Abstract
Replant disease significantly hinders the development of the grape industry, and the imbalance of the rhizosphere microecological environment is one of the fundamental reasons hindering grape replants. Peanut is a common intercropping crop, and whether the root exudates of peanut can alleviate grape replant obstacles is still unknown. In this study, the effects of exogenous peanut root exudates on replanting grapevine growth, and the microbial community structure of grapevine replant soils were studied. The results showed that peanut root exudates could promote the growth of replanting grapevine seedlings; enhance root vigor and SOD activity, increasing 55.18% and 95.71%, respectively; and reduce the MDA content of root, decreasing 31.10%. After peanut exudate treatment, the growth of Fusarium solanum, an important harmful fungus that is an obstacle to grape replant, was inhibited. The relative abundances of Gaiella in bacteria and Cystobasidium and Mortierella in fungi increased, and the potential pathogen fungi Fusicolla decreased. Peanut root exudates also modified the soil bacterial and fungal community in a certain range and increased the interaction among the bacteria of grapevine rhizosphere soil. However, they loosened the interaction among fungi. There are extensive mutualistic interactions among bacteria or fungi in grape rhizosphere assemblages after peanut exudates treatment. Therefore, peanut root exudates might be helpful in changing the soil microbial environment and alleviating the grape replanting obstacle.
1. Introduction
The grapevine is widely distributed globally. However, the replant obstacle is widespread in many vineyards, especially in nurseries, and this significantly hinders the healthy growth of young grapevines. Replant obstacle is the phenomenon of weak growth and reduced yield and quality when the grapevines are replanted. Previous studies pointed out that there were three possible reasons for replant obstacles: nutritional imbalance [1], autotoxicity of root secretion [2,3], and shifts in the microbial community [4,5]. With further research, it was found that rhizosphere microecological disorder caused by self-toxic substances was an important cause of replant obstacles.
The common methods to alleviate the replant obstacle include soil disinfection, biological agents, and intercropping or rotation. Intercropping or rotation, which is one of the effective and simple methods to alleviate replant obstacles, has attracted the attention of researchers. Yu et al. [6] found that under Fusarium oxysporum f. sp. niveum invasion, compared with the watermelon monoculture system, the watermelon–wheat intercropping rhizosphere bacterial community was more stable. Wu et al. [7] showed that intraspecific intercropping alleviated the severe replanting disease of R. pseudostellariae, increasing the relative abundance of potentially beneficial taxa and decreasing the relative abundance of pathogenic taxa. Atractylodes lancea intercropping significantly changed the composition of peanut rhizosphere fungal community, alleviated Fusarium root rot, and promoted peanut growth. Volatiles derived from A. lancea rhizome material markedly suppressed the growth of F. oxysporum [8]. Interplanting Chinese onion was able to increase the yield per plant of cucumber and alleviate the incidence of cucumber Fusarium wilt [9]. Yuan et al. [10] reported that the pineapple–banana rotation decreased banana Fusarium wilt disease incidence. Zhou et al. [11] found that crop rotation resulted in a higher abundance of potential plant-growth-promoting microorganisms and increased cucumber yield. Therefore, intercropping and rotation can alleviate the replant obstacle by regulating the rhizosphere soil microecological environment.
Peanut is a common intercropping crop. It is still unclear whether peanut root exudates have a positive effect on the microbial community of the grape rhizosphere soil. Beta (V. riparia × V. labrusca) seedlings were used as test materials in the experiment, which were planted in the replanting soil of grapevine, and exogenous peanut root exudates were added. The purpose of this study was to investigate the effect of exogenous peanut root exudates on soil microbial community structure in grape replanted soils and analyze the potential and ways of peanut exudates in alleviating grape replant obstacles.
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
Seed-derived seedlings of the grape rootstock “Beta” (Vitis riparia × Vitis labrusca) were used in the experiments, and the seeds of Peanut Luhua No. 1 (Arachis hypogaea Linn.) were purchased from Hebei Qingfeng Seed Industry Co. (Cangzhou City, Hebei Province, China).
2.1.2. Soil Materials
The soil materials used in the experiment were sampled from the vineyard that has been planting grapevines for 14 years in Jiulongdi street, Gaizhou county, Yingkou City, Liaoning Province, China (40°21′ N, 122°13′ E), which experiences temperate monsoon climate, that is rain and heat in the same season.
The root zone soil was randomly selected and collected by the multi-point sampling method. Top soil (0–20 cm) was removed, and 20–40 cm root layer soil was collected. After removing leaves, rocks, and roots, each sample was completely homogenized and divided into two parts, one was stored at room temperature for pot experiments, and the other was stored at 4 °C for soil physical and chemical analysis. The physical and chemical properties are shown in Table 1.

Table 1.
Physicochemical properties of grape replant soils.
2.1.3. Strain Material
The Fusarium solani is an important harmful fungus that had been isolated from grape replant soil in our laboratory.
2.2. Experimental Design
2.2.1. Collection of Peanut Root Secretion
Peanut root exudates were collected by the water culture method. The details were as follows: germinated peanut seeds were grown in nursery trays filled with seedling substrate. When the 45 seedlings grew to a 15–20 cm height, they were taken out of the plate, and carefully, the substrate attached to the root was removed and rinsed with distilled water. Transfer 3 seedlings to a glass bottle that was opaque containing 250 mL of distilled water. Use an air pump to aerate the water for 15 min at 30 min intervals. Root exudates were collected every 12 h and replaced with an equal volume of fresh distilled water. After 6 collections, the underground part of the seedlings was collected for root dry weight measurement to determine the concentration of root exudates, and the root exudates (stored at 4 °C) were pooled, mixed, and then concentrated at 40 °C using a rotary evaporator (BUCHI R-220 SE, Switzerland).
The concentrated peanut root exudates were used for exogenous application (0.75 g·L−1 dry root) and antifungal activity tests (0.5 g·mL−1 dry root).
2.2.2. Peanut Root Exudates Treatment in Grape Replant Soil
There were two treatments: the replant soil was irrigated with peanut root exudates (E) or with distilled water (W). The pot (8.5 cm× 8.5 cm × 7 cm) was filled with 230 g of the replant soil, and both treatments were performed in 15 pots and cultured at room temperature.
From 2 December to 29 December in 2021, 30 mL of peanut root exudates or 30 mL of distilled water were used to replant soil every 3 days. After 28 days of cultivation, 30 g of soil was taken from each pot, mixed, and stored at −80 °C for DNA and sequencing analysis. This experiment was performed on three replicates.
2.2.3. Pot Experiment
Beta seeds were grown in nursery trays filled with seedling matrix, and when the seedlings developed 4–5 leaves, they were transferred to the potted replant soil previously treated with peanut root exudates (E) or distilled water (W) after washing the matrix attached to the roots. These seedlings were then put into the plant growth incubator (28 ℃, relative air humidity—70%, light intensity—4000Lx, 16 h light/8 h dark) and applied with 50 mL of peanut root exudates (EP) or distilled water (WP) respectively, once every 2 d. The position of different pots was changed every week, and the plant height was measured once a week. After 24 d of treatment, the fresh weight of above-ground and underground was measured, and the roots were isolated and washed with distilled water. Surface water was dried with absorbent paper, and the samples were divided into two parts; one part was used for the determination of root vigor, and the other part were pre-cooled with liquid nitrogen, and stored at −80 °C until physiological parameters were measured. The rhizosphere soil attached to the roots was brushed off, collected, and stored at −80 °C for DNA and sequencing analysis. Each 5 grapevine root or rhizosphere soil was mixed as a biological replicate, and the experiment was repeated three times in total.
2.3. The Effect of Peanut Secretions on the Growth of Fusarium solani
The concentration (0.5 g·mL−1 root dry weight) of peanut root exudates was diluted to 0.25 g·mL−1, 0.1 g·mL−1, 0.05 g·mL−1, 0.025 g·mL−1, and 0.01 g·mL−1 and filtered by millipore filtration (pore size: 0.22 μm) under aseptic conditions, then stored at 4 °C.
A 9 mm round hole was punched in the center of the media (PDA: 20 g·L−1 glucose, 20 g·L−1 agar, and 200 g·L−1 potato), and 200 μL of root exudates of different concentrations (0.5 g·mL−1, 0.25 g·mL−1, 0.1 g·mL−1, 0.05 g·mL−1, 0.025 g·mL−1, 0.01 g·mL−1, and 0.00 g·mL−1) were added into the hole.
Taken out the mycelium of F. solani with a diameter of 8 mm, inoculated it in the hole of the petri dish, and recorded the growth of the fungus after 4 days of culture at 28 °C. The colony diameter was determined by the crossover method, and percent inhibition of growth was calculated by the following equation:
Inhibition rate (%) = (control colony diameter − treated colony diameter)/control colony diameter × 100
The experiments were performed with eight replicates.
2.4. Soil Bacterial and Fungal Community Analyses
Total soil microbial DNA (E, W, EP, and WP treatments) was extracted from soil samples using a FastDNA Spin Kit (MP Biomedicals, Cleveland, OH, USA). DNA extracts were analyzed on 1% agarose electrophoresis gels, and DNA concentration and purity were detected using the NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, NC, USA).
Bacterial 16S rRNA was amplified using universal primers (515F-GTGCCAGCMGCCGCGG and 907R-CCGTCAATTCMTTTRAGTTT). Fungal internal transcribed spacer amplification was performed using primers (ITS1F-CTTGGTCATTTAGAGGAAGTAA and ITS2R-GCTGCGTTCTTCATCGATGC).
The 16S rRNA PCR protocol was set as follows: initial denaturation at 95 °C for 3 min, 27–35 cycles of denaturing at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 45 s, and final extension at 72 °C for 10 min. The PCR was performed in triplicate with a final volume of 20 μL containing 2–4 μL of buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of 5 μM forward primer, 0.8 μL of 5 μM reverse primer, 0.2–0.4 μL of polymerase, 10 ng of template DNA, 0.2 μL of BSA, and ddH2O. The PCR products were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (AxygenBiosciences, Union City, CA, USA).
Purified amplicons were then sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA) according to the standard protocol of MajorbioBio-Pharm Technology Co., Ltd. (Shanghai, China). The raw reads were deposited into the NCBI Sequence Read Archive (SRA) (Accession Number: PRJAN859424).
2.5. Determination of Physiological Indexes
The root vigor was assessed by measuring the triphenyl tetrazolium chloride (TTC) levels in the root [12]. Superoxide dismutase (SOD) activity was determined by the nitro blue tetrazolium (NBT) reduction method, and the content of malondialdehyde (MDA) was determined by the thiobarbituric acid colorimetric method [13].
2.6. Statistical Analysis
The original FastQ files were quality controlled using Fastp (version 0.19.6) and merged with FLASH (version 1.2.11). Operational taxonomic units (OTUs) with a 97% similarity cutoff were clustered using the UPARSE11 version. To minimize the impact of sequencing depth, we used a subsampling procedure to normalize the number of reads to the lowest number of reads observed across all samples. Bioinformatics analysis was performed using the MajorbioCloud platform (https://cloud.majorbio.com, access on 22 March 2022). According to the OTU information, Mothur (version 1.30.2) was used to calculate rarefaction curves and the alpha diversity index. Microbial community similarity was determined by principal coordinate analysis (PCA) using the R package (version 3.3.1). Co-occurrence network analysis was performed on each sample using Cytoscape 3.7.1 to analyze the relationships among OTUs, and OTUs with an average relative abundance of less than 0.001 in each group were removed from the network analysis. If Spearman’s correlation coefficient was >0.8 or <−0.8 and the p-value < 0.05, the correlation between the two nodes was considered statistically stable.
Multiple comparisons used one-way analysis of variance (ANOVA) and Tukey’s HSD multiple range test. The Student’s t-test was used to show the difference in growth and physiological indexes between EP and WP, and it was also used to show differences in alpha diversity indices and relative abundances of bacteria and fungi at the genus level for E and W or EP and WP. One-way ANOVA and Student’s t-test were performed using SPSS 20.0 statistical software (SPSS, version 26, IBM, Armonk, NY, USA).
3. Results
3.1. Effects of Peanut Root Exudates on the Growth and Physiological Index of Grapevine Seedlings
It can be seen from Figure 1 that after the treatment with peanut root exudates, the plant height of grape seedlings (14 d and 21 d) was significantly higher than that of the control, and the fresh weight of seedlings also increased. Root vigor and SOD enzyme activity were significantly enhanced, increasing 55.18% and 95.71%, respectively, and reduced MDA content of root, decreasing 31.10%. All of these results suggest that root exudates promote the growth of replanting grapevine seedlings.
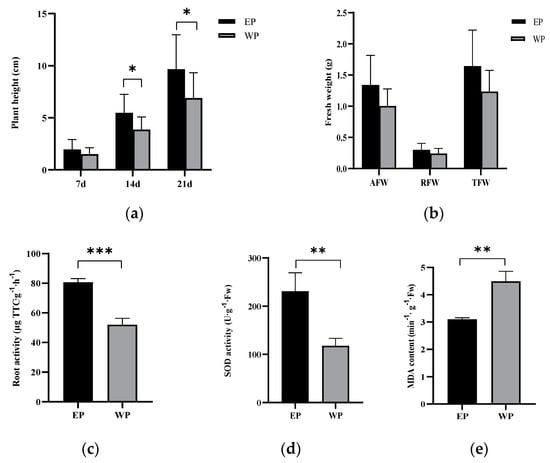
Figure 1.
Effects of peanut root exudates on the growth and physiological indexes of grapevine seedlings. EP: rhizosphere soil of grapevine seedling irrigated with peanut root exudates for 28d+24d, WP: rhizosphere soil of grapevine seedling irrigated with distilled water for 28 d+24 d. *, ** or *** indicated significant difference at p < 0.05, p < 0.01, or p < 0.001 between EP and WP (Student’s t-test). (a) Plant height. (b) Fresh weight. AFW represents above-ground fresh weight, RFW represents root fresh weight, and TFW represents total fresh weight. (c) Root activity, (d) SOD activity of root, and (e) MDA content of root.
3.2. Effects of Peanut Root Exudates on the Growth of Harmful Fungus (Fusarium solanum)
Peanut root exudates with different concentrations can considerably inhibit the growth of Fusarium solanum. In the range of 0.01–0.25 dry root ·mL−1, the inhibition rate enhanced with the increase in peanut root exudates concentration. The maximum inhibition rate reached 11.06% at the concentration of peanut root exudate of 0.25 g dry root ·mL−1. The inhibition rate decreased when the concentration was 0.5 g dry root ·mL−1 (Figure 2).
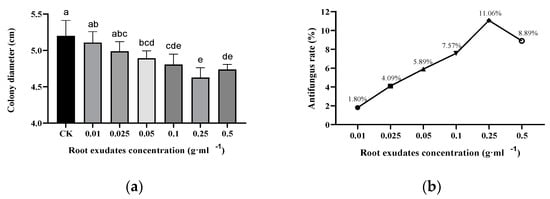
Figure 2.
Effect of peanut root exudates on Fusarium solani growth, (a) colony diameter of Fusarium solani after peanut root exudates treating, (b) inhibition rate of peanut root exudates in petri dish experiments. The different letters represent significant differences at p < 0.05 (Tukey’s test).
The effect of peanut root exudates on Fusarium solani colony growth is shown in Figure S1.
3.3. Composition and Diversity of Bacteria and Fungi in Grapevine Replanted Soil following Application of Peanut Root Exudates
The rarefaction curves for all samples were close to saturation, showing that the depth was sufficient to capture the overall bacterial and fungal diversity in the four samples (Figure 3).
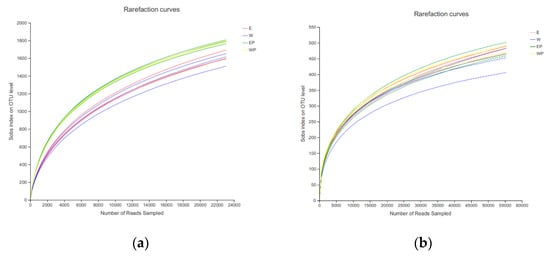
Figure 3.
The rarefaction curve. (a) Bacteria, (b) fungi, E: grapevine replant soil irrigated with peanut root exudates, W: grapevine replant soil irrigated with distilled water. EP: rhizosphere soil of grapevine seedling irrigated with peanut root exudates for 28 d + 24 d; WP: rhizosphere soil of grapevine seedling irrigated with distilled water for 28d+24d.
The diagram (Figure 4a) showed that the number of bacterial OTUs in E, W, EP, WP were 2301, 2260, 2330, and 2382, respectively, and the number of their combined OTUs was 1538. The number of fungal OTUs in the E, W, EP, and WP was 706, 653, 698, and 736, respectively, and the number of their combined OTUs was 371 (Figure 4b).
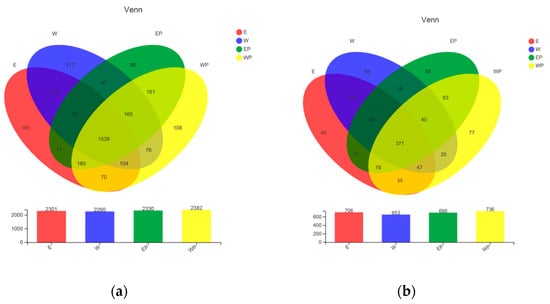
Figure 4.
Venn diagram of OTUs in four samples. (a) Bacteria; (b) fungi.
The bacterial alpha diversity is shown in Table 2. Compared with the EP and WP groups, the rhizosphere Shannon’s index of the E and W groups decreased significantly (p < 0.05), and the Simpson’s index in EP and WP groups was significantly lower than that E and W groups. Chao1 and Ace indexes have no significant differences among the four groups.

Table 2.
Alpha diversity indices of bacterial communities in grapevine replanting soil applied with peanut root exudates.
Fungal alpha diversity is shown in Table 3. Compared with the E, W, and WP groups, the rhizosphere Shannon’s index in the EP group decreased significantly (p < 0.05), and the Simpson index in the EP group was significantly higher than that in the other groups. Chao1 and Ace indexes have no significant differences among the four treatments.

Table 3.
Alpha diversity indices of fungal communities of grapevine replanting soil after the application of peanut root exudation.
PCA analysis indicated that the four samples were clearly separated from each other. This result demonstrated that exogenous peanut root exudates could modify the soil bacterial and fungal community in a certain range (Supplementary Figure S2).
Proteobacteria (29.87–56.87%), Actinobacteriota (18.96–20.76%), Acidobacteriota (6.62–12.07%), and Firmicutes (3.47–13.19%) were the four most dominant phyla, accounting for approximately 75–86% of the relative abundance of the bacterial communities (Figure 5a). Proteobacteria dominated the soil, and its relative abundance in E and W soils was higher than that in EP and WP soils. In contrast, the relative abundances of Acidobacteriota, Firmicutes, Chloroflexi, Planctomycetota, and Gemmatimonadota were observed to decrease in E and W soils.
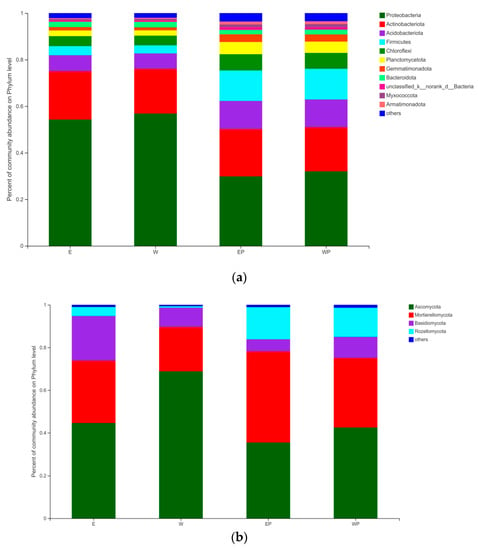
Figure 5.
Relative abundances of different bacterial and fungal taxa at the phylum level in grapevine replanting soil by applying peanut root exudates or distilled water. (a) Bacteria; (b) fungi. E: grapevine replanting soil irrigated with peanut root exudates, W: grapevine replanting soil irrigated with distilled water, EP: rhizosphere soil of grapevine seedling irrigated with peanut root exudates for 28 d+24 d, WP: rhizosphere soil of grapevine seedling irrigated with distilled water for 28 d+24 d. Those with a relative abundance of less than 1% were grouped as “Others.”.
Only four phyla of the fungus, which were more than 98% of the relative abundance of fungal communities, were identified: Ascomycota (35.54–68.82%), Mortierellomycota (20.80–42.66%), Basidiomycota (5.65–20.93%), and Rozellomycota (0.68–15.06%). The relative abundances of Ascomycota and Basidiomycota in E and W soils were higher than those in EP and WP soils. In contrast, a decrease was observed in the relative abundances of Mortierellomycota and Rozellomycota in E and W soils (Figure 5b).
The relative abundance of the top 15 bacterial taxa at the genus level can be seen in Figure 6a. The relative abundances of norank_f__norank_o__Gaiellales, Tumebacillus, norank_f__SC-I-84, Bacillus, norank_f__JG30-KF-AS9, norank_f__norank_o__Acidobacteriales, Bryobacter, and Gaiella were significantly higher in EP and WP treatments than in E and W treatments. The relative abundances of Massilia and unclassified_f__Comamonadaceae were lower in E than W (p < 0.01). The relative abundances of Massilia and norank_f__JG30-KF-AS9 were lower in EP than WP (p < 0.01 or p < 0.05), whereas Gaiella increased in the soil of EP treatment compared with WP treatment (p < 0.05).
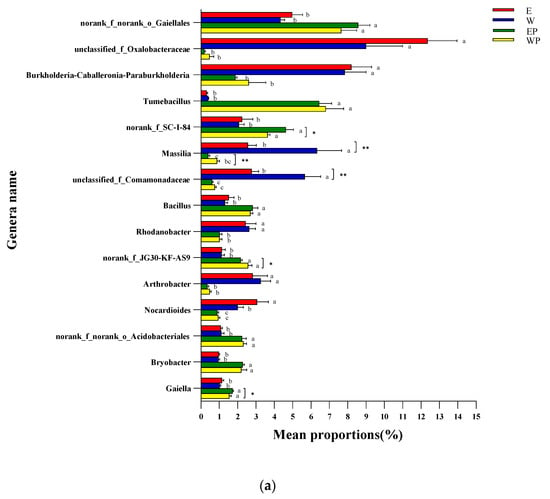
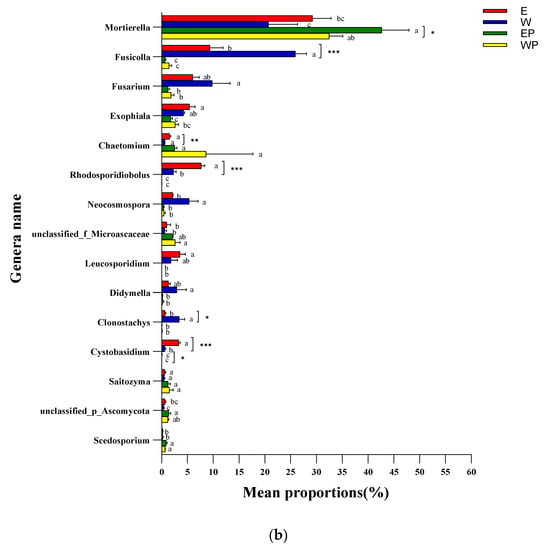
Figure 6.
Genus-level relative abundance and major changes of the top 15 bacterial and fungal taxa in soil samples applied to peanut root exudates or distilled water. (a) Bacteria; (b) fungi. E: grapevine replanting soil irrigated with peanut root exudates, W: grapevine replanting soil irrigated with distilled water, EP: rhizosphere soil of grapevine seedling irrigated with peanut root exudates for 28d+24d, WP: rhizosphere soil of grapevine seedling irrigated with distilled water for 28d+24d. *, ** or *** indicated significant difference at p < 0.05, p < 0.01, or p < 0.001 between E and W or between EP and WP (Student’s t-test). The different letters represent significant differences at p < 0.05 (Tukey’s test).
Figure 6b shows the relative abundance of the top 15 fungal taxa at the genus level. The relative abundance of most genera in EP and WP was lower than that in E and W; however, the relative abundances of Mortierella, unclassified_f__Microascaceae, unclassified_p__Ascomycota, and Scedosporium showed an increasing trend after planting grapevine seedlings. The relative abundances of Cystobasidium, Rhodosporidiobolus, and Chaetomium were higher in E than W (p < 0.01 or p < 0.001), whereas those of Clonostachy and Fusicolla decreased in soils from E treatment compared with those in the soil of W treatment (p < 0.05 or p < 0.001). The relative abundances of Mortierella and Cystobasidium were higher in EP than in WP (p < 0.05).
3.4. Bacteria and Fungi Co-Occurrence Network
Co-occurrence networks were constructed to reveal the connectivity among bacterial OTUs (Figure 7a) and fungal OTUs (Figure 7b) in soil samples by applying peanut root exudates or distilled water. The OTUs with relative abundance > 0.1% from the soils were used to construct ecological network of microbial communities (p < 0.05, R > 0.8 or <−0.8).
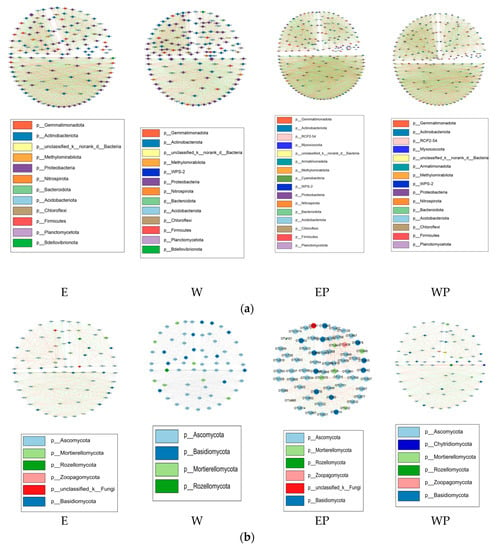
Figure 7.
The co-occurrence network of soil bacteria (a) and fungi (b) in soil samples by applying peanut root exudates or distilled water. E: grapevine replanting soil irrigated with peanut root exudates, W: grapevine replanting soil irrigated with distilled water, EP: rhizosphere soil of grapevine seedling irrigated with peanut root exudates for 28d+24d, WP: rhizosphere soil of grapevine seedling irrigated with distilled water for 28d+24d.
Among treatments, the topological properties of the networks showed clear differences (Table 4). The density of the bacterial co-occurrence network was the highest in E treatment, followed by WP, EP, and W treatments. The ratio of positive to negative correlations was higher in EP treatment than in WP treatment and lower in E treatment than in W treatment. The clustering coefficient and average number of neighbors in E and EP treatments were higher than in W and WP treatments, respectively.

Table 4.
Topological properties of co-occurring bacterial and fungal networks in soil samples from applying peanut root exudates or distilled water.
For fungal co-occurrence networks, the highest number of nodes were detected in E treatment, followed by WP, EP, and W treatment. The edges and average number of neighbors were higher in E than in W treatment. The network density and average number of neighbors were lower in EP than in WP treatment. The positive and negative correlation ratio of EP treatment was higher than that of WP treatment.
4. Discussion
4.1. Peanut Root Exudates Can Promote the Growth of Grapevine Seedlings in Replanting Soil
Grape replanting diseases are common in vineyards and nurseries and can seriously affect the healthy growth of young trees [14]. Grapevine seedlings grow weakly in replanting soil, and soil sterilization can significantly reduce or eliminate this inhibitory effect, which was manifested as increased plant height, stem diameter, above-ground fresh mass and underground fresh mass, enhanced SOD enzyme activity in leaves, and decreased MDA content. This indicated that the explosion of soil pathogens under replant was one of the important reasons for grape replant disease [15]. In the experiment, after peanut exudates treating, the growth of grapevine seedlings enhanced, the root vigor and SOD enzyme activity increased significantly, and the MDA content decreased. The results showed that peanut root exudates could promote the growth of replanting grapevine seedlings, which might affect seedlings’ growth by changing the structure and function of the rhizosphere microbial community.
4.2. Root Exudates from Peanuts Can Affect the Compositions and Functions of Bacteria and Fungi in Soil
In this study, peanut root exudate markedly affected the structure and function of the bacterial and fungal communities.
In bacteria, the relative abundances of norank_f__norank_o__Gaiellales, Bacillus, and Gaiella in grapevine rhizosphere soil treated with EP and WP were markedly higher than those with E and W treatments, and the relative abundances of Gaiella, norank_f__SC-I-84 in the EP treatment was markedly higher than in the WP treatment. Zhao et al. [16] reported a strong positive relationship between the relative abundance of Gaiella and Fusarium oxysporum f. sp. lycopersici inhibition. Norank_o_Gaiellales played a crucial role in suppressing the occurrence of root rot [17]. Bacillus is a kind of PGPR that has been widely studied. Certain strains of spore-forming Bacillus spp. can elicit ISR (induced systemic resistance) and reduce the severity of disease by a wide range of pathogens [18]. The increase in the relative abundance of the above genera after treating peanut exudates might be helpful in changing the rhizosphere microbial environment and alleviating the grape replanting disease. The specific functions of norank_f__SC-I-84 are barely reported and ought to be explored [19].
The relative abundances of Massilia markedly decreased in soils after peanut root secretion treatment. The relative abundances of unclassified_f__Comamonadaceae were markedly lower in the E treatment than in the W treatment. The members of Massilia have functions of dissolving phosphorus [20] and degrading phenanthrene [21]. The family Comamonadaceae belongs to the Beta subdivision of the phylum Proteobacteria, and its members exhibit biological characteristics mainly related to physiological and/or ecological properties [22]. The relative abundances of norank_f__ JG30-KF-AS9 were considerably lower in the EP treatment than in the WP treatment. Yu et al. [23] reported that the enrichment of JG30-KF-AS9 may be related to the decreasing enzyme activities in the garlic cropping obstacle soil. In the experiment, the function of the three genera needs further research.
In fungi, the relative abundances of Clonostachys and Fusicolla considerably decreased in soils after peanut root exudate treatment (E) compared with those in the soil after water treatment (W). EP treatment also showed a decreasing tendency compared with WP treatment. Clonostachys fungi have many classes of secondary metabolites, many of which have biological activities such as phytotoxic and cytotoxic activities et al. [24]. Fusicolla has generally been considered a synonym of Fusarium [25]. The relative abundances of Fusarium and Didymella also showed a decreasing tendency in the soil after peanut root secretion treatment compared with those in the soil of water treatment. Fusarium contains many important agronomic plant pathogens and producers of mycotoxins [26]. F. solanum might be an important harmful fungus related to grape replant disease [27]. The plate confrontation test indicated that peanut root secretions had the ability to inhibit the growth of F. solanum, which showed similar results to the sequencing analysis. The results indicated that peanut root exudate had the potential to alleviate grape replant disease. Didymella belongs to Didymellaceae, which contains many plant pathogenic, saprobic, and endophytic species related to a variety of hosts [28].
The relative abundances of Rhodosporidiobolus were significantly higher in E than in W, and Mortierella and Cystobasidium showed an increasing trend in soils after peanut root exudate treatment compared with those in the soil after water treatment. Margesin et al. [29] reported that members of the genus Rhodosporidiobolus have the ability to utilize ligin-derived compounds and demonstrated that R. colostri DBVPG 10655 could completely degrade a series of aromatic monomers derived from lignin, including 4-hydroxybenzoic acid. Furthermore, 4-hydroxybenzoic acid is an important autotoxin of grape [14], and the relative abundances of Rhodosporidiobolus increasing in the soil after peanut root exudate treatment might have the potential to degrade autotoxin. Mortierella has been shown in various environments, and some strains are plant-growth-promoting fungi (PGPF) [30]. Lipids produced by some Mortierella species are known to increase plant resistance to phytopathogens [31]. The disease-free (Fusarium wilt of banana) soils were dominated by Mortierella species, which accounted for more than 36% of the total soil abundance [32]. Xiong et al. [33] reported that suppressive soil of vanilla was mainly Mortierella, suggesting that this taxon can be used as an indicator and enhancer of Fusarium wilt disease suppression. F. solanum might be an important harmful fungus related to grape replant disease. The relative abundance of Mortierella had an increasing trend, which might have the potential ability to inhibit the growth of F. solanum. Sobrino-Plata et al. [34] reported that Cystobasidium sp. pre-inoculation reduced the abundance of Ophiostoma. novo-ulmi (a hemi-biotrophic pathogen) in the whole plant.
In fungi, among the top 15 fungal genera, the relative abundances of Clonostachys, Fusicolla, and Fusarium showed a decreasing trend after peanut root secretion treatment, while Mortierella and Cystobasidium showed an upward trend, which might be beneficial to the growth of replanting grapevine seedlings.
4.3. The Peanut Root Exudate Changed the Topological Properties of Bacterial and Fungal Co-Occurrence Networks
Network analysis provides a means to examine the ecological understanding of microbial communities by revealing non-random co-variation patterns in community organizations [35,36]. Liu et al. [37] reported that the average number of neighbors in the hilltop network was higher, suggesting closer interactions among bacterial communities at this elevation. Wu et al. [7] considered that intercropping increased the clustering coefficients of the bacterial and fungal networks, which indicated that the soil microbiome was more closely related under the intercropping system. In our experiment, the clustering coefficient and average number of neighbors of rhizosphere soil bacterial co-occurrence network increased in the EP treatment compared with the WP treatment. This indicated that the bacterial networks in the grapevine rhizospheres soils after peanut root exudate treatment showed much more interactions among bacteria communities. Compared with the WP network, the fungal rhizosphere network of EP contains fewer connections (edges) between nodes, reducing the connection density and the average number of neighbors, thus creating a less complex rhizosphere network pattern. In addition, the rhizosphere microbe network of EP had a higher ratio of positive to negative correlations compared with WP. The positive features of the co-occurrence patterns in rhizosphere microbiomes after peanut exudate suggests the potential for extensive mutualistic interactions among bacteria or fungi in rhizosphere assemblages.
5. Conclusions
Peanut root exudates could promote the growth of replanting grapevine seedlings, modify the soil bacterial and fungal communities in a certain range, and increase the interaction among the bacteria of grapevine rhizosphere soil. However, it eased the interaction among fungi. There are extensive mutualistic interactions among bacteria or fungi in grape rhizosphere assemblages after peanut exudate treatment. Peanut root exudates can markedly inhibit the growth of Fusarium solanum, an important harmful fungus related to grape replant disease. The relative abundances of Gaiella in bacteria, Cystobasidium, and Mortierella in fungi increased, and that of potential pathogen fungi Fusicolla decreased, which might be helpful in changing the soil microbial environment and alleviating the grape replanting disease. In the future, it should be strengthened to explore the function components of peanut root exudates which play an important role in changing the microbial community structure and function of grape-replanting soil.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8100892/s1, Figure S1: Antifungal activity of peanut root exudates with different concentrations (n = 8); Figure S2: PCA of microbial communities in grapevine replant soil obtained by applying peanut root exudates or distilled water.
Author Contributions
Formal analysis, J.Z. and Q.L.; investigation, J.Z. and L.M.; writing—original draft preparation, J.Z., K.L. and L.M.; writing—review and editing, K.L.; supervision, K.L.; funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation of Liaoning Province Education Administration, grant number LJKZ0636.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rao, D.; Liu, P.Y.; Zou, L.Y.; Teng, Y.; Yu, H.Y. Microbial dysbiosis together with nutrient imbalance cause the replant problem of upper six flue-cured tobacco in Central Henan. J. Plant Dis. Prot. 2021, 128, 1487–1500. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.Z.; Wang, F.Q.; Li, M.J.; Zhang, J.Y.; Gu, L.; Zhang, L.J.; Tu, W.Q.; Zhang, Z.Y. Assaying the potential autotoxins and microbial community associated with Rehmannia glutinosa replant problems based on its ‘autotoxic circle’. Plant Soil 2016, 407, 307–322. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zhang, Z.D.; Han, X.Y.; Wu, J.H.; Zhang, L.Z.; Wang, J.R.; Gefu, W.P. Specific response mechanism to autotoxicity in melon (Cucumis melo L.) root revealed by physiological analyses combined with transcriptome profiling. Ecotoxicol. Environ. Saf. 2020, 200, 110779. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Ding, C.F.; Hua, K.; Zhang, T.L.; Zhang, Y.N.; Zhao, L.; Yang, Y.R.; Liu, J.G.; Wang, X.X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Lin, S.; Chu, L.X.; Gao, J.T.; Azeem, S.; Lin, W.X. Insight into structure dynamics of soil microbiota mediated by the richness of replanted Pseudostellaria heterophylla. Sci. Rep. 2016, 6, 26175. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Chen, S.C.; Zhang, X.X.; Zhou, X.G.; Wu, F.Z. Rhizosphere bacterial community in watermelon-wheat intercropping was more stable than in watermelon monoculture system under Fusarium oxysporum f. sp. niveum invasion. Plant Soil Int. J. Plant Soil 2019, 445, 369–381. [Google Scholar] [CrossRef]
- Wu, H.M.; Lin, M.H.; Christopher, R.; Qin, X.J.; Zhang, S.K.; Chen, J.; Wu, L.K.; Zhao, Y.L.; Lin, S.; Lin, W.X. Plant-mediated rhizospheric interactions in intraspecific intercropping alleviate the replanting disease of Radix pseudostellariae. Plant Soil 2020, 454, 411–430. [Google Scholar] [CrossRef]
- Li, X.G.; Wietse, D.B.; Zhang, Y.N.; Ding, C.F.; Zhang, T.L.; Wang, X.X. Suppression of soil-borne Fusarium pathogens of peanut by intercropping with the medicinal herb Atractylodes lancea. Soil Biol. Biochem. 2018, 116, 120–130. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, F.Z.; Liu, S.W. Allelopathic effects of root exudates of Chinese onion accessions on cucumber yield and Fusarium oxysporum f. sp. cucumerinum. Allelopath. J. 2011, 27, 75–85. [Google Scholar]
- Yuan, X.F.; Wang, B.B.; Hong, S.; Xiong, W.; Shen, Z.Z.; Ruan, Y.Z.; Li, R.; Shen, Q.R.; Dini, A.F. Promoting soil microbial-mediated suppressiveness against Fusarium wilt disease by the enrichment of specifc fungal taxa via crop rotation. Biol. Fertil. Soils 2021, 57, 1137–1153. [Google Scholar] [CrossRef]
- Zhou, X.G.; Liu, J.; Wu, F.Z. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 2017, 415, 507–520. [Google Scholar] [CrossRef]
- Ma, S.C.; Wang, T.C.; Guan, X.K.; Zhang, X. Effect of sowing time and seeding rate on yield components and water use efficiency of winter wheat by regulating the growth redundancy and physiological traits of root and shoot. Field Crop. Res. 2018, 221, 166–174. [Google Scholar] [CrossRef]
- Li, H.S.; Sun, Q.; Zhao, S.J.; Zhang, W.H. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Liu, Q.W.; Li, K.; Guo, X.W.; Ma, L.; Guo, Y.S.; Liu, Z.D. Developmental characteristics of grapevine seedlings root border cells and their response to ρ-hydroxybenzoic acid. Plant Soil 2019, 443, 199–218. [Google Scholar] [CrossRef]
- Guo, X.W.; Li, K.; Xie, H.G.; Sun, Y.N.; Hu, X.X.; Zhang, L.H. Effect of sterilized replant soil on grape growth and root exudation characteristics. J. Fruit Sci. 2010, 27, 29–33. (In Chinese) [Google Scholar]
- Zhao, F.Y.; Zhang, Y.Y.; Dong, W.G.; Zhang, Y.Q.; Zhang, G.X.; Sun, Z.P.; Yang, L.J. Vermicompost can suppress Fusarium oxysporum f. sp. lycopersici via generation of beneficial bacteria in a long-term tomato monoculture soil. Plant Soil 2019, 440, 491–505. [Google Scholar] [CrossRef]
- Ding, Y.R.; Chen, Y.L.; Lin, Z.Q.; Tuo, Y.Y.; Li, H.L.; Wang, Y. Differences in soil microbial community composition between suppressive and root rot-conducive in tobacco fields. Curr. Microbiol. 2021, 78, 624–633. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S.A. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Muhammad, R.; Zhang, L.; El-Desouki, Z.; Jiang, C.C. Biochar induces changes to basic soil properties and bacterial communities of different soils to varying degrees at 25 mm rainfall: More effective on acidic soils. Front. Microbiol. 2019, 10, 1321. [Google Scholar] [CrossRef]
- Zheng, B.X.; Bi, Q.F.; Hao, X.L.; Zhou, G.W.; Yang, X.R. Massilia phosphatilytica sp. nov., a phosphate solubilizing bacteria isolated from a long-term fertilized soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 2514–2519. [Google Scholar] [CrossRef]
- Lou, J.; Gu, H.P.; Wang, H.Z.; An, Q.L.; Xu, J.M. Complete genome sequence of Massilia sp. WG5, an efficient phenanthrene-degrading bacterium from soil. J. Biotechnol. 2016, 218, 49–50. [Google Scholar] [CrossRef]
- Jara, E.; Morel, M.A.; Lamolle, G.; Castro-Sowinski, S.; Simón, D.; Iriarte, A.; Musto, H. The complex pattern of codon usage evolution in the family Comamonadaceae. Ecol. Genet. Genom. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Yu, J.Y.; Liu, Y.H.; Wang, Z.Y.; Huang, X.H.; Chai, D.; Gu, Y.F.; Zhao, K.; Yu, X.M.; Shuai, Z.B.; Liu, H.J.; et al. The cropping obstacle of garlic was associated with changes in soil physicochemical properties, Enzymatic activities and bacterial and fungal communities. Front. Microbiol. 2022, 13, 828196. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Zhang, X.P.; Xu, D.; Zhang, B.W.; Lai, D.W.; Zhou, L.G. Metabolites from Clonostachys Fungi and their biological activities. J. Fungi 2020, 6, 229. [Google Scholar] [CrossRef] [PubMed]
- Gräfenhan, T.; Schroers, H.J.; Nirenberg, H.I.; Seifert, K.A. An overview of the taxonomy, phylogeny, and typifcation of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011, 68, 79–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; David, M.G.; Robert, H.P.; Alejandro, P.R.; Kerry, O.D.; Frances, T.; Donald, M.G.; John, M.M.; Kemal, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.W.; Wang, S.X.; Li, K.; Qiao, J.; Guo, Y.S.; Liu, Z.D.; Guo, X.W. Responses of soil bacterial and fungal communities to the long-term monoculture of grapevine. Appl. Microbiol. Biotechnol. 2021, 105, 7035–7050. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Cai, L.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef]
- Margesin, R.; Ludwikowski, T.M.; Kutzner, A.; Wagner, A.O. Low-temperature biodegradation of lignin-derived aromatic model monomers by the cold-adapted yeast Rhodosporidiobolus colostri isolated from alpine forest soil. Microorganisms 2022, 10, 515. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Eroshin, V.; Dedyukhina, E. Effect of lipids from Mortierella hygrophila on plant resistance to phytopathogens. World J. Microbiol. Biotechnol. 2002, 18, 165–167. [Google Scholar] [CrossRef]
- Zhou, D.B.; Tao, J.; Chen, Y.F.; Wang, F.; Qi, D.F.; Feng, R.J.; Xie, J.H.; Li, H.P. Deciphering microbial diversity associated with Fusarium wilt-diseased and disease-free banana rhizosphere soil. BMC Microbiol. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, R.; Ren, Y.; Liu, C.; Zhao, Q.Y.; Wu, H.S.; Jousset, A.; Shen, Q.R. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Sobrino-Plata, J.; Martinez-Arias, C.; Ormeno-Moncalvillo, S.; Fernandez, I.; Collada, C.; Gil, L.; Pieterse, C.M.J.; Martin, J. No priming, just fighting–endophytic yeast attenuates the defense response and the stress induced by Dutch elm disease in Ulmus minor Mill. Tree Physiol. 2022, tpac062. [Google Scholar] [CrossRef]
- Shi, S.J.; Nuccio, E.E.; Shi, Z.J.; He, Z.L.; Zhou, J.Z.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Tariq, A.; Zeng, F.J.; Graciano, C.; Sun, F.; Chai, X.T.; Ahmed, Z. Nitrogen and water addition regulate fungal community and microbial co-occurrence network complexity in the rhizosphere of Alhagi sparsifolia seedlings. Appl. Soil Ecol. 2021, 164, 103940. [Google Scholar] [CrossRef]
- Liu, X.; Yang, T.; Shi, Y.; Zhu, Y.C.; He, M.L.; Zhao, Y.K.; Adams, J.M.; Chu, H.Y. Strong partitioning of soil bacterial community composition and co-occurrence networks along a small-scale elevational gradient on Zijin Mountain. Soil Ecol. Lett. 2021, 3, 290–302. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).