Abstract
Phytoremediation is an effective method used to control the accumulation of certain contaminants found in industrial or city wastewater. Among the species with high efficacy are Eichhornia crassipes (water hyacinth), Lemna minor (common duckweed), and Pistia stratiotes (water lettuce). In this study, the application of these species in the context of two municipal wastewater treatment facilities in Cluj County, Romania, is evaluated. To determine the efficacy of bioaccumulation, we measured the content of nitrogen species (ammoniacal nitrogen, nitrites, and nitrates), phosphorous, iron, and chromium before and after the addition of plant material to effluent and treated wastewater. The results showed that E. crassipes, L. minor, and P. stratiotes presented high phytoremediation yields for these common wastewater pollutants after one week of contact, with yields as high as 99–100% for ammoniacal nitrogen, 95% for phosphorous, 96% for iron, and 94% for chromium. However, the remediation capacity for nitrate and nitrite was less significant.
1. Introduction
Clean water is extremely important for human life so a suitable process for wastewater treatment is needed before its discharge into the natural environment [1]. One of the major concerns is the toxicity of wastewater, as it contains many harmful chemical compounds such as heavy metals, detergents, and sanitizers [2]. Moreover, remanent nutrients in the effluents of the secondary municipal wastewater can cause eutrophication, and nitrogen and phosphorous, in particular, can induce a bloom in microalgal populations [3,4]. Different methods are used to decontaminate wastewater, but one of the best approaches involves using low-cost, sustainable, and green technologies such as bioremediation. In this category, phytoremediation involves the potential of plants and microorganisms to remove pollutant species of an organic or inorganic nature [1,5]. As a method used to control pollution, phytoremediation is based on the capacity of plants to tolerate and accumulate high amounts of chemical elements and pollutants from the environment [6].
Nitrogen can be found in wastewater as ammoniacal nitrogen (NH4+, AN). It is considered a common contaminant and it can lead to excessive eutrophication, water anoxia, and the formation of chloramine. Therefore, the removal of this species is necessary and highly enforced by authorities. At the same time, phytoremediation is one of the most suitable and effective methods for removing AN from wastewater [4,7]. Inorganic nitrogen is also represented by nitrates (NO3−) and nitrites (NO2−), and as the latter presents higher toxicity to aquatic life forms, it can also be remediated [8,9,10].
Another major nutrient that contributes to the increase in the eutrophication of water is phosphorous, which has a similar mechanism to nitrogen, and it can be removed by the incorporation of phosphate, for example, into biological solids [11]. Unfortunately, phosphorus is found in many forms in water, including particulate and dissolved, and dissolved inorganic phosphorous is the only form that can be bio-absorbed by plants during phytoremediation [12].
An important concern regarding wastewater is that it contains different concentrations of metals, which can act as environmental pollutants and increase toxicity [13]. Metal-accumulating plants can be used in the phytoremediation of contaminated water and agricultural land [14,15] and aquatic macrophytes have shown high potential as heavy metal bio-accumulators [16].
Among the species known for phytoremediation, some have a high potential and others are currently undergoing research. One such species is Eichhornia crassipes (Mart.) Solms (water hyacinth, Pontederiaceae), a floating macrophyte that can absorb a wide range of pollutants from wastewater, inducing an acceleration of its proliferation as a result of intense photosynthesis [4,16,17]. Water hyacinth has been studied in the phytoremediation of AN [4] and other nitrogen species [18,19], phosphorous [20], heavy metals [21], cyanide [22], etc. This species has the advantage of having one of the highest growth rates, with the potential for rapid development and accumulation [23] and it also presents a high tolerance for metals [24].
Another extensively studied and used species is Lemna minor L. (common duckweed, Araceae), with a high bioremediation efficiency. This species can reduce the concentration of many aquatic pollutants, including heavy metals, nanomaterials, and organic compounds such as pharmaceuticals, hydrocarbons, toxins, and dyes [25,26,27]. The absorption capacity is based on the passive uptake that takes place in the cell walls of the plant root, followed by storage or reduction. The advantage of the species is that it can work in wetland or stationary settings and it also shows good adaptation potential in comparison to other Lemnaceae species [25,28].
Lastly, Pistia stratiotes L. (water lettuce, Araceae), a perennial plant species with long roots, is also useful for the phytoremediation of nitrogen and phosphorous compounds, but at the same time, it can be applied to the bioaccumulation of potentially toxic metals [29,30,31]. This is due to the metal-binding affinity of P. stratiotes, which can capture and transport certain metals in its organism [32]. The plant grows easily, with reduced requirements for light, and it is suitable for ponds and aquariums [33].
Considering the usefulness of the species mentioned above, the purpose of the present study is to determine the possible phytoremediation application of the three well-known species in the context of municipal wastewater in Cluj County, Romania, by studying the bioaccumulation of AN, NO3−, NO2−, phosphorous, and two common metals, Fe and Cr, from two different treatment facilities.
2. Materials and Methods
2.1. Chemicals and Equipment
The chemicals used in this study were purchased from Merck (Darmstadt, Germany). For the determinations, the following equipment was used: 25 UV/Vis spectrophotometer (PerkinElmer, Waltham, MA, USA); PinAAcle 900T atomic absorption spectrometer (PerkinElmer, Waltham, MA, USA); SpeedWave Four microwave digestor (Berghof Products + Instruments GmbH, Eningen unter Achalm, Germany); Milli-Q IQ 7005 water purification system (Merck, Darmstadt, Germany).
2.2. Location, Plant Material, and Growing Conditions
The present study was conducted in Cluj County and the phytoremediation experiments were conducted at two treatment facilities in Romania, Cluj-Napoca (46°47′35.0″ N, 23°41′3.0″ E) and Sânpaul (46°51′38.7″ N, 23°24′24.4″ E).
The aquatic plants were obtained from the aquatic collection displayed in a greenhouse that belongs to the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca. The plants were identified in this institution.
The plant materials were conditioned as follows:
- For E. crassipes, 20 to 25 units of the plant were used for 50 L of wastewater and treated wastewater, with a determination after 48 h and 7 days of contact.
- For P. stratiotes, 30 units of the plant were used for 20 L of wastewater and treated wastewater, with a determination after 48 h and 7 days of contact.
- For L. minor, 1000 g of the plant was used for 10 L of wastewater and treated wastewater (10 g for 100 mL of water), with a determination after 48 h and 7 days of contact.
The wastewater sampling was carried out according to the standard SR ISO 5667-10/2021 [34]. For the assessment of the parameters, a water sample from each probe was homogenized with a laboratory blender and filtered through blue band filter paper or glass fiber filters to eliminate any solid particles that can interfere with the determination. The conservation of water samples was carried out at 1–5 °C for a maximum of 24 h according to the standard SR ISO 5667-3/2018 [35].
In this study, we assessed several phytoremediation parameters, specifically AN, total nitrogen, phosphorous, and two metals (Fe and Cr). The duration of the study was four months (between the 10th of May 2020 and the 3rd of September 2020) to progressively determine the concentrations of the mentioned pollutant species. The effect on bioremediation was determined for the effluent wastewater (“Entry”) and the treated wastewater (“Evacuation”).
The methodology used to determine the measurements in the current study followed the Specifical Laboratory Procedures (SLP) according to the standards for water quality used in Romanian legislation (given by ASRO—The Romanian Standardization Association).
The determinations were made in three cycles on different dates (May, August, and September 2020). During each cycle, the parameters were analyzed at the initial time, after 48 h, and after another 5 days (7 days in total). The results were expressed as the phytoremediation yield, which is the average percentage of analyte eliminated from the tested wastewater over the three periods considered. The standard deviation for the triplicate data was also included in the results. For the determinations of iron and chromium, only two determinations were carried out, corresponding to only two periods (May and August 2020).
2.3. Determination of Ammoniacal Nitrogen, Nitrites, and Nitrates
The ammoniacal nitrogen (AN) content was determined through a spectrophotometric assay according to SR ISO 7150-1: 2001 [36]. The ammonium ions generated reacted with salicylate and hypochlorite (generated from alkaline hydrolysis of sodium dichloroisocyanurate) in the presence of nitrosopentacyanoferrate(III), forming a blue compound. The absorbance was measured at 663 nm, and the results are expressed as mg NH4+/L of wastewater (with a limit of quantification of 0.036 mg/L). The determinations were carried out in triplicate.
The equation used for the quantification was:
where A and A0 represent the absorbances of the sample and blank, Vf and Vs represent the final and sample volume in the volumetric flask (capacity of 50 mL, expressed as mL), and b represents the calibration curve slope.
mg N/L = [(A − A0) × Vf]/b × Vs
To determine the nitrites, a method based on molecular absorption spectrometry was applied according to SR EN 26777: 2002/C91: 2006 [37]. Specifically, the method is based on the reaction of diazotization of nitrite ions and 4-amino-benzene sulphonamide at pH = 1.9, forming a diazonium salt that can be azo coupled with N-(1-naphthyl)-ethylene-diamine dihydrochloride. The absorbance was measured at 540 nm and the results are expressed as mg nitrites/L of wastewater (with a limit of quantification of 0.002 mg/L). The determinations were carried out in triplicate.
The equation used for the quantification was:
where A and A0 represent the absorbances of the sample and blank, Vf and Vs represent the final and sample volume in the volumetric flask (capacity of 50 mL, expressed as mL), and b represents the calibration curve slope.
mg N/L = [(A − A0) × Vf]/b × Vs
A spectrophotometric assay based on 2,6-dimethylphenol was used to determine the nitrates, according to SR ISO 7890-1: 1998 [38]. With this method, a mix of 2,6-dimethylphenol, sulfuric, and phosphoric acid reacted with nitrate ions through nitration, generating 4-nitro-2,6-dimethylphenol. The absorbance of the obtained colored product was measured at 324 nm and the results are expressed as mg nitrates/L of wastewater (with a limit of quantification of 0.06 mg/L). The determinations were carried out in triplicate.
The equation used for the quantification was:
where A and A0 represent the absorbances of the sample and blank and b represents the calibration curve slope.
mg N/L = (A − A0)/b
2.4. Determination of Phosphorus
The phosphorous content, originating in orthophosphates, was determined through a spectrophotometric assay according to SR EN ISO 6878: 2005 Cap. 7 [39]. The conversion of polyphosphates and organic phosphorous into orthophosphate was carried out through hydrolysis with sulfuric acid and the complete transformation was conducted with potassium persulfate. The orthophosphate ions reacted with antimony molybdate forming a phosphomolybdic complex, which was reduced by ascorbic acid. The absorbance of the obtained blue Mo complex was measured at 880 nm and the results are expressed as mg P/L of wastewater (with a limit of quantification of 0.016 mg/L). The determinations were carried out in triplicate.
The equation used for the quantification was:
where A and A0 represent the absorbances of the sample and blank, Vf and Vs represent the final and sample volume in the volumetric flask (capacity of 50 mL, expressed as mL), and b represents the calibration curve slope.
mg P/L = [(A − A0) × Vf]/b × Vs
2.5. Determination of Metals: Iron and Chromium
The determination of iron (Fe) was achieved through flame atomic absorption spectrometry using samples mineralized with nitric acid in Berghoff equipment according to SR 13315: 1996/C91: 2008 [40]. The samples were pulverized in air-acetylene flame produced inside the spectrometer and then the atomic absorption spectra were recorded. The results are expressed as mg Fe/L (with a limit of quantification of 0.05 mg/L).
The determination of chromium (Cr) was achieved through atomic absorption spectrometry with an electrically heated graphite furnace using samples mineralized with nitric acid in Berghoff equipment according to SR EN ISO 15586: 2004 [41]. The samples were injected into the graphite furnace, where the atomic absorption spectra were recorded. The results are expressed as μg Cr/L (with a limit of quantification of 0.1 μg/L).
The microwave-assisted mineralization process was carried out by mixing 40 mL of the sample with 4 mL of 60% nitric acid directly in the Berghoff mineralization flask according to the EPA 3015A:2007 standard (microwave-assisted acid digestion of aqueous samples and extracts). In the case of effervescence, nitric acid was added gradually. The mineralized samples were left for one hour and then the contents were added to a 100 mL volumetric flask with ultra-pure water. For each sample, a blank containing only water was used.
Both determinations of the metal amounts were carried out in duplicate. The concentration of each metal is expressed using the formula:
where Ct and Cb represent the concentration of metal in the sample and blank (expressed as mg/L) and F represents the dilution factor.
mg metal/L = (Ct − Cb) × F
3. Results
3.1. Phytoremediation of Ammonia, Nitrites, and Nitrates
The determination of AN is presented in Table 1. As can be seen, the highest phytoremediation yields for AN were identified in the wastewater from the Sânpaul Treatment Facility, where the average yields were between 97 and 100% for the three plant species in both the entry and evacuation water after 7 days. The lowest yield was identified for E. crassipes, accumulating only 79% of AN in the wastewater collected from the entry to the facility after 7 days. Considering a remediation period of 48 h, the most effective species was L. minor, with yields of between 91 and 98%. The lowest yield after 48 h was identified for E. crassipes, which eliminated only 28% of AN, although it also had an increase in the yield of up to 79% after another 5 days of contact. In comparison with the other two species, L. minor showed the highest yields (in a shorter time), as can be seen in Figure 1a.

Table 1.
Determination of ammoniacal nitrogen in the wastewater samples before and after phytoremediation. Results are expressed as mg NH4+/L and remediation yields (%).
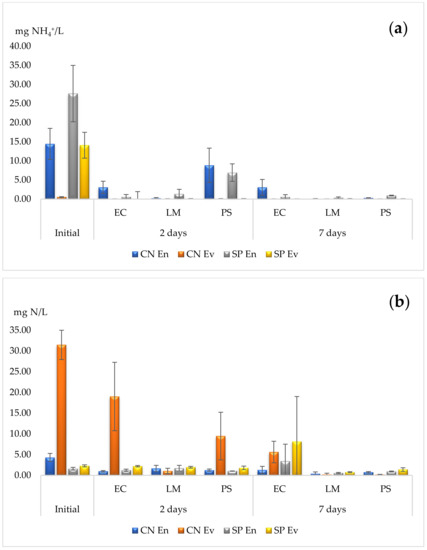
Figure 1.
Phytoremediation capacities for two nitrogen species (a) AN, and (b) nitrate, for the three species. EC—E. crassipes, LM—L. minor, PS—P. stratiotes. Results are expressed as mean and standard deviation (n = 3).
Regarding the content of nitrite and nitrate ions, the results are presented in Table 2 and Table 3, respectively. In comparison to the remediation yields for the ammoniacal nitrogen, the three species showed a lower capacity to eliminate nitrite ions from the wastewater solution. Moreover, in the case of E. crassipes, no phytoremediation potential was observed, and the identified nitrite values exhibited an increase after a longer period of contact. For L. minor, yields of 97 and 90 were determined for the entry water but for the evacuation water, the yields decreased to approximately 50%. A similar trend was observed for P. stratiotes, which showed a higher remediation capacity for nitrite in entry water in comparison to evacuation water.

Table 2.
Determination of nitrite (NO2−) in the wastewater samples, before and after phytoremediation. Results are expressed as mg N/L and remediation yields (%).

Table 3.
Determination of nitrate (NO3−) in the wastewater samples before and after phytoremediation. Results are expressed as mg N/L and remediation yields (%).
The phytoremediation capacity for nitrate showed high variability (Figure 1b). In this case, E. crassipes reduced the nitrate content by 70 to 82% in the Cluj-Napoca Treatment Facility; however, in the Sânpaul Treatment Facility, the same species showed no capacity. Nevertheless, L. minor showed a significant reduction in nitrate in the Cluj-Napoca Treatment Facility, with yields of 91 and 99%, but a smaller capacity in the other facility. P. stratiotes influenced the concentration of nitrate ions in an analogous matter, with yields more than double for the facility in Cluj-Napoca. Generally, the remediation yields seemed to be higher after 7 days in comparison to only 2 days, except for E. crassipes.
3.2. Phytoremediation of Phosphorous
The determination of phosphorous is presented in Table 4. The accumulation yields are lower in comparison to the AN accumulation and the results show that P. stratiotes samples have been the most effective in the accumulation of P, with yields as high as 95%, followed by L. minor, after 7 days of contact (Figure 2). On the other hand, E. crassipes was less effective in the phytoaccumulation process and was only able to absorb between 63 and 77% of P from the wastewater. In a shorter time (48 h), L. minor was the most effective species, showing yields of up to 66%. Overall, the remediation yields showed an improvement over time, reaching maximal values after one week.

Table 4.
Determination of phosphorous in the wastewater samples before and after phytoremediation. Results are expressed as mg P/L and remediation yields (%).
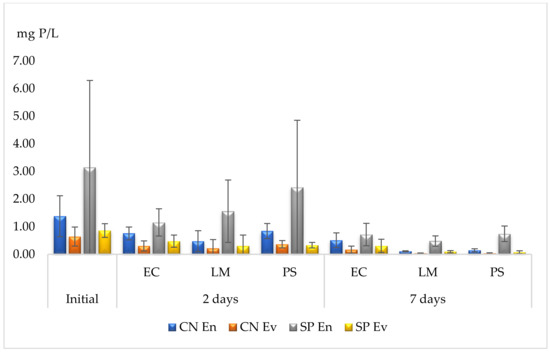
Figure 2.
Phytoremediation capacities for phosphorous. EC—E. crassipes, LM—L. minor, PS—P. stratiotes. Results are expressed as mean and standard deviation (n = 3).
3.3. Phytoremediation of Iron and Chromium
The determinations of iron (Fe) and chromium (Cr) are presented in Table 5 and Table 6, respectively, as well as in Figure 3 For Fe accumulation, the yields are similar for the three species after one week of contact, with slightly higher effectiveness for L. minor (maximal yield of 96%). Considering 48 h only, P. stratiotes was able to eliminate the highest amounts of Fe (with a maximal yield of 77%), whereas L. minor was the least effective in a short time (with a maximal yield of 58%). Nevertheless, all three species showed an average reduction of more than 90% of Fe from the two types of wastewater after 7 days of remediation, which was significantly higher in comparison to only 2 days of remediation.

Table 5.
Determination of Fe in the wastewater samples before and after phytoremediation. Results are expressed as mg Fe/L and remediation yields (%).

Table 6.
Determination of Cr in the wastewater samples before and after phytoremediation. Results are expressed as μg Cr/L and remediation yields (%).
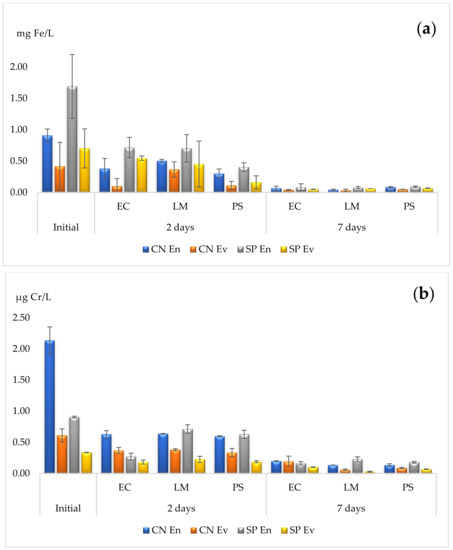
Figure 3.
Phytoremediation capacities for (a) iron—Fe, and (b) chromium—Cr. EC—E. crassipes, LM—L. minor, PS—P. stratiotes. Results are expressed as mean and standard deviation (n = 3).
During one week of contact, L. minor showed the highest accumulation power for Cr, with yields of 90 and 94% for the Cluj-Napoca facility wastewater. P. stratiotes showed intermediate values ranging from 80 to 94%. E. crassipes accumulated 91% of Cr from the entry wastewater; however, the other yields were much lower, ranging from 68 to 82%. For a shorter period of time, E. crassipes showed similar efficacy to P. stratiotes; however, the yields for L. minor were lower. In a similar matter to Fe remediation, Cr elimination capacities improved over time, and, consequently, maximal values were reached after one week of contact for all the considered cases.
Figure 4 shows a global representation of the phytoremediation yields for the most significant results: AN, P, Fe, and Cr. The comparative view shows that L. minor had the highest remediation capacity, immediately followed by P. stratiotes. Among the three species, E. crassipes was the least effective, but even its remediation yields were higher than 70% on average. However, we did not include the nitrite and nitrate remediation yields in the figure, as the results showed high variability and the efficacy was less significant.
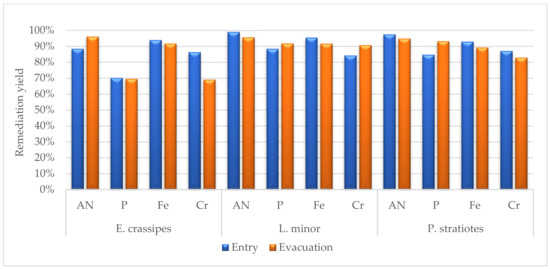
Figure 4.
Average phytoremediation yields for four representative analytes, AN, P, Fe, and Cr, after 7 days of contact. The results were considered for the entry and evacuation wastewater.
4. Discussion
Overall, the present study’s findings highlight the fact that the examined species of aquatic macrophytes present high phytoremediation yields for certain common pollutants, such as AN, phosphorous, and especially Fe and Cr, where the bioaccumulation resulted in concentrations in wastewater samples under the limit of quantification.
A large number of previous studies have assessed the potential of the aquatic macrophyte species in the phytoremediation of wastewater. As a pollutant, AN usually results from the degradation of organic matter rich in nitrogen and it must be kept under strict limits because it prevents nitrification and diminishes the capacity of water purification [42]. For AN, some species present remarkable accumulation efficacy, with the most notable example being E. crassipes, which is considered a nutrient hyperaccumulator. This species has an amazing capacity to accumulate total nitrogen, with values as high as 50 g/kg of the plant [4,18]. L. minor can also remove AN efficiently, and Aziz et al. [42] reported an 80.4% reduction in AN after 8 days of phytoremediation. Consequently, Ayu Hazmi et al. found a reduction in AN of 66.4% by L. minor after 7 days [43]. Finally, Mohd Nizam et al. determined a reduction in AN by a maximal 74% and 78% for E. crassipes and P. stratiotes in catfish aquaculture wastewater after two weeks of remediation [44]. Our results showed a similar trend, with a high capacity of the three species considered to eliminate AN from wastewater after 7 days. E. crassipes was observed to be less effective after only 48 h. Nonetheless, L. minor showed a significant reduction in capacity after both 2 days and 7 days. The applicability of these species was proven at the two treatment facilities and the results were consistent with the data published in the literature.
Another study by Fang et al. showed that E. crassipes showed a high potential to remove total nitrogen from eutrophicated water after a total of 44 days, with a decrease from 2.1 to 0.5 mg/L [19]. Although the authors found that E. crassipes can remediate nitrite, our results showed no such capacity at the 7-day mark. Interestingly, the nitrite remediation yields for the entry wastewater were significantly higher after 48 h, which suggests that this could be the period with the maximal efficiency and that after one week, the nitrite is released back into the medium. On the other hand, this tendency was not noticed for L. minor and P. stratiotes. In the case of these species, the nitrite elimination yields became higher after 7 days of contact (for example, from 36 to 48% and 28 to 51%). These results suggest a difference in the phytoremediation mechanism used by different species.
The nitrate anions present relatively low toxicity; however, the problem is related to their capacity to produce other species, such as nitrite, nitric oxide, and nitroso derivatives [45]. Moreover, its presence can be harmful to aquatic ecosystems [46]. E. crassipes removed 40 and 77% of nitrate after 48 h of contact and P. stratiotes removed 71 and 70% of nitrogen species for the same period. For P. stratiotes, the remediation yield increased after another 5 days of contact. The results were similar to those of a previous study, which found a potential to remove 40–63.5% of nitrate (after 3 weeks) and 61.5–91.8% (after 6 weeks) for E. crassipes and P. stratiotes [45].
As a contaminant in water, phosphorous acts as a stimulus for algal growth and the increase in the cyanobacteria population, which contributes to eutrophication [47]. In this study, the phosphorous remediation capacity was higher after 7 days in comparison to only 2 days for the three species. For E. crassipes, the yields were as high as 77% after one week, which suggests that the maximal capacity was not achieved during this period. Indeed, a previous study reported that a 100% elimination of total phosphorous by water hyacinth could be obtained after 9 weeks of contact, after which time plant harvesting can be applied. Consequently, this species could be used as an efficient remediator for very low-polluting phosphorous-rich wastewater [20]. Moreover, another study found that E. crassipes can eliminate 75 to 97.2% of phosphorous from water in three weeks [45]. Ceschin et al. showed that L. minor, as well as L. minuta, can increase the accumulation of phosphate for up to three weeks; after four weeks, the potential is smaller [48]. Consequently, we found a maximal remediation capacity of between 85 and 95% after 7 days and it is possible that the yield could increase further with a longer contact time. P. stratiotes showed a high remediation capacity after 7 days (79 to 95%), and similarly, a study found the removal of 90–99% after up to six weeks of contact [45]. Nonetheless, Ntakiyiruta et al. showed that the species E. crassipes was more efficient than P. stratiotes for phosphorous removal [49].
Regarding the heavy metals accumulated in wastewater, they have the disadvantage of being non-degradable, which leads to the contamination of water sources, including underground water [50,51]. Moreover, contaminated water consumption is one of the major paths of exposure to heavy metals, and has shown a risk of bioaccumulation in living organisms [52]. Chromium (Cr) is one of the metals that can be found in wastewater and it is considered an important pollutant given its high toxicity and implications for carcinogenicity. Environmental Cr is accumulated from natural sources; however, a significant quantity of Cr originates from anthropogenic activities such as electroplating, tanning, petroleum refinery, and the alloy industry [50,53]. The removal of Cr from contaminated water can be carried out using different techniques such as membrane filtration, adsorption, and ion exchange [54]. Still, one of the most efficient methods could be represented by phytoremediation [55].
Water hyacinth is known for its capacity to accumulate heavy metals, potentially removing from 70% to 90% in a short time [50]. It can effectively absorb high levels of Zn, Cr, Cu, and Cd [55]. Mishra et al. found that E. crassipes can accumulate up to 84% of Cr after 11 days of incubation [56]. Comparing E. crassipes to P. stratiotes, water hyacinth has a better bioaccumulation capacity for Cr (80.90%), whereas the other species are more efficient for accumulating Cu than Cr (77.30%) [57]. Furthermore, L. minor has also been studied, showing a high removal capacity of Cr, Ni, and Co from mining water [58] and Cr from wastewater with continuous flow [59]. Our results are consistent with the literature, and after 7 days of contact, L. minor showed the highest remediation yields. For water hyacinth, we identified a Cr remediation capacity of 70–91% and 80–94% for P. stratiotes, which is consistent with the previous data from the literature.
E. crassipes can also remove another common metal pollutant, iron (Fe), and Ndimele et al. showed that plant roots could accumulate approximately 11.22 ± 6.69 mg Fe/kg [23]. P. stratiotes, on the other hand, showed an 83.20% removal of Fe3+ after treatment with 5 mg/L in an aqueous solution, which was the concentration with the highest remediation efficiency [32]. The same species has been proven by Lu et al. to reduce the concentration of Al, Fe, and Mn in water by more than 20%, showing a hyperaccumulator capacity for Fe [60]. Regarding L. minor, Teixeira et al. showed that this species is efficient for Fe bioaccumulation from mine effluent and that the highest capacity of remediation occurs during the first days of the experiment [61]. Our results support these previous findings, showing that all three species can accumulate high amounts of Fe after 7 days of contact with the residual water. In this short time, all species showed a capacity to reduce solubilized Fe by at least 90% on average, again highlighting their hyperaccumulation nature. Remediation is applicable for the wastewater considered in this study, which showed a concentration of Fe as high as 1.69 ± 0.51 mg Fe/L.
The results related to metal phytoremediation suggest that the three species considered showed a hyperaccumulation potential and could accumulate Fe and Cr up to 80–90% after 7 days of contact. Moreover, as shown in Figure 3a,b, the elimination patterns were similar, indicating that the phytoremediation capacity is dependent on the contact time. This can be explained by the high tolerance of certain plant species to metals since their organisms present different binding mechanisms, such as complexation with organic acids and chelation by proteins [62].
The applicability of the phytoremediation of wastewater stems from the fact that it helps with the removal of compounds that are toxic for the aquatic environment but which can serve as a source of important nutrients after proper recovery. For example, nitrogen and phosphorus are present in high amounts in municipal wastewater, and if recovered, they can be used as bioenergy, fertilizers, and in the production of chemicals [1,5]. High bioaccumulation activities can also be achieved using dried plant material such as roots [63]. Moreover, at the end of the vegetative period of plants when the phytoremediation capacity has ended, the exhausted biomass can be used in the recovery of metals, for example, through incineration or in biogas production, using anaerobic fermentation [64]. Effective disposal strategies are necessary to prevent environmental pollution from bioaccumulated species. Other disposal methods that are possible include composting, pyrolysis, and leaching [65]. As tertiary decantation, the three macrophyte species could be used in artificial lakes (in warmer periods and with reduced flow) or in multi-level greenhouses in which the flow of water travels through a labyrinth filled with plants as bio accumulators.
5. Conclusions
The present study’s findings emphasize the fact that E. crassipes, L. minor, and P. stratiotes have high phytoremediation yields for certain common pollutants, such as AN, phosphorous, and especially Fe and Cr, in the context of wastewater collected from two treatment facilities in Cluj County, Romania. The remediation yields were as high as 99–100% for ammoniacal nitrogen, 95% for phosphorous, 96% for iron, and 94% for chromium. However, the remediation effect on nitrite and nitrate was generally less significant. The elimination of different species of pollutants from wastewater showed high variability and was dependent on the plant used, the period of contact, the type of analyte, and the location. The three species showed similar efficacies, although E. crassipes was less effective in phytoremediation. This work emphasizes the utility of the species in bioremediation and the possibility of integrating them after the last phase of depuration, which is the secondary decantation.
Author Contributions
Conceptualization, E.B. and I.L.B.; methodology, E.B., I.L.B., M.O. and E.B.T.; software, I.L.B.; validation, M.O., I.L.B. and E.T; investigation, I.L.B.; resources, M.O. and S.R.; data curation, E.B. and I.L.B.; writing—original draft preparation, A.N. and F.D.B.; writing—review and editing, A.N., A.M. and F.D.B.; visualization, F.D.B.; supervision, C.I.B.; project administration, M.O.; funding acquisition, E.B. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consulting project no. 5934/17.03.2022 of the UASVM Cluj-Napoca, Romania.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to Călin Vasile Neamțu at “Apele Române” Company (Someș S.A Water Company) for the technical support and for providing the space and equipment necessary for every determination assessed for the present study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mustafa, H.M.; Hayder, G. Recent Studies on Applications of Aquatic Weed Plants in Phytoremediation of Wastewater: A Review Article. Ain Shams Eng. J. 2021, 12, 355–365. [Google Scholar] [CrossRef]
- Victor, K.K.; Séka, Y.; Norbert, K.K.; Sanogo, T.A.; Celestin, A.B. Phytoremediation of Wastewater Toxicity Using Water Hyacinth (Eichhornia Crassipes) and Water Lettuce (Pistia Stratiotes). Int. J. Phytoremediat. 2016, 18, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, T.Y.; Dao, G.H.; Xu, X.Q.; Wang, X.X.; Hu, H.Y. Microalgae-Based Advanced Municipal Wastewater Treatment for Reuse in Water Bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Ting, W.H.T.; Tan, I.A.W.; Salleh, S.F.; Wahab, N.A. Application of Water Hyacinth (Eichhornia Crassipes) for Phytoremediation of Ammoniacal Nitrogen: A Review. J. Water Process Eng. 2018, 22, 239–249. [Google Scholar] [CrossRef]
- Hu, H.; Li, X.; Wu, S.; Yang, C. Sustainable Livestock Wastewater Treatment via Phytoremediation: Current Status and Future Perspectives. Bioresour. Technol. 2020, 315, 123809. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, H.; Cai, T.; Chen, K.; Lin, Y.; Xi, Y.; Chhuond, K. Effects of Phytoremediation on Industrial Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2019, 371, 032011. [Google Scholar] [CrossRef]
- Kinidi, L.; Salleh, S. Phytoremediation of Nitrogen as Green Chemistry for Wastewater Treatment System. Int. J. Chem. Eng. 2017, 2017, 1961205. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, J.; Feng, J.; Liu, Q.; Nan, F.; Xie, S. Treatment of Real Aquaculture Wastewater from a Fishery Utilizing Phytoremediation with Microalgae. J. Chem. Technol. Biotechnol. 2019, 94, 900–910. [Google Scholar] [CrossRef]
- Gomes, R.S.; de Lima, J.P.V.; Cavalli, R.O.; Correia, E.D.S. Acute Toxicity of Ammonia and Nitrite to Painted River Prawn, Macrobrachium Carcinus, Larvae. J. World Aquac. Soc. 2016, 47, 239–247. [Google Scholar] [CrossRef]
- Petrov, D.S.; Kuznecov, V.S.; Suprun, I.K.; Zhuravkova, M.A.; Solnyshkova, M.A. Phytoremediation Efficiency of Duckweed Communities for Mining Enterprises Wastewater Treatment from Nitrogen Compounds. J. Phys. Conf. Ser. 2019, 1399, 055044. [Google Scholar] [CrossRef]
- Ojoawo, S.O.; Udayakumar, G.; Naik, P. Phytoremediation of Phosphorus and Nitrogen with Canna x Generalis Reeds in Domestic Wastewater through NMAMIT Constructed Wetland. Aquat. Procedia 2015, 4, 349–356. [Google Scholar] [CrossRef]
- Kumar, S.; Deswal, S. Phytoremediation Capabilities of Salvinia Molesta, Water Hyacinth, Water Lettuce, and Duckweed to Reduce Phosphorus in Rice Mill Wastewater. Int. J. Phytoremediat. 2020, 22, 1731729. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pandey, A.K.; Udayan, A.; Kumar, S. Role of Microbial Community and Metal-Binding Proteins in Phytoremediation of Heavy Metals from Industrial Wastewater. Bioresour. Technol. 2021, 326, 124750. [Google Scholar] [CrossRef]
- Raskin, I.; Smith, R.D.; Salt, D.E. Phytoremediation of Metals: Using Plants to Remove Pollutants from the Environment. Curr. Opin. Biotechnol. 1997, 8, 221–226. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, J.; Lombi, E. Phytoremediation of Metals, Metalloids, and Radionuclides. Adv. Agron. 2002, 75, 1–56. [Google Scholar] [CrossRef]
- Rezania, S.; Ponraj, M.; Talaiekhozani, A.; Mohamad, S.E.; Md Din, M.F.; Taib, S.M.; Sabbagh, F.; Sairan, F.M. Perspectives of Phytoremediation Using Water Hyacinth for Removal of Heavy Metals, Organic and Inorganic Pollutants in Wastewater. J. Environ. Manag. 2015, 163, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Kruatrachue, M.; Pokethitiyook, P.; Homyok, K. Removal of Cadmium and Zinc by Water Hyacinth, Eichhornia Crassipes. Sci. Asia 2004, 30, 93. [Google Scholar] [CrossRef]
- Fox, L.J.; Struik, P.C.; Appleton, B.L.; Rule, J.H. Nitrogen Phytoremediation by Water Hyacinth (Eichhornia Crassipes (Mart.) Solms). Water. Air. Soil Pollut. 2008, 194, 199–207. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Yang, X.E.; Chang, H.Q.; Pu, P.M.; Ding, X.F.; Rengel, Z. Phytoremediation of Nitrogen-Polluted Water Using Water Hyacinth. J. Plant Nutr. 2007, 30, 1753–1765. [Google Scholar] [CrossRef]
- Jayaweera, M.W.; Kasturiarachchi, J.C. Removal of Nitrogen and Phosphorus from Industrial Wastewaters by Phytoremediation Using Water Hyacinth (Eichhornia Crassipes (Mart.) Solms). Water Sci. Technol. 2004, 50, 217–225. [Google Scholar] [CrossRef]
- Odjegba, V.J.; Fasidi, I.O. Phytoremediation of Heavy Metals by Eichhornia Crassipes. Environmentalist 2007, 27, 349–355. [Google Scholar] [CrossRef]
- Ebel, M.; Evangelou, M.W.H.; Schaeffer, A. Cyanide Phytoremediation by Water Hyacinths (Eichhornia Crassipes). Chemosphere 2007, 66, 816–823. [Google Scholar] [CrossRef]
- Ndimele, P.E.; Kumolu-Johnson, C.A.; Chukwuka, K.S.; Adaramoye, O.R. Phytoremediation of Iron (Fe) and Copper (Cu) by Water Hyacinth (Eichhornia Crassipes). Trends Appl. Sci. Res. 2013, 9, 485–493. [Google Scholar]
- Thapa, G.; Das, D.; Gunupuru, L.R.; Tang, B. Endurance Assessment of Eichhornia Crassipes (Mart.) Solms, in Heavy Metal Contaminated Site–A Case Study. Cogent Environ. Sci. 2016, 2, 1215280. [Google Scholar] [CrossRef]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of Common Duckweed (Lemna Minor) in Phytoremediation of Chemicals in the Environment: State and Future Perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.H.; Ahmad, I.; Mahmood-Ul-Hassan, M.; Mohammad, A. Phytoremediation Potential of Lemna Minor L. for Heavy Metals. Int. J. Phytoremediat. 2015, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Banerjee, A.; Sarkar, S. Phytoremediation Potential of Duckweed (Lemna Minor L.) On Steel Wastewater. Int. J. Phytoremediat. 2015, 17, 589–596. [Google Scholar] [CrossRef]
- Ubuza, L.J.A.; Padero, P.C.S.; Nacalaban, C.M.N.; Tolentino, J.T.; Alcoran, D.C.; Tolentino, J.C.; Ido, A.L.; Mabayo, V.I.F.; Arazo, R.O. Assessment of the Potential of Duckweed (Lemna Minor L.) in Treating Lead-Contaminated Water through Phytoremediation in Stationary and Recirculated Set-Ups. Environ. Eng. Res. 2020, 25, 977–982. [Google Scholar] [CrossRef]
- Schwantes, D.; Gonçalves, A.C.; da Paz Schiller, A.; Manfrin, J.; Campagnolo, M.A.; Somavilla, E. Pistia Stratiotes in the Phytoremediation and Post-Treatment of Domestic Sewage. Int. J. Phytoremediat. 2019, 21, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Goswami, S.; Talukdar, A. Das A Study on Cadmium Phytoremediation Potential of Water Lettuce, Pistia Stratiotes L. Bull. Environ. Contam. Toxicol. 2014, 92, 169–174. [Google Scholar] [CrossRef]
- Odjegba, V.J.; Fasidi, I.O. Accumulation of Trace Elements by Pistia Stratiotes: Implications for Phytoremediation. Ecotoxicology 2004, 13, 637–646. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.; Saini, A.; Kumar, P. Phytoremediation of Copper, Iron and Mercury from Aqueous Solution by Water Lettuce (Pistia Stratiotes L.). Environ. Sustain. 2019, 2, 55–65. [Google Scholar] [CrossRef]
- Leblebici, Z.; Dalmiş, E.; Andeden, E.E. Determination of the Potential of Pistia Stratiotes L. in Removing Nickel from the Environment by Utilizing Its Rhizofiltration Capacity. Brazilian Arch. Biol. Technol. 2019, 62, 1–12. [Google Scholar] [CrossRef]
- ASRO SR ISO 5667; Standard—Quality of Water. Sampling—Part 10. ISO: Geneva, Switzerland, October 2021.
- ASRO SR ISO 5667; Standard—Quality of Water. Sampling—Part 3. ISO: Geneva, Switzerland, March 2018.
- ASRO SR ISO 7150-1; Standard—Quality of Water. Determination of Ammonia Content. Part 1. ISO: Geneva, Switzerland, 2001.
- ASRO SR EN 26777: 2002/C91; Standard—Quality of Water. Determination of Nitrite Content. ISO: Geneva, Switzerland, 2006.
- ASRO SR ISO 7890-1; Standard—Quality of Water. Determination of Nitrate Content. Part 1. ISO: Geneva, Switzerland, 1998.
- ASRO SR EN ISO 6878; Cap. 7 Standard—Quality of Water. Determination of Phosphorous. ISO: Geneva, Switzerland, 2005.
- ASRO SR 13315: 1996/C91; Standard—Quality of Water. Determination of Iron Content. ISO: Geneva, Switzerland, 2008.
- ASRO SR EN ISO 15586; Standard—Quality of Water. Determination of Traces of Elements. ISO: Geneva, Switzerland, 2004.
- Aziz, N.I.H.A.; Hanafiah, M.M.; Halim, N.H.; Fidri, P.A.S. Phytoremediation of TSS, NH3-N and COD from Sewage Wastewater by Lemna Minor L., Salvinia Minima, Ipomea Aquatica and Centella Asiatica. Appl. Sci. 2020, 10, 5397. [Google Scholar] [CrossRef]
- Ayu Hazmi, N.I.; Hanafiah, M. Phytoremediation of Livestock Wastewater Using Azolla Filiculoides and Lemna Minor. Environ. Ecosyst. Sci. 2018, 2, 13–16. [Google Scholar] [CrossRef]
- Nizam, N.U.M.; Hanafiah, M.M.; Noor, I.M.; Karim, H.I.A. Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater. Appl. Sci. 2020, 10, 2712. [Google Scholar] [CrossRef]
- Sundaralingam, T.; Gnanavelrajah, N. Phytoremediation Potential of Selected Plants for Nitrate and Phosphorus from Ground Water. Int. J. Phytoremediat. 2013, 16, 275–284. [Google Scholar] [CrossRef]
- Mook, W.T.; Chakrabarti, M.H.; Aroua, M.K.; Khan, G.M.A.; Ali, B.S.; Islam, M.S.; Abu Hassan, M.A. Removal of Total Ammonia Nitrogen (TAN), Nitrate and Total Organic Carbon (TOC) from Aquaculture Wastewater Using Electrochemical Technology: A Review. Desalination 2012, 285, 1–13. [Google Scholar] [CrossRef]
- Rubin, J.A.; Görres, J.H. Potential for Mycorrhizae-Assisted Phytoremediation of Phosphorus for Improved Water Quality. Int. J. Environ. Res. Public Health 2020, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Ceschin, S.; Crescenzi, M.; Iannelli, M.A. Phytoremediation Potential of the Duckweeds Lemna Minuta and Lemna Minor to Remove Nutrients from Treated Waters. Environ. Sci. Pollut. Res. 2020, 27, 15806–15814. [Google Scholar] [CrossRef]
- Ntakiyiruta, P.; Briton, B.G.H.; Nsavyimana, G.; Adouby, K.; Nahimana, D.; Ntakimazi, G.; Reinert, L. Optimization of the Phytoremediation Conditions of Wastewater in Post-Treatment by Eichhornia Crassipes and Pistia Stratiotes: Kinetic Model for Pollutants Removal. Environ. Technol. 2020, 43, 1805–1818. [Google Scholar] [CrossRef]
- Panneerselvam, B.; Priya, K.S. Phytoremediation Potential of Water Hyacinth in Heavy Metal Removal in Chromium and Lead Contaminated Water. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy Metals in Drinking Water: Occurrences, Implications, and Future Needs in Developing Countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Elahi, A.; Arooj, I.; Bukhari, D.A.; Rehman, A. Successive Use of Microorganisms to Remove Chromium from Wastewater. Appl. Microbiol. Biotechnol. 2020, 104, 3729–3743. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water. Air. Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Yapoga, S.; Ossey, Y.B.; Kouamé, V. Phytoremediation of Zinc, Cadmium, Copper and Chrome from Industrial Wastewater by Eichhornia Crassipes. Int. J. Conserv. Sci. 2013, 4, 81–86. [Google Scholar]
- Mishra, V.K.; Tripathi, B.D. Accumulation of Chromium and Zinc from Aqueous Solutions Using Water Hyacinth (Eichhornia Crassipes). J. Hazard. Mater. 2009, 164, 1059–1063. [Google Scholar] [CrossRef]
- Tabinda, A.B.; Irfan, R.; Yasar, A.; Iqbal, A.; Mahmood, A. Phytoremediation Potential of Pistia Stratiotes and Eichhornia Crassipes to Remove Chromium and Copper. Environ. Technol. 2018, 41, 1514–1519. [Google Scholar] [CrossRef]
- Sasmaz, A.; Dogan, I.M.; Sasmaz, M. Removal of Cr, Ni and Co in the Water of Chromium Mining Areas by Using Lemna Gibba L. and Lemna Minor L. Water Environ. J. 2016, 30, 235–242. [Google Scholar] [CrossRef]
- Uysal, Y. Removal of Chromium Ions from Wastewater by Duckweed, Lemna Minor L. by Using a Pilot System with Continuous Flow. J. Hazard. Mater. 2013, 263, 486–492. [Google Scholar] [CrossRef]
- Lu, Q.; He, Z.L.; Graetz, D.A.; Stoffella, P.J.; Yang, X. Uptake and Distribution of Metals by Water Lettuce (Pistia Stratiotes L.). Environ. Sci. Pollut. Res. 2011, 18, 978–986. [Google Scholar] [CrossRef]
- Teixeira, S.; Vieira, M.N.; Marques, J.E.; Pereira, R. Bioremediation of an Iron-Rich Mine Effluent by Lemna Minor. Int. J. Phytoremediat. 2014, 16, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.R.; Schröder, P. Implications of Metal Accumulation Mechanisms to Phytoremediation. Environ. Sci. Pollut. Res. 2009, 16, 162–175. [Google Scholar] [CrossRef]
- Jahangiri, F.M.; Moutushi, H.T.; Moniruzzaman, M.; Hoque, S.; Hossain, M.E. Removal of Lead from Aqueous Solutions and Wastewaters Using Water Hyacinth (Eichhornia Crassipes) Roots. Water Pract. Technol. 2021, 16, 404–419. [Google Scholar] [CrossRef]
- Hejna, M.; Onelli, E.; Moscatelli, A.; Bellotto, M.; Cristiani, C.; Stroppa, N.; Rossi, L. Heavy-Metal Phytoremediation from Livestock Wastewater and Exploitation of Exhausted Biomass. Int. J. Environ. Res. Public Health 2021, 18, 2239. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A Critical Review on the Phytoremediation of Heavy Metals from Environment: Performance and Challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).