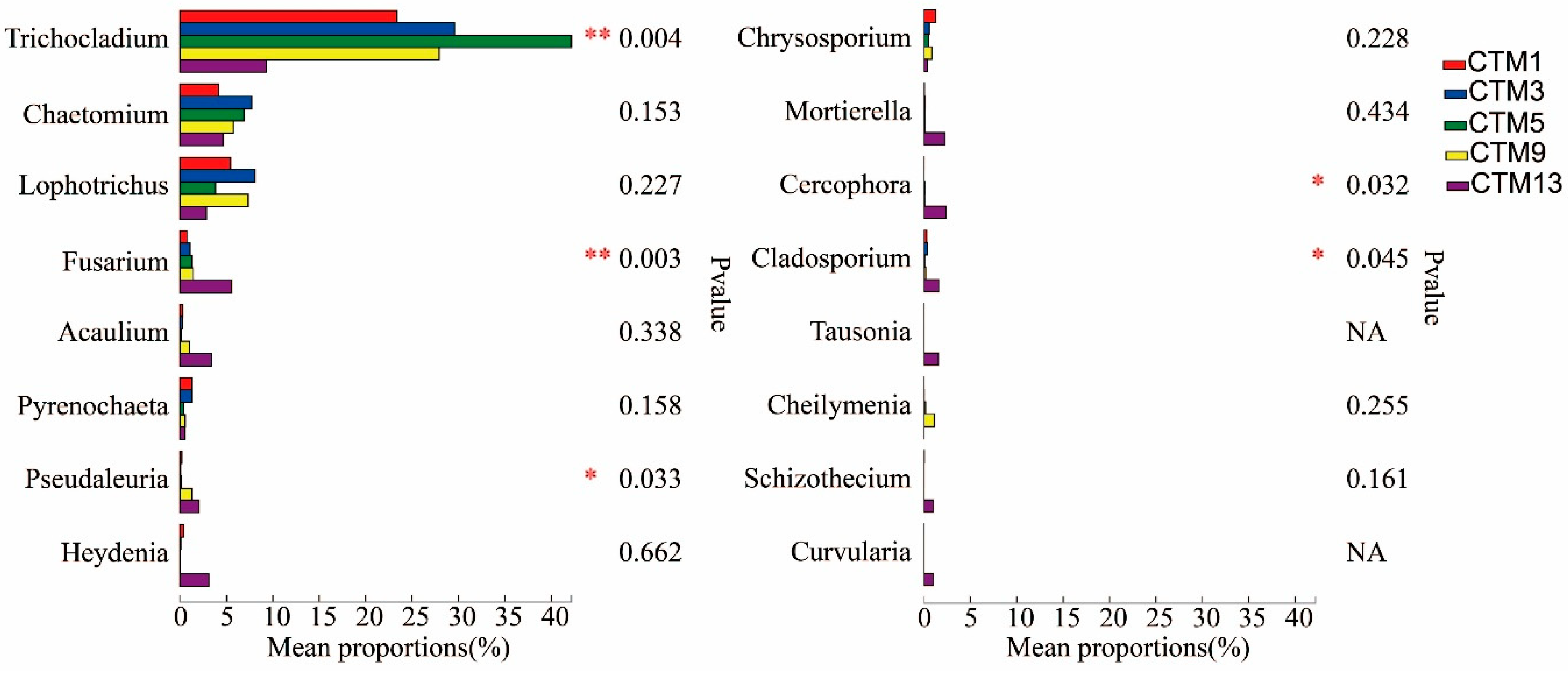
3.3. Soil Fungal Community Composition
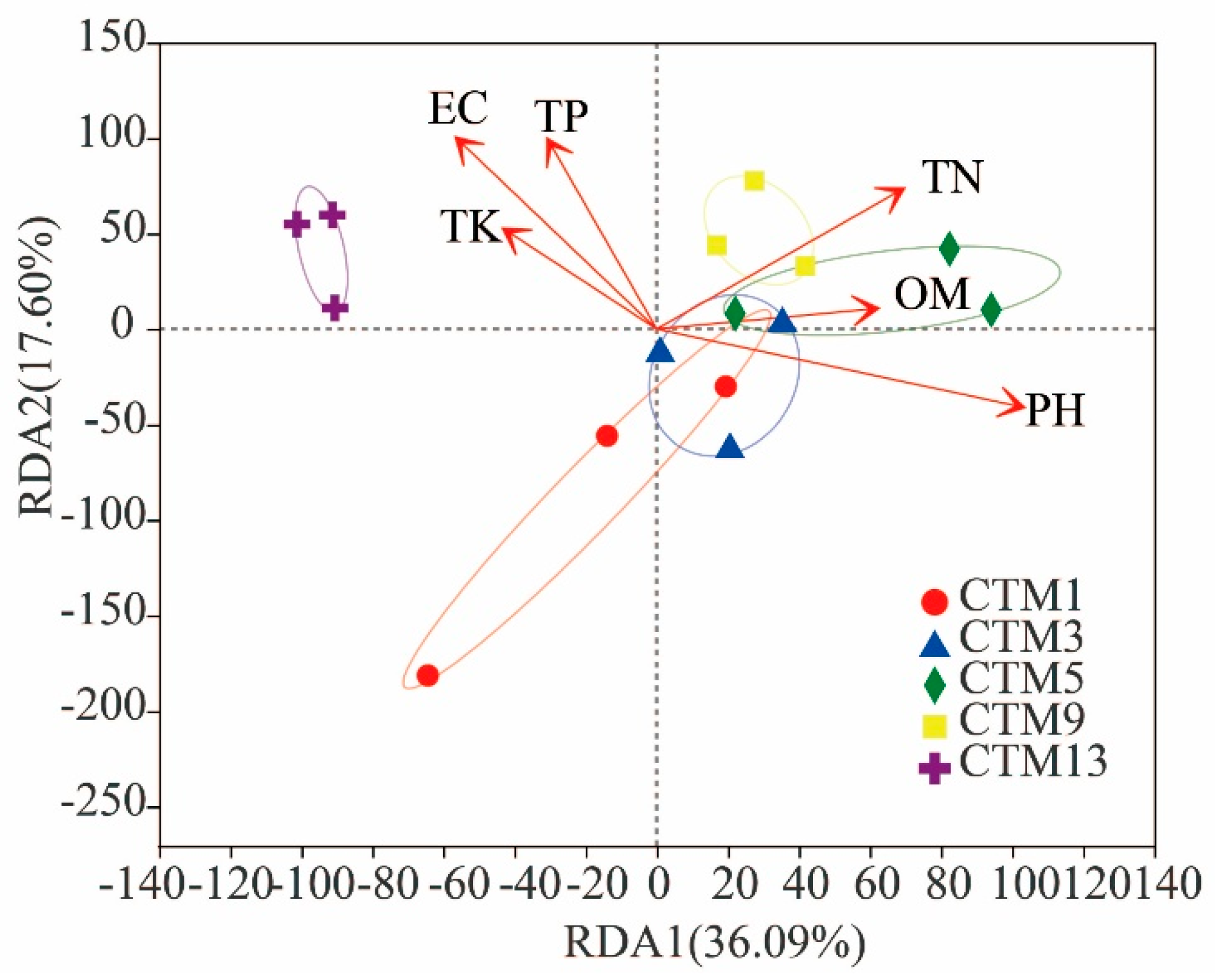
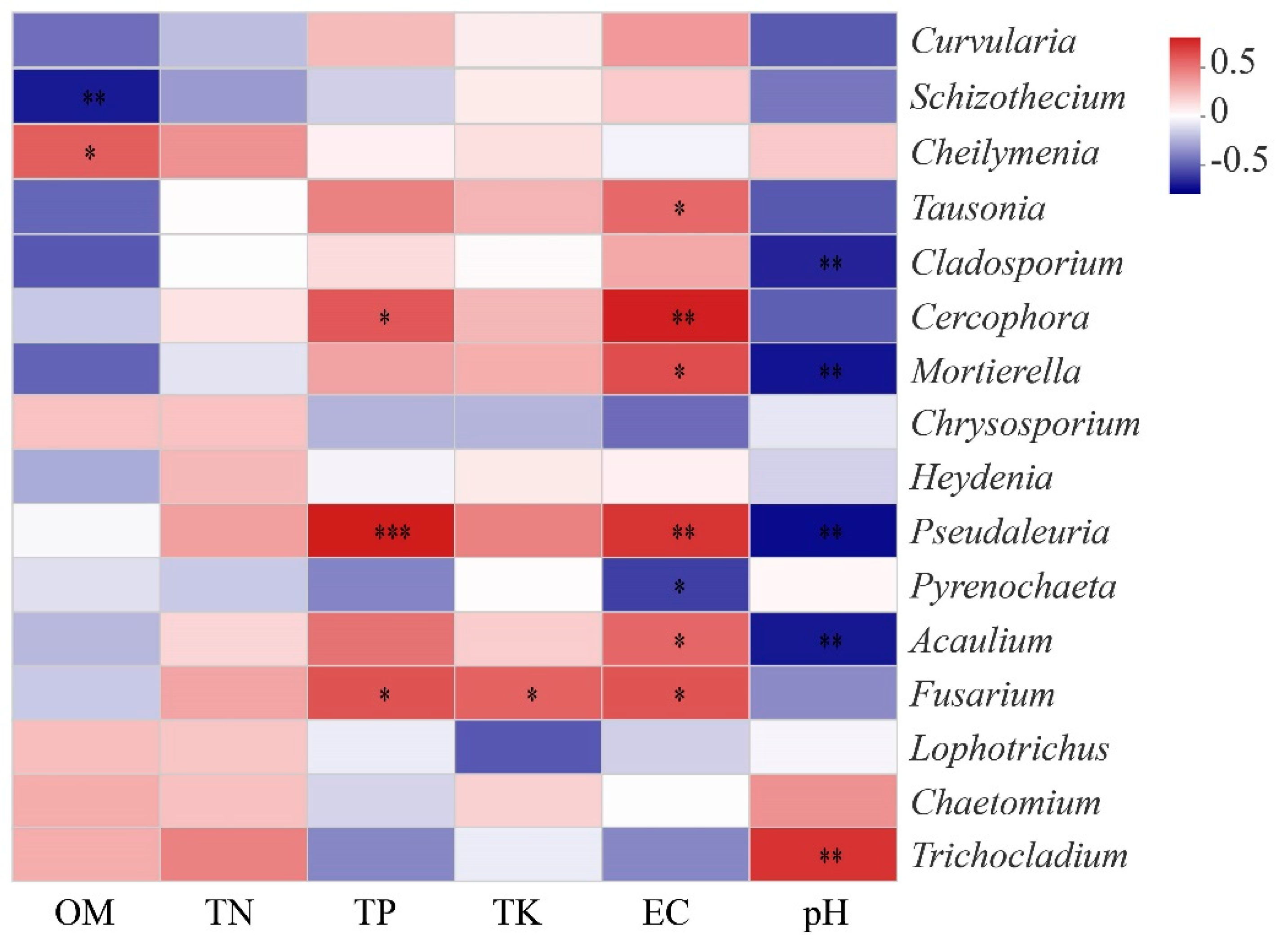
3.4. Effects of Soil Chemical Properties on Soil Fungal Community Composition
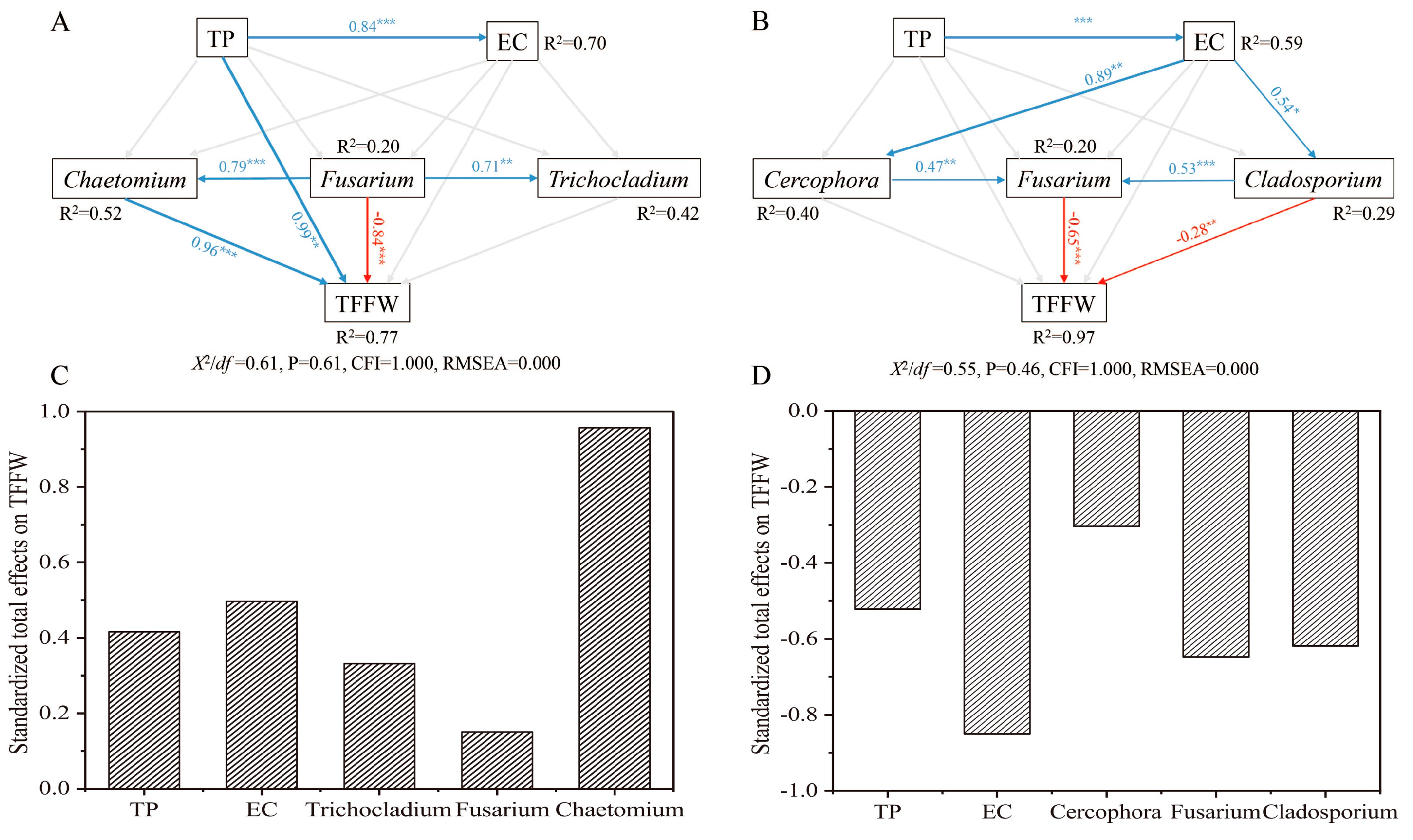
3.5. Linking TFFW to Soil Chemical Properties and Soil Fungal Genera
4. Discussion
4.1. Effects of 13 Cycles of Consecutive Tomato Monoculture on Soil Chemical Properties
4.2. Effects of 13 Cycles of Consecutive Tomato Monoculture Cropping on Soil Fungal Community
4.3. Effects of Soil Chemical Properties and Soil Fungal Community on TFFW
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Abbreviation | Definition |
| CTM | Consecutive tomato monoculture cropping |
| TN | Soil total nitrogen |
| TP | soil total phosphorus |
| TK | Soil total potassium |
| EC | soil electrical conductivity |
| OM | soil organic matter content |
| TFFW | tomato fruit fresh weight per plant |
| RDA | Redundancy analysis |
| SEM | Structural-equation-model |
References
- Tilahun, S.; Seo, M.H.; Park, D.S.; Jeong, C.S. Effect of Cultivar and Growing Medium on the Fruit Quality Attributes and Antioxidant Properties of Tomato (Solanum lycopersicum L.). Hortic. Environ. Biotechnol. 2018, 59, 215–223. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sanchez-Zapata, E.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A.; Fernández-López, J. Tomato and Tomato Byproducts. Human Health Benefits of Lycopene and Its Application to Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1032–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, Q.; Qi, X. Present situation and development countermeasure of tomato production in Liaoning Province. China Veg. 2015, 10, 6–9. (In Chinese) [Google Scholar]
- Zhao, F.; Zhang, Y.; Dong, W.; Zhang, Y.; Zhang, G.; Sun, Z.; Yang, L. Vermicompost Can Suppress Fusarium oxysporum f. sp. lycopersici via Generation of Beneficial Bacteria in a Long-Term Tomato Monoculture Soil. Plant Soil 2019, 440, 491–505. [Google Scholar] [CrossRef]
- Lyu, J.; Jin, L.; Jin, N.; Xie, J.; Xiao, X.; Hu, L.; Tang, Z.; Wu, Y.; Niu, L.; Yu, J. Effects of Different Vegetable Rotations on Fungal Community Structure in Continuous Tomato Cropping Matrix in Greenhouse. Front. Microbiol. 2020, 11, 829. [Google Scholar] [CrossRef]
- Li, W. Horticultural Plant Cultivation Techniques, 2nd ed.; Chongqing University Press: Chongqing, China, 2013. [Google Scholar]
- Cui, R.; Geng, G.; Wang, G.; Stevanato, P.; Dong, Y.; Li, T.; Yu, L.; Wang, Y. The Response of Sugar Beet Rhizosphere Micro-Ecological Environment to Continuous Cropping. Front. Microbiol. 2022, 13, 956785. [Google Scholar] [CrossRef]
- Zhao, H.T.; Li, T.P.; Zhang, Y.; Hu, J.; Bai, Y.C.; Shan, Y.H.; Ke, F. Effects of Vermicompost Amendment as a Basal Fertilizer on Soil Properties and Cucumber Yield and Quality under Continuous Cropping Conditions in a Greenhouse. J. Soils Sediments 2017, 17, 2718–2730. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.; Gu, L. Impact of Continuous Cucumis sativus L. Cropping on the Incidence of Root Diseases and Bacterial Community Structure. Adv. Mater. Res. 2012, 518, 5472–5479. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, F. Dynamics of the Diversity of Fungal and Fusarium Communities during Continuous Cropping of Cucumber in the Greenhouse. FEMS Microbiol. Ecol. 2012, 80, 469–478. [Google Scholar] [CrossRef]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-Soil Feedbacks and Soil Sickness: From Mechanisms to Application in Agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ramesh, A.; Sharma, M.P.; Joshi, O.P.; Govaerts, B.; Steenwerth, K.L.; Karlen, D.L. Microbial Community Structure and Diversity as Indicators for Evaluating Soil Quality. In Biodiversity, Biofuels, AgroForestry and Conservation Agriculture; Lichtfouse, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 317–358. [Google Scholar]
- Fu, H.; Zhang, G.; Zhang, F.; Sun, Z.; Geng, G.; Li, T. Effects of Continuous Tomato Monoculture on Soil Microbial Properties and Enzyme Activities in a Solar Greenhouse. Sustainability 2017, 9, 317. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Y.; Li, Z.; Shi, J.; Zhang, G.; Zhang, H.; Yang, L. Vermicompost Improves Microbial Functions of Soil with Continuous Tomato Cropping in a Greenhouse. J. Soils Sediments 2020, 20, 380–391. [Google Scholar] [CrossRef]
- Min, H.; Sheng, J.F.; Chen, L.; Han, C.J.; Tang, N.; Liu, H.J. Effects of Continuous Cropping of Tomato and Strawberry on Soil Bacterial Community Composition. Allelopath. J. 2016, 38, 25–40. [Google Scholar]
- Chen, L.; Zhang, J.; Zhao, B.; Yan, P.; Zhou, G.; Xin, X. Effects of Straw Amendment and Moisture on Microbial Communities in Chinese Fluvo-Aquic Soil. J. Soils Sediments 2014, 14, 1829–1840. [Google Scholar] [CrossRef]
- Ning, Q.; Chen, L.; Jia, Z.; Zhang, C.; Ma, D.; Li, F.; Zhang, J.; Li, D.; Han, X.; Cai, Z.; et al. Multiple Long-Term Observations Reveal a Strategy for Soil PH-Dependent Fertilization and Fungal Communities in Support of Agricultural Production. Agric. Ecosyst. Environ. 2020, 293, 106837. [Google Scholar] [CrossRef]
- Li, W.-H.; Liu, Q.-Z. Changes in Fungal Community and Diversity in Strawberry Rhizosphere Soil after 12 Years in the Greenhouse. J. Integr. Agric. 2019, 18, 677–687. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, X.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. Response of Soil Microbial Communities to Continuously Mono-Cropped Cucumber under Greenhouse Conditions in a Calcareous Soil of North China. J. Soils Sediments 2020, 20, 2446–2459. [Google Scholar] [CrossRef]
- Yang, R.; Mo, Y.; Liu, C.; Wang, Y.; Ma, J.; Zhang, Y.; Li, H.; Zhang, X. The Effects of Cattle Manure and Garlic Rotation on Soil under Continuous Cropping of Watermelon (Citrullus lanatus L.). PLoS ONE 2016, 11, e0156515. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, X.; Fu, H.; Sun, Z. Effects of Artificially-Simulated Acidification on Potential Soil Nitrification Activity and Ammonia Oxidizing Microbial Communities in Greenhouse Conditions. PeerJ 2022, 10, e14088. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Science and Technique Press: Beijing, China, 2013. [Google Scholar]
- Wu, L.; Chen, J.; Wu, H.; Wang, J.; Wu, Y.; Lin, S.; Khan, M.U.; Zhang, Z.; Lin, W. Effects of Consecutive Monoculture of Pseudostellaria Heterophylla on Soil Fungal Community as Determined by Pyrosequencing. Sci. Rep. 2016, 6, 26601. [Google Scholar] [CrossRef] [PubMed]
- Potdar, R.P.; Shirolkar, M.M.; Verma, A.J.; More, P.S.; Kulkarni, A. Determination of Soil Nutrients (NPK) Using Optical Methods: A Mini Review. J. Plant Nutr. 2021, 44, 1826–1839. [Google Scholar] [CrossRef]
- Tian, S.; Dong, X.; Guo, H.; Dong, L.; Zhang, Y.; Liu, S.; Luo, J. Key Soil Nutrient Requirements for Different Yield Levels in North China. Soil Sci. Plant Nutr. 2019, 65, 519–524. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.; Li, Z.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Variations of Rhizosphere Bacterial Communities in Tea (Camellia sinensis L.) Continuous Cropping Soil by High-Throughput Pyrosequencing Approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Y.; Dong, W.; Zhou, C.; Zhang, G.; Yang, L. Effects of Amendments on Base Cation and Micronutrient Availabilities in Soils Planted with Tomato in a Solar Greenhouse. Soil Sci. Plant Nutr. 2018, 64, 782–792. [Google Scholar] [CrossRef]
- Jiao, H.; Chen, Y.; Lin, X.; Liu, R. Diversity of Arbuscular Mycorrhizal Fungi in Greenhouse Soils Continuously Planted to Watermelon in North China. Mycorrhiza 2011, 21, 681–688. [Google Scholar] [CrossRef]
- Huang, S.; Tang, J.; Li, C.; Zhang, H.; Yuan, S. Reducing potential of chemical fertilizers and scientific fertilization countermeasure in vegetable production in China. J. Plant Nutr. Fertil. 2017, 23, 1480–1493. (In Chinese) [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Han, J.; Luo, Y.; Yang, L.; Liu, X.; Wu, L.; Xu, J. Acidification and Salinization of Soils with Different Initial PH under Greenhouse Vegetable Cultivation. J. Soils Sediments 2014, 14, 1683–1692. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q. Analysis of Soil EC Value Dynamic and Salt Sensitivity for Facility Fruity Vegetable. China Veg. 2014, 2, 15–20. [Google Scholar]
- Gao, Z.; Han, M.; Hu, Y.; Li, Z.; Liu, C.; Wang, X.; Tian, Q.; Jiao, W.; Hu, J.; Liu, L.; et al. Effects of Continuous Cropping of Sweet Potato on the Fungal Community Structure in Rhizospheric Soil. Front. Microbiol. 2019, 10, 2269. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Sun, K.; Fu, L.; Song, Y.; Yuan, L.; Zhang, H.; Wen, D.; Yang, N.; Wang, X.; Yue, Y.; Li, X.; et al. Effects of Continuous Cucumber Cropping on Crop Quality and Soil Fungal Community. Environ. Monit. Assess. 2021, 193, 436. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, X.; Jiang, A.; Fan, J.; Lan, T.; Zhang, J.; Cai, Z. Distinct Impacts of Reductive Soil Disinfestation and Chemical Soil Disinfestation on Soil Fungal Communities and Memberships. Appl. Microbiol. Biotechnol. 2018, 102, 7623–7634. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, L.; Wen, T.; Zhang, J.; Wang, F.; Cai, Z. Changes in the Soil Microbial Community after Reductive Soil Disinfestation and Cucumber Seedling Cultivation. Appl. Microbiol. Biotechnol. 2016, 100, 5581–5593. [Google Scholar] [CrossRef]
- Hamed, S.A.M. In-Vitro Studies on Wood Degradation in Soil by Soft-Rot Fungi: Aspergillus Niger and Penicillium Chrysogenum. Int. Biodeterior. Biodegrad. 2013, 78, 98–102. [Google Scholar] [CrossRef]
- Collemare, J.; Griffiths, S.; Iida, Y.; Karimi Jashni, M.; Battaglia, E.; Cox, R.J.; De Wit, P.J.G.M. Secondary Metabolism and Biotrophic Lifestyle in the Tomato Pathogen Cladosporium Fulvum. PLoS ONE 2014, 9, e85877. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Pan, Y.; Li, L.; Wu, X.; Wang, Y. Composition and Diversity of Rhizosphere Fungal Community in Coptis Chinensis Franch. Continuous Cropping Fields. PLoS ONE 2018, 13, e0193811. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Rethinasamy, V.; Al-Mahrouqi, H.; Al-Sadi, A.M. Fungal Diversity in Tomato Rhizosphere Soil under Conventional and Desert Farming Systems. Front. Microbiol. 2017, 8, 1462. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Manici, L.M.; Insam, H.; Stres, B. Rhizosphere Bacteria and Fungi Associated with Plant Growth in Soils of Three Replanted Apple Orchards. Plant Soil 2015, 395, 317–333. [Google Scholar] [CrossRef]
- Li, X.-G.; Ding, C.-F.; Zhang, T.-L.; Wang, X.-X. Fungal Pathogen Accumulation at the Expense of Plant-Beneficial Fungi as a Consequence of Consecutive Peanut Monoculturing. Soil Biol. Biochem. 2014, 72, 11–18. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Liu, Q.; Zhou, Z.; Chen, F.; Xiang, D. Effects of Consecutive Monoculture of Sweet Potato on Soil Bacterial Community as Determined by Pyrosequencing. J. Basic Microbiol. 2019, 59, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, X.; Huang, H.; Jing, J.; Zhao, H.; Wang, L.; Long, X.E. Contrasting Beneficial and Pathogenic Microbial Communities across Consecutive Cropping Fields of Greenhouse Strawberry. Appl. Microbiol. Biotechnol. 2018, 102, 5717–5729. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Lu, X.H.; Guo, R.J.; Hao, J.; Miao, Z.Q.; Yang, L.; Li, S.D. Responses of Soil Abiotic Properties and Microbial Community Structure to 25-Year Cucumber Monoculture in Commercial Greenhouses. Agriculture 2021, 11, 341. [Google Scholar] [CrossRef]
- Zhong, S.; Mo, Y.; Guo, G.; Zeng, H.; Jin, Z. Effect of Continuous Cropping on Soil Chemical Properties and Crop Yield in Banana Plantation. J. Agric. Sci. Technol. 2014, 16, 239–250. [Google Scholar]
- Wei, W.; Xu, Y.; Li, S.; Zhu, L.; Song, J. Developing Suppressive Soil for Root Diseases of Soybean with Continuous Long-Term Cropping of Soybean in Black Soil of Northeast China. Acta Agric. Scand. B Soil Plant Sci. 2015, 65, 279–285. [Google Scholar] [CrossRef]
- Dong, L.; Xu, J.; Feng, G.; Li, X.; Chen, S. Soil Bacterial and Fungal Community Dynamics in Relation to Panax Notoginseng Death Rate in a Continuous Cropping System. Sci. Rep. 2016, 6, 31802. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Yang, K.; Wang, J.; Jousset, A.; Xu, Y.; Shen, Q.; Friman, V.P. Phage Combination Therapies for Bacterial Wilt Disease in Tomato. Nat. Biotechnol. 2019, 37, 1513–1520. [Google Scholar] [CrossRef]
- Susilo, F.X.; Neutel, A.M.; Noordwijk, M. Soil biodiversity and food webs. In Below-Groundinteractions in Tropical Agroecosystems: Concepts and Models with Multiple Plant Components; Van Noordwijk, M., Cadisch, G., Ong, C.K., Eds.; CABI International: Wallingford, UK, 2004; pp. 285–307. [Google Scholar]
- Gu, X.; Yang, N.; Zhao, Y.; Liu, W.; Li, T. Long-Term Watermelon Continuous Cropping Leads to Drastic Shifts in Soil Bacterial and Fungal Community Composition across Gravel Mulch Fields. BMC Microbiol. 2022, 22, 189. [Google Scholar] [CrossRef]
| TFFW (g·Plant−1) | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | EC (μs·cm−1) | |
|---|---|---|---|---|---|
| CTM1 | 1495.60 ± 42.85 b | 1.13 ± 0.08 b | 2.34 ± 0.17 c | 14.12 ± 0.64 a | 520.30 ± 17.49 c |
| CTM3 | 2014.87 ± 33.58 a | 1.17 ± 0. 03 b | 2.51 ± 0.18 c | 14.54 ± 0.45 a | 556.97 ± 7.53 c |
| CTM5 | 1969.55 ± 14.22 a | 1.43 ± 0.23 ab | 2.98 ± 0.26 bc | 14.91 ± 0.31 a | 673.47 ± 57.50 b |
| CTM9 | 1936.78 ± 63.68 a | 1.67 ± 0.08 a | 3.84 ± 0.29 a | 15.48 ± 0.34 a | 795.37 ± 12.76 a |
| CTM13 | 1466.24 ± 32.00 b | 1.20 ± 0.08 b | 3.61 ± 0.08 ab | 16.61 ± 1.99 a | 887.30 ± 42.66 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Guo, M.; Shan, X.; Zhang, X.; Sun, Z.; Liu, Y.; Li, T. 13 Cycles of Consecutive Tomato Monoculture Cropping Alter Soil Chemical Properties and Soil Fungal Community in Solar Greenhouse. Horticulturae 2023, 9, 505. https://doi.org/10.3390/horticulturae9040505
Fu H, Guo M, Shan X, Zhang X, Sun Z, Liu Y, Li T. 13 Cycles of Consecutive Tomato Monoculture Cropping Alter Soil Chemical Properties and Soil Fungal Community in Solar Greenhouse. Horticulturae. 2023; 9(4):505. https://doi.org/10.3390/horticulturae9040505
Chicago/Turabian StyleFu, Hongdan, Meiqi Guo, Xuan Shan, Xiaolan Zhang, Zhouping Sun, Yufeng Liu, and Tianlai Li. 2023. "13 Cycles of Consecutive Tomato Monoculture Cropping Alter Soil Chemical Properties and Soil Fungal Community in Solar Greenhouse" Horticulturae 9, no. 4: 505. https://doi.org/10.3390/horticulturae9040505
APA StyleFu, H., Guo, M., Shan, X., Zhang, X., Sun, Z., Liu, Y., & Li, T. (2023). 13 Cycles of Consecutive Tomato Monoculture Cropping Alter Soil Chemical Properties and Soil Fungal Community in Solar Greenhouse. Horticulturae, 9(4), 505. https://doi.org/10.3390/horticulturae9040505





