Effects of Crystal Lime Sulfur Fumigation and Application of Root-Growth-Promoting Agents on the Control of Apple Replant Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Treatment
2.3. Measured Indicators and Methods
2.3.1. Determination of Malus hupehensis Rehd. Seedling Biomass
2.3.2. Determination of Chlorophyll Content and Photosynthetic Parameters of Malus hupehensis Rehd. Seedlings
2.3.3. Determination of Root Respiration Rate and Root Protective Enzyme Activity
2.3.4. Determination of Soil Enzyme Activity
2.3.5. Determination of Soil Microbial Quantity
2.3.6. DNA Extraction and Real-Time Quantitative Analysis of Fusarium
2.3.7. High-Throughput Sequencing of Soil Fungi and Bacteria
2.4. Statistical Analysis
3. Results
3.1. Effect of Different Treatments on the Growth of Malus hupehensis Rehd. Seedlings
3.2. Effect of Different Treatments on Soil Enzyme Activity
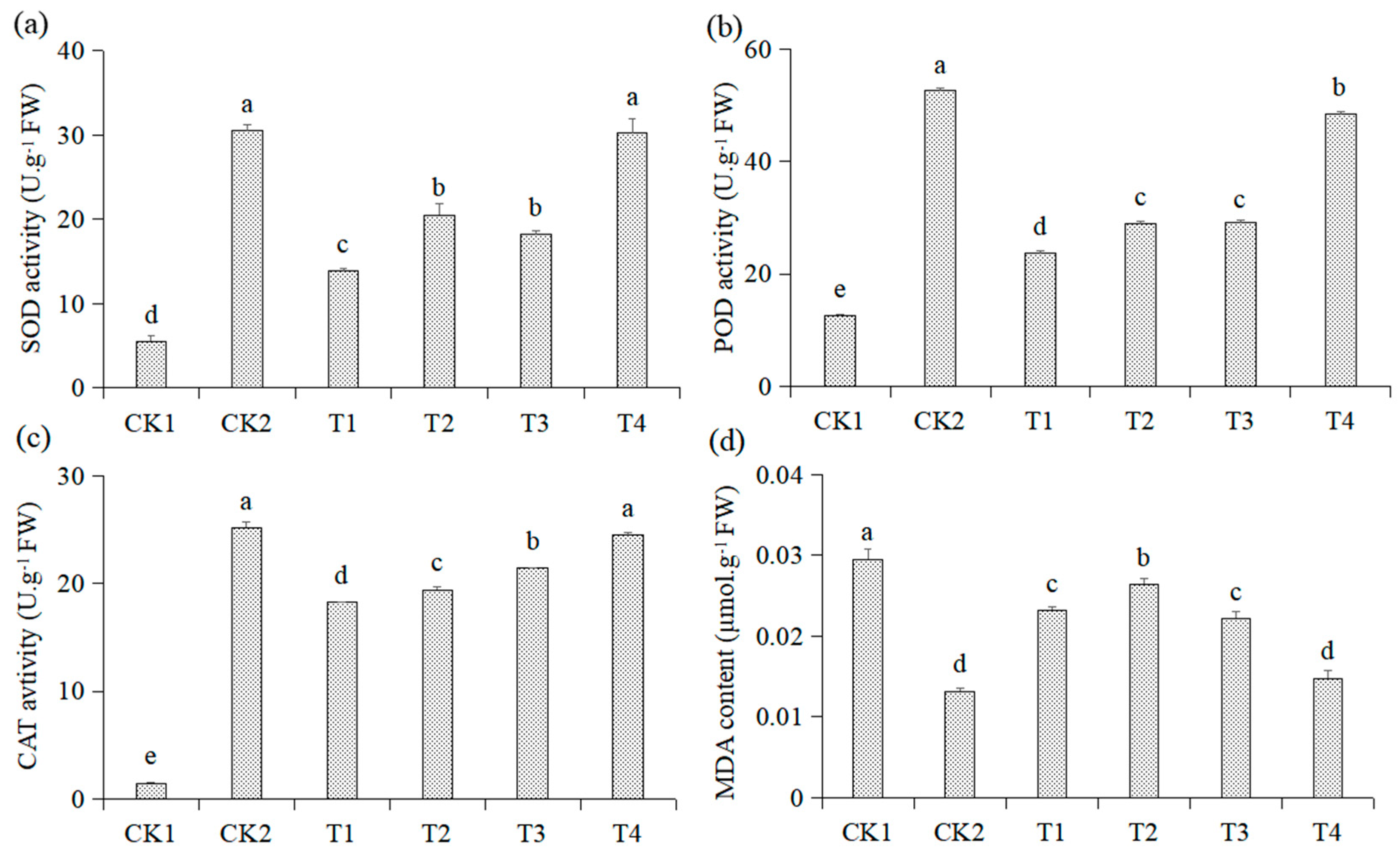
3.3. Effects of Different Treatments on Activities of Antioxidant Enzymes and the MDA Content in the Roots of Malus hupehensis Rehd. Seedlings
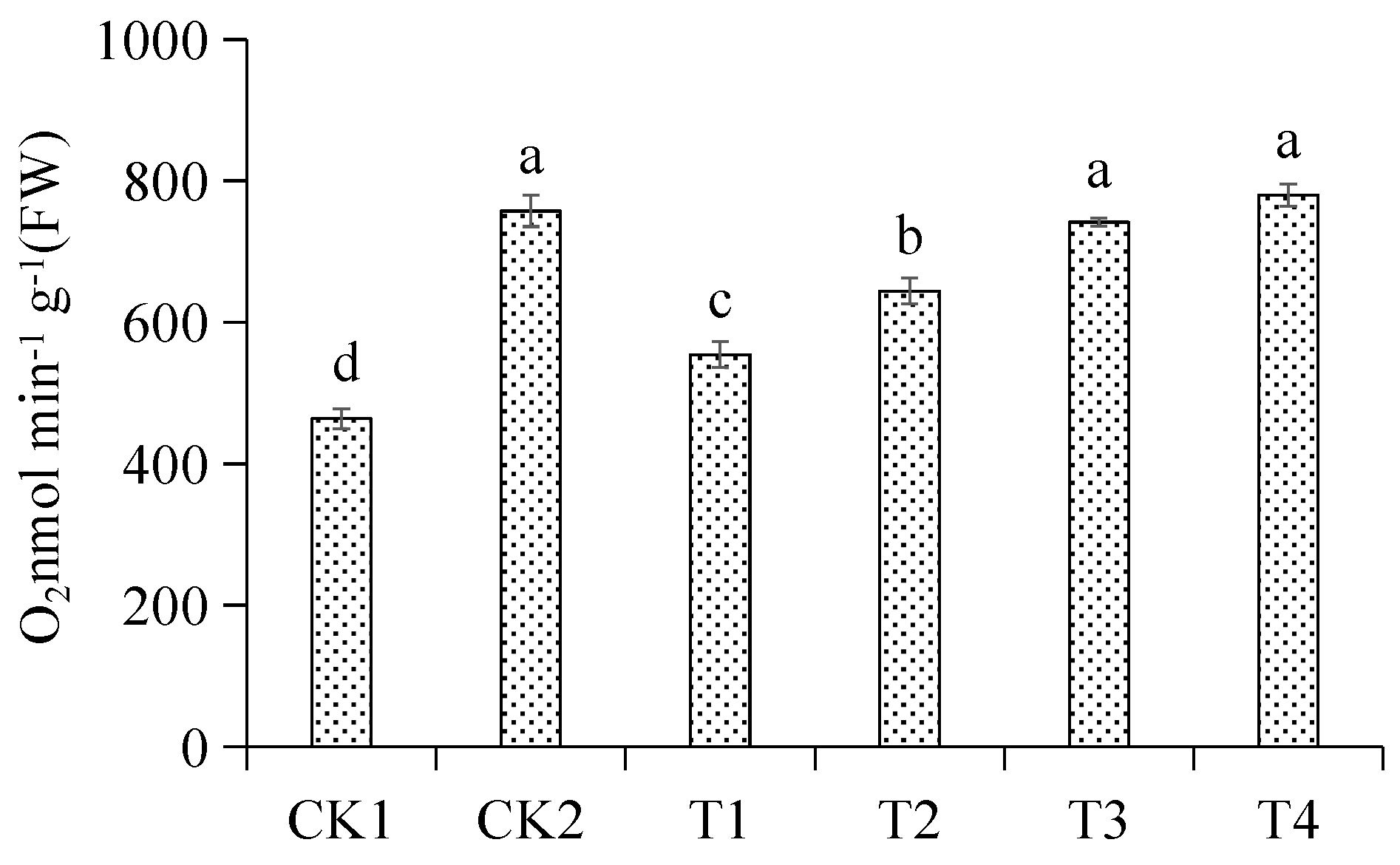
3.4. Effects of Different Treatments on Root Respiration Rate of Malus hupehensis Rehd. Seedling
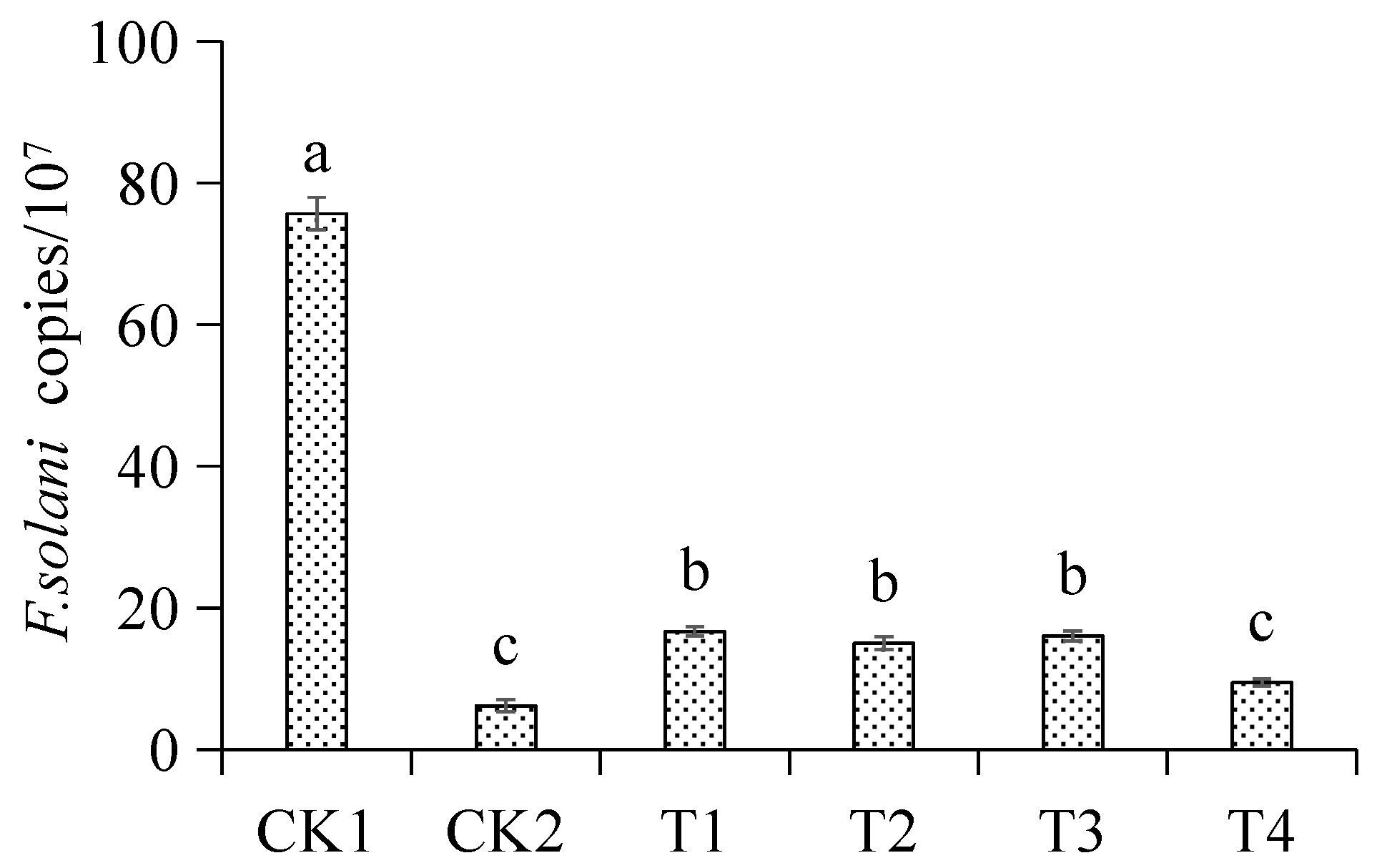
3.5. Inhibitory Effect of Different Treatments on Fusarium oxysporum in the Seedling Rhizosphere Soil
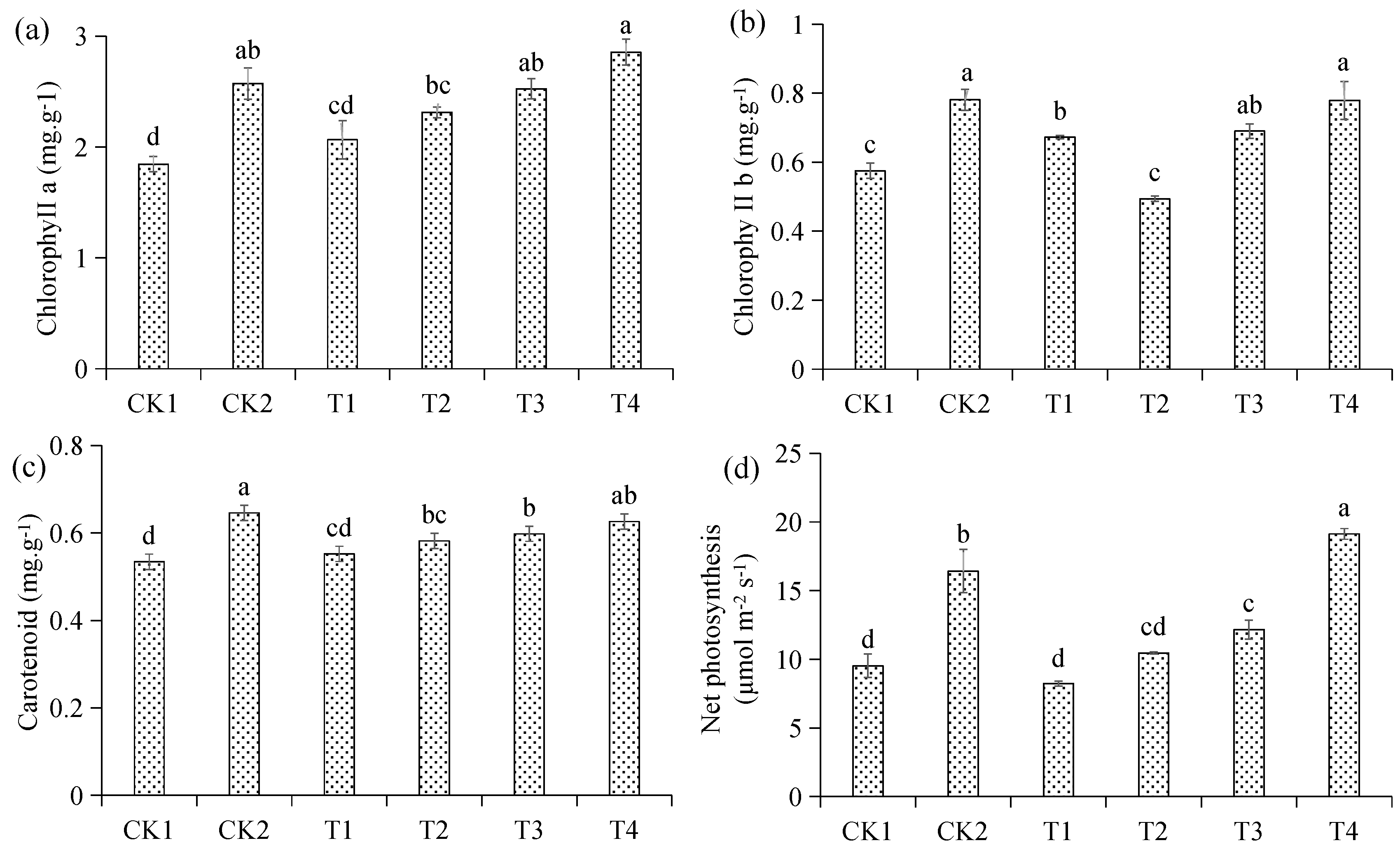
3.6. Effects of Different Treatments on Photosynthesis and Chlorophyll Content of Malus hupehensis Rehd. Seedling
3.7. Effects of Different Treatments on the Number of Culturable Microorganisms in the Soil
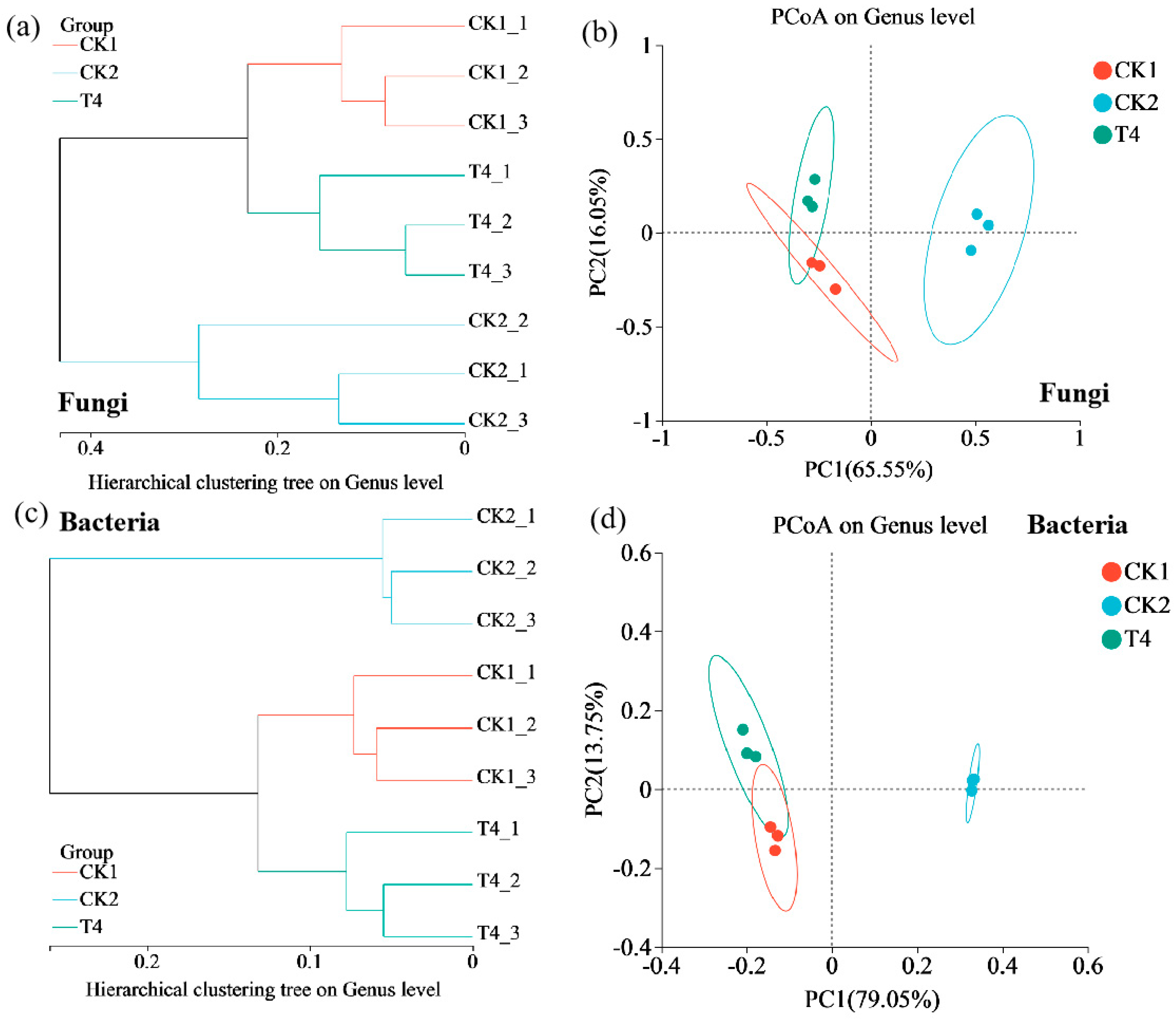
3.8. Effects of Different Treatments on Soil Microbial Community Diversity
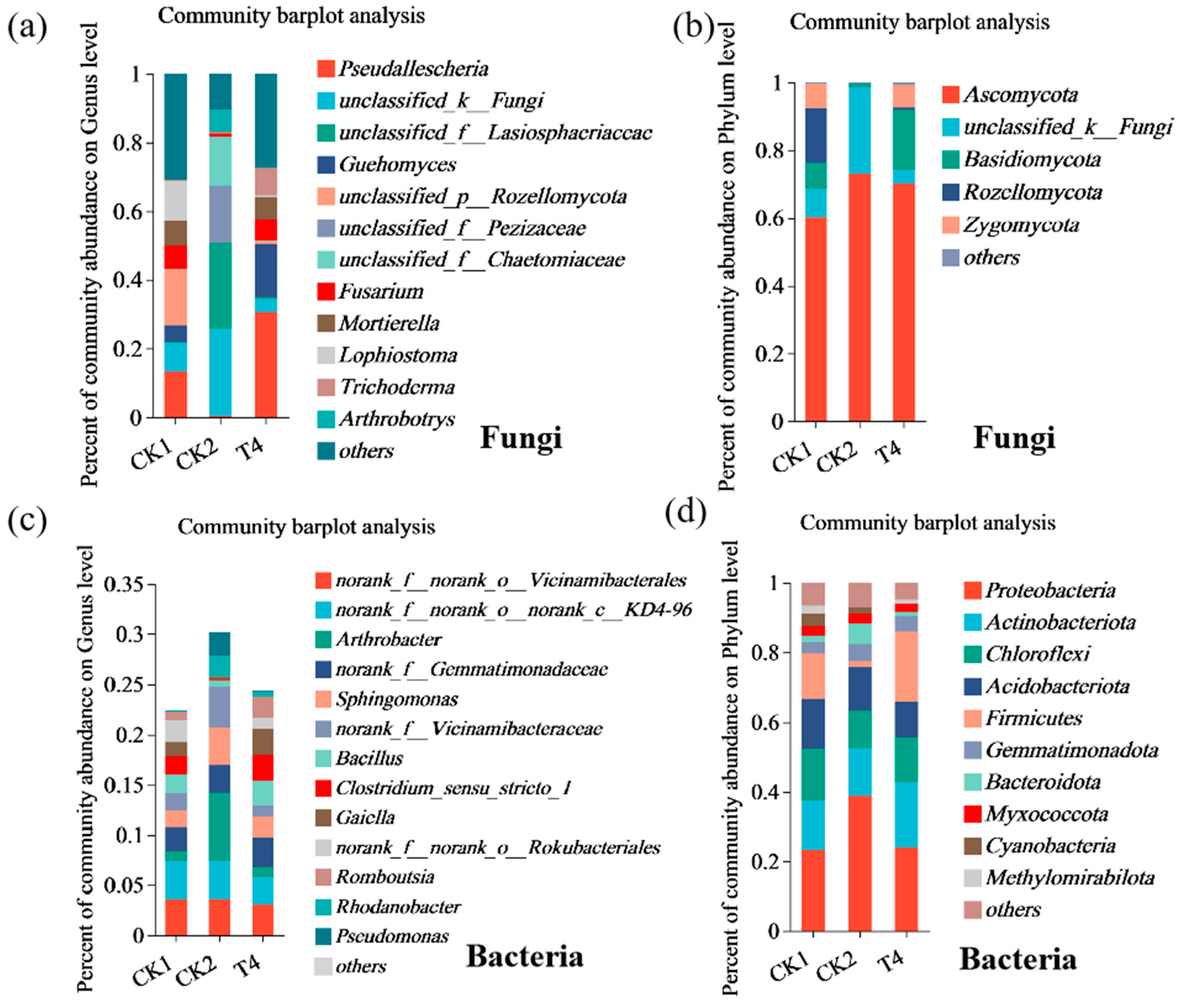
3.9. Effects of Different Treatments on Species Composition of Soil Microbial Community
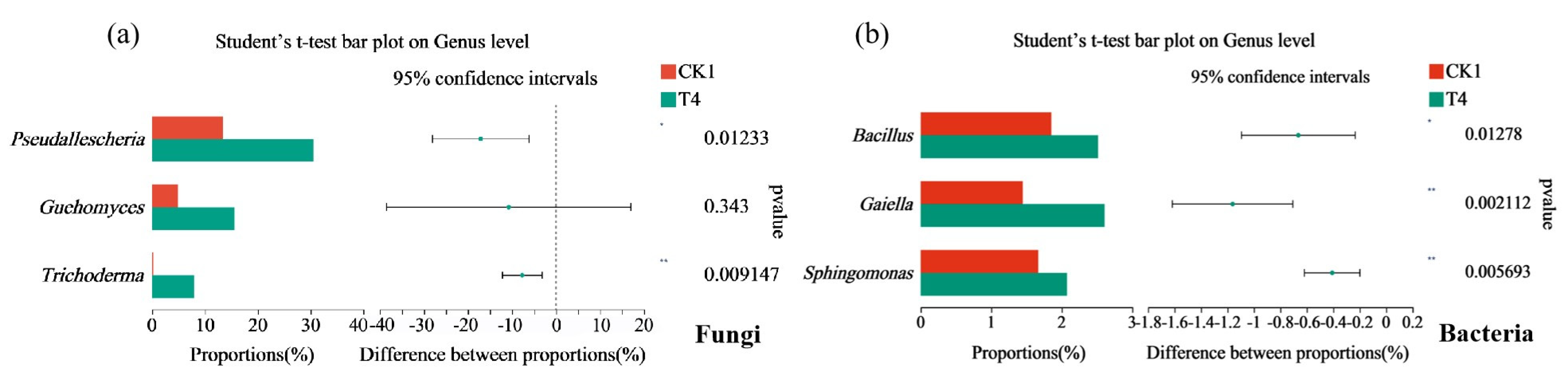
3.10. Differences between Soil Microbial Species and Their Correlation with Physical and Chemical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, C.M.; Xiang, L.; Wang, G.S.; Wang, Y.F.; Shen, X.; Chen, X.S.; Mao, Z.Q. Phloridzin promotes the growth of Fusarium moniliforme (Fusarium verticillioides). Sci. Hortic. 2017, 214, 187–194. [Google Scholar] [CrossRef]
- Jaffee, B.A.; Abawi, G.S.; Mai, W.F. Fungi associated with roots of apple seedlings grown in soil from an apple replant site. Plant Dis. 1982, 66, 942–944. [Google Scholar] [CrossRef]
- Weiß, S.; Bartsch, M.; Winkelmann, T. Transcriptomic analysis of molecular responses in Malus domestica ‘M26’ roots affected by apple replant disease. Plant Mol. Biol. 2017, 943, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Grunewaldt-Stöcker, G.F.; Mahnkopp, C.; Popp, E.M.; Winkelmann, T. Diagnosis of apple replant disease (ARD): Microscopic evidence of early symptoms in fine roots of different apple rootstock genotypes. Sci. Hortic. 2019, 243, 583–594. [Google Scholar] [CrossRef]
- St. Laurent, A.; Merwin, I.A.; Fazio, G.; Thies, J.E.; Brown, M.G. Rootstock genotype succession influences apple replant disease and root-zone microbial community composition in an orchard soil. Plant Soil 2010, 337, 259–272. [Google Scholar] [CrossRef]
- Mazzola, M. Elucidation of the microbial complex having a causal role in the development of Apple replant diseases in Washington. Phytopathology 1998, 88, 930–938. [Google Scholar] [CrossRef]
- Manici, L.M.; Ciavatta, C.; Kelderer, M.; Erschbaumer, G. Replant problems in south tyrol: Role of fungal pathogens and microbial population in conventional and organic apple orchards. Plant Soil 2003, 256, 315–324. [Google Scholar] [CrossRef]
- Schoor, L.V.; Denman, S.; Cook, N.C. Characterisation of apple replant disease under South African conditions and potential biological management strategies. Sci. Hortic. 2009, 119, 153–162. [Google Scholar] [CrossRef]
- Wang, G.S.; Yin, C.M.; Pan, F.B.; Wang, X.B.; Xiang, L.; Wang, Y.F.; Wang, J.Z.; Tian, C.P.; Chen, J.; Mao, Z.Q. Analysis of the fungal community in apple replanted soil around Bohai Gulf. Hortic. Plant J. 2018, 4, 175–181. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, M.; Jiang, W.T.; Wang, Y.F.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. Key indicators for renewal and reconstruction of perennial trees soil: Microorganisms and phloridzin. Ecotox. Environ. Saf. 2021, 225, 112723. [Google Scholar] [CrossRef]
- Nicola, L.; Turco, E.; Albanese, D.; Donati, C.; Thalheimer, M.; Pindo, M.; Insam, H.; Cavalieri, D.; Pertot, I. Fumigation with dazomet modifies soil microbiota in apple orchards affected by replant disease. Appl. Soil Ecol. 2017, 113, 71–79. [Google Scholar] [CrossRef]
- Zasada, I.A.; Halbrendt, J.M.; Kokalis-burelle, N. Managing nematodes without methyl bromide. Annu. Rev. Phytopathol. 2010, 48, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Montag, J.; Schreiber, L.; Schönherr, J. An in vitro study on the postinfection activities of hydrated lime and lime sulphur against apple scab (Venturia inaequalis). J. Phytopathol. 2005, 153, 485–491. [Google Scholar] [CrossRef]
- Hu, S.Y.; Li, D.; Man, Y.D.; Wen, Y.Y.; Huang, C. Evaluation of remediation of Cr(VI)-contaminated soils by calcium polysulfide: Long-term stabilization and mechanism studies. Sci. Total Environ. 2021, 790, 148140. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, T.L.; Eissenstat, D.M.; Granett, J.; Gardner, D.M.; Smart, D.R. Consequences of insectherbivory on grape fine root systems with different growth rates. Plant Cell Environ. 2007, 30, 786–795. [Google Scholar] [CrossRef]
- Chen, L.L.; Wang, X.F.; Liu, M.; Yang, F.J.; Shi, Q.H.; Wei, M.; Li, Q.M. Effects of calcium and ABA on photosynthesis and related enzymes activities in cucumber seedlings under drought stress. J. Appl. Ecol. 2016, 27, 3996–4002. [Google Scholar]
- Kanazawa, S.; Sano, S.; Koshiba, T.; Ushimaru, T. Changes in antioxidative enzymes in cucumber cotyledons during natural senescence: Comparison with those during dark-induced senescence. Physiol. Plant. 2000, 109, 211–216. [Google Scholar] [CrossRef]
- St Clair, S.B.; Lynch Jonathan, P. Base cation stimulation of mycorrhization and photosynthesis of sugar maple on acid soils are coupled by foliar nutrient dynamics. New Phytol. 2004, 165, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.; Delong, J.; Lada, R.; Panage, R. The relationship between water status and chlorophyll a fluorescence in grapes (Vitis spp.). Postharvest Biol. Technol. 2009, 51, 193–199. [Google Scholar] [CrossRef]
- Kaznina, N.M.; Titov, A.F.; Laĭdinen, G.F.; Talanov, A.V. Setaria viridis tolerance of high zinc concentrations. Biol. Bull. 2009, 36, 575–581. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Tan, Z.L. Molybdenum affects photosynthesis and ionic homeostasis of Chinese cabbage under salinity stress. Commun. Soil Sci. Plan Anal. 2014, 45, 2660–2672. [Google Scholar] [CrossRef]
- Antonietti, M. On the way to artificial photosynthesis: Simple materials and system designs for photoelectrodes. Angew. Chem. Int. Ed. 2013, 52, 1086–1087. [Google Scholar] [CrossRef] [PubMed]
- Bloem, E.; Haneklaus, S.; Schnug, E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front. Plant Sci. 2015, 5, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Zhang, Y.; Wang, X.W.; Han, X.; An, Y.; Lin, S.W.; Shen, C.; Wen, J.L.; Liu, C.; Yin, W.L.; et al. Root-specific NF-Y family transcription factor, PdNF-YB21, positively regulates root growth and drought resistance by abscisic acid-mediated indoylacetic acid transport in Populus. New Phytol. 2020, 227, 407–426. [Google Scholar] [CrossRef]
- Raspor, M.; Motyka, V.; Ninković, S.; Dobrev, P.; Malbeck, J.; Ćosić, T.; Cinge, A.; Savić, J.; Tadić, V.; Dragićević, I. Endogenous levels of cytokinins, indole-3-acetic acid and abscisic acid in in vitro grown potato: A contribution to potato hormonomics. Sci. Rep. 2020, 10, 3437. [Google Scholar] [CrossRef]
- Thoa, N.T.K.; Mai, D.T.H.; Hiu, B.L.; Duong, C.A.; Chau, N.N.B.; Nghiep, N.M.; Van, M.N.; Quoc, N.B. Roles of β-Indole acetic acid (IAA) producing endophytic bacteria on the recovery of plant growth and survival ability of sugarcane infected white leaf disease (SWLD). Curr Microbiol. 2022, 79, 389. [Google Scholar] [CrossRef]
- Jiang, W.T.; Chen, R.; Ming, C.J.; Wang, H.Y.; Shen, X.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. Effects of crystal sulfur mixture fumigation on the soil environment of apple replant and the growth of Malus hupehensis Rehd. seedling. J. Plant Physiol. 2020, 56, 1825–1832. (In Chinese) [Google Scholar]
- Zhao, H.W.; Li, Q.L.; Jin, X.T.; Li, D.; Zhu, Z.Q.; Li, Q.X. Chiral enantiomers of the plant growth regulator paclobutrazol selectively affect community structure and diversity of soil microorganisms. Sci. Total Environ. 2021, 797, 148942. [Google Scholar] [CrossRef]
- Khan, N. Application of Plant Growth Promoting Microorganism and Plant Growth Regulators in Agricultural Production and Research. Agronomy 2021, 11, 524. [Google Scholar] [CrossRef]
- Jiang, W.T.; Chen, R.; Zhao, L.; Qin, L.; Fan, H.; Chen, X.S.; Wang, Y.F.; Yin, C.M.; Mao, Z.Q. Chemical fumigants control apple replant disease: Microbial community structure-mediated inhibition of Fusarium and degradation of phenolic acids. J. Hazard. Mater. 2022, 440, 129786. [Google Scholar] [CrossRef]
- Fu, F.Y.; Xiang, L.; Xu, S.Z.; Liu, X.L.; Shen, X.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. Effects of carbendazim and microbial organic fertilizer on replant soil. Indian. J. Hortic. 2016, 43, 1452–1462. (In Chinese) [Google Scholar]
- Wang, M.; Yin, C.M.; Sun, M.M.; Yang, M.F.; Feng, F.L.; Chen, X.S.; Shen, X.; Mao, Z.Q. Effects of fulvic acid microbial inoculum on photosynthetic characteristics of replant soil. J. Plant Physiol. 2019, 55, 99–106. (In Chinese) [Google Scholar]
- Zheng, L.I.; Wang, P.; Shen, Y.X.; Wei, M.F.; He, Y.M. Immobilization and properties of polyphenol oxidase. Biocatal. Agric. Biotechnol. 2021, 10, 79–83. [Google Scholar]
- Singh, B.K.; Sharma, S.R.; Singh, B. Antioxidant enzymes in cabbage: Variability and inheritance of superoxide dismutase, peroxidase and catalase. Sci. Hortic. 2010, 124, 9–13. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, W.T.; Xu, S.Z.; Fan, H.; Chen, X.S.; Shen, X.; Yin, C.M.; Mao, Z.Q. An emerging chemical fumigant: Two-sided effects of dazomet on soil microbial environment and plant response. Environ. Sci. Pollut. Res. 2022, 29, 3022–3036. [Google Scholar] [CrossRef]
- Duan, Y.N.; Zhao, L.; Jiang, W.T.; Chen, R.; Zhang, R.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. The phlorizin-degrading Bacillus licheniformis XNRB-3 mediates soil microorganisms to alleviate apple replant disease. Front. Microbiol. 2022, 13, 839484. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Du, K.Q.; Sun, C.; Vimalanathan, A.; Liang, X.L.; Li, Y.; Wang, B.H.; Lu, X.M.; Li, L.J.; Shao, Y.Q. Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry feeding relatives. ISME J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bi, H.; Xu, H.; Duan, H.; Peng, R.; Wang, J. Variation of fine roots distribution in apple (Malus pumila M.)—Crop intercropping systems on the loess Plateau of China. Agronomy 2018, 8, 280. [Google Scholar] [CrossRef]
- Wang, G.S.; Mao, Z.Q.; Pan, F.B.; Tian, C.P.; Wang, J.Z.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. Effect of apple replanting on soil microorganism and nitrogen absorption, distribution, utilization of Malus hupehensis Rehd. seedlings. Plant Nutr. Fertil. Sci. 2019, 25, 481–488. (In Chinese) [Google Scholar]
- Jiang, W.T.; Chen, R.; Zhao, L.; Duan, Y.N.; Wang, H.Y.; Yan, Z.B.; Shen, X.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. Isolation of phloridzin-degrading, IAA-producing bacterium Ochrobactrum haematophilum and its effects on the apple replant soil environment. Hortic. Plant J. 2023, 9, 199–208. [Google Scholar] [CrossRef]
- Margaux, S.; Eva, L.; Andreas, W.; Wulf, A. In-field heterogeneity of apple replant disease: Relations to abiotic soil properties. Sci. Hortic. 2020, 259, 108809. [Google Scholar]
- Wang, Y.F.; Pan, F.B.; Wang, G.S.; Zhang, G.D.; Wang, Y.L.; Chen, X.S.; Mao, Z.Q. Effects of biochar on photosynthesis and antioxidative system of Malus hupehensis Rehd. seedlings under replant conditions. Sci. Hortic. 2014, 175, 9–15. [Google Scholar] [CrossRef]
- Gull, M.; Sajid, Z.A.; Aftab, F. Alleviation of salt stress in Solanum tuberosum L. by exogenous application of indoleacetic acid and L-tryptophan. J. Plant Growth Regul. 2023, 42, 3257–3273. [Google Scholar] [CrossRef]
- Pan, C.J.; Du, X.G. Effects of the best combination of copper, zinc, iron, and manganese on the relationship of lettuce resistance to Botrytis cinerea and its antioxidant system. Emir. J. Food Agric. 2017, 29, 330–338. [Google Scholar] [CrossRef]
- Balwant, V.; Reddy, M.S. Biochar augmentation improves ectomycorrhizal colonisation, plant growth and soil fertility. Soil Res. 2020, 58, 673–682. [Google Scholar]
- Shao, X.X.; Yang, W.Y.; Wu, M. Seasonal dynamics of soil labile organic carbon and enzyme activities in relation to vegetation types in hangzhou bay tidal flat wetland. PLoS ONE 2015, 10, e0142677. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.J.; Fultz, L.M.; White, P.; Jeong, C.Y. Application of biochar in estrogen hormone contaminated and manure affected soils: Impact on soil respiration, microbial community and enzyme activity. Chemosphere 2021, 270, 128625. [Google Scholar] [CrossRef]
- Albornoz, F.E.; Prober, S.M.; Ryan, M.H.; Standish, R.J. Ecological interactions among microbial functional guilds in the plant-soil system and implications for ecosystem function. Plant Soil 2022, 476, 301–313. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Guo, H.; Mao, Z.Q.; Jiang, H.X.; Liu, P.; Zhou, B.Q.; Bao, Z.Z.; Sui, J.K.; Zhou, X.Y.; Liu, X.L. Community analysis of plant growth promoting rhizobacteria for apple trees. Crop Prot. 2014, 62, 1–9. [Google Scholar] [CrossRef]
- Maron, P.A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High microbial diversity promotes soil ecosystem functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef] [PubMed]
- Vinas, M.; Sabate, J.; Espuny, M.J.; Solanas, A.M. Bacterial community dynamics and polycyclic aromatic hydrocarbon degradation during bioremediation of heavily creosote-contaminated soil. Appl. Environ. Microbiol. 2005, 71, 7008–7018. [Google Scholar] [CrossRef] [PubMed]
- Horemans, B.; Vandermaesen, J.; Vanhaecke, L.; Smolders, E.; Springael, D. Variovorax sp.-mediated biodegradation of the phenyl urea herbicide linuron at micropollutant concentrations and effects of natural dissolved organic matter as supplementary carbon source. Appl. Microbiol. Biotechnol. 2013, 97, 9837–9846. [Google Scholar] [CrossRef] [PubMed]
- Wasi, S.; Tabrez, S.; Ahmad, M. Use of Pseudomonas spp. for the bioremediation of environmental pollutants: A review. Environ. Monit. Assess. 2013, 185, 8147–8155. [Google Scholar] [CrossRef]
- Villaverde, J.; Rubio-Bellido, M.; Merchan, F.; Morillo, E. Bioremediation of diuron contaminated soils by a novel degrading microbial consortium. J. Environ. Manag. 2017, 188, 379–386. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Gdanetz, K.; Benucci, G.M.N.; Pol, N.V.; Bonito, G. CONSTAX: A tool for improved taxonomic resolution of environmental fungal ITS sequences. BMC Bioinform. 2017, 18, 538. [Google Scholar] [CrossRef]
- Huang, Y.H. Illumina-based Analysis of Endophytic Bacterial Diversity of four Allium species. Sci. Rep. 2019, 9, 15271. [Google Scholar] [CrossRef]


| Abbreviation | Treatment |
|---|---|
| CK1 | Replanted soil |
| CK2 | Methyl-bromide-fumigated soil |
| T1 | Crystal lime sulfur |
| T2 | Crystal lime sulfur and indoleacetic acid |
| T3 | Crystal lime sulfur with nutritional elements |
| T4 | Crystal lime sulfur, indoleacetic acid, and nutrient elements |
| Time | Treatment | Plant Height (cm) | Stem Diameter (mm) | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|---|---|---|
| CK1 | 30.40 ± 0.31 d | 3.67 ± 0.17 d | 6.76 ± 1.66 d | 3.13 ± 0.42 d | |
| CK2 | 67.67 ± 3.71 a | 7.84 ± 0.41 a | 35.25 ± 7.11 b | 17.86 ± 1.96 b | |
| July | T1 | 46.00 ± 0.58 c | 5.01 ± 0.17 c | 17.10 ± 2.73 c | 10.09 ± 1.22 cd |
| T2 | 53.67 ± 1.76 b | 6.28 ± 0.43 b | 23.68 ± 5.90 c | 11.21 ± 2.02 bc | |
| T3 | 56.00 ± 1.73 b | 7.70 ± 0.24 a | 23.12 ± 1.72 c | 18.53 ± 4.24 ab | |
| T4 | 66.33 ± 1.20 a | 7.83 ± 0.28 a | 43.80 ± 3.46 a | 25.79 ± 2.69 a | |
| CK1 | 49.97 ± 1.36 b | 6.14 ± 0.02 c | 25.02 ± 0.20 d | 6.36 ± 0.10 f | |
| CK2 | 66.37 ± 2.12 a | 8.16 ± 0.12 a | 105.00 ± 0.58 a | 43.82 ± 1.26 a | |
| August | T1 | 55.77 ± 3.24 b | 4.88 ± 0.17 d | 28.36 ± 0.51 d | 13.71 ± 0.34 e |
| T2 | 53.60 ± 1.46 b | 6.13 ± 0.10 c | 40.24 ± 1.80 c | 18.02 ± 0.83 d | |
| T3 | 53.17 ± 0.64 b | 6.34 ± 0.22 c | 42.03 ± 3.37 c | 23.88 ± 0.31 c | |
| T4 | 65.07 ± 1.43 a | 7.00 ± 0.03 b | 70.22 ± 1.80 b | 32.70 ± 0.91 b |
| Treatment | Bacteria (×105 CFU·g−1) | Actinomyces (×105 CFU·g−1) |
|---|---|---|
| CK1 | 8.67 ± 0.33 cd | 15.00 ± 0.58 b |
| CK2 | 3.00 ± 0.58 e | 8.67 ± 0.88 d |
| T1 | 7.00 ± 0.58 d | 11.33 ± 0.88 c |
| T2 | 10.33 ± 0.88 c | 15.00 ± 0.58 b |
| T3 | 12.67 ± 0.33 b | 18.00 ± 0.58 a |
| T4 | 15.00 ± 0.58 a | 19.00 ± 0.58 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Q.; Jiang, W.; Liu, S.; Qin, L.; Zhao, G.; Li, Z.; Yin, C.; Mao, Z.; Wang, Y. Effects of Crystal Lime Sulfur Fumigation and Application of Root-Growth-Promoting Agents on the Control of Apple Replant Disease. Horticulturae 2023, 9, 901. https://doi.org/10.3390/horticulturae9080901
Xia Q, Jiang W, Liu S, Qin L, Zhao G, Li Z, Yin C, Mao Z, Wang Y. Effects of Crystal Lime Sulfur Fumigation and Application of Root-Growth-Promoting Agents on the Control of Apple Replant Disease. Horticulturae. 2023; 9(8):901. https://doi.org/10.3390/horticulturae9080901
Chicago/Turabian StyleXia, Qun, Weitao Jiang, Shaochun Liu, Lei Qin, Guangyu Zhao, Zhao Li, Chengmiao Yin, Zhiquan Mao, and Yanfang Wang. 2023. "Effects of Crystal Lime Sulfur Fumigation and Application of Root-Growth-Promoting Agents on the Control of Apple Replant Disease" Horticulturae 9, no. 8: 901. https://doi.org/10.3390/horticulturae9080901
APA StyleXia, Q., Jiang, W., Liu, S., Qin, L., Zhao, G., Li, Z., Yin, C., Mao, Z., & Wang, Y. (2023). Effects of Crystal Lime Sulfur Fumigation and Application of Root-Growth-Promoting Agents on the Control of Apple Replant Disease. Horticulturae, 9(8), 901. https://doi.org/10.3390/horticulturae9080901





