Comprehensive Metabolomic and Transcriptomic Analysis of the Regulatory Network of Volatile Terpenoid Formation during the Growth and Development of Pears (Pyrus spp. ‘Panguxiang’)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Physiological Parameters
2.3. Sample Treatment and GC-MS Conditions
2.4. Integrative Analysis of Metabolome and Transcriptome
2.5. Statistical Analysis
3. Results
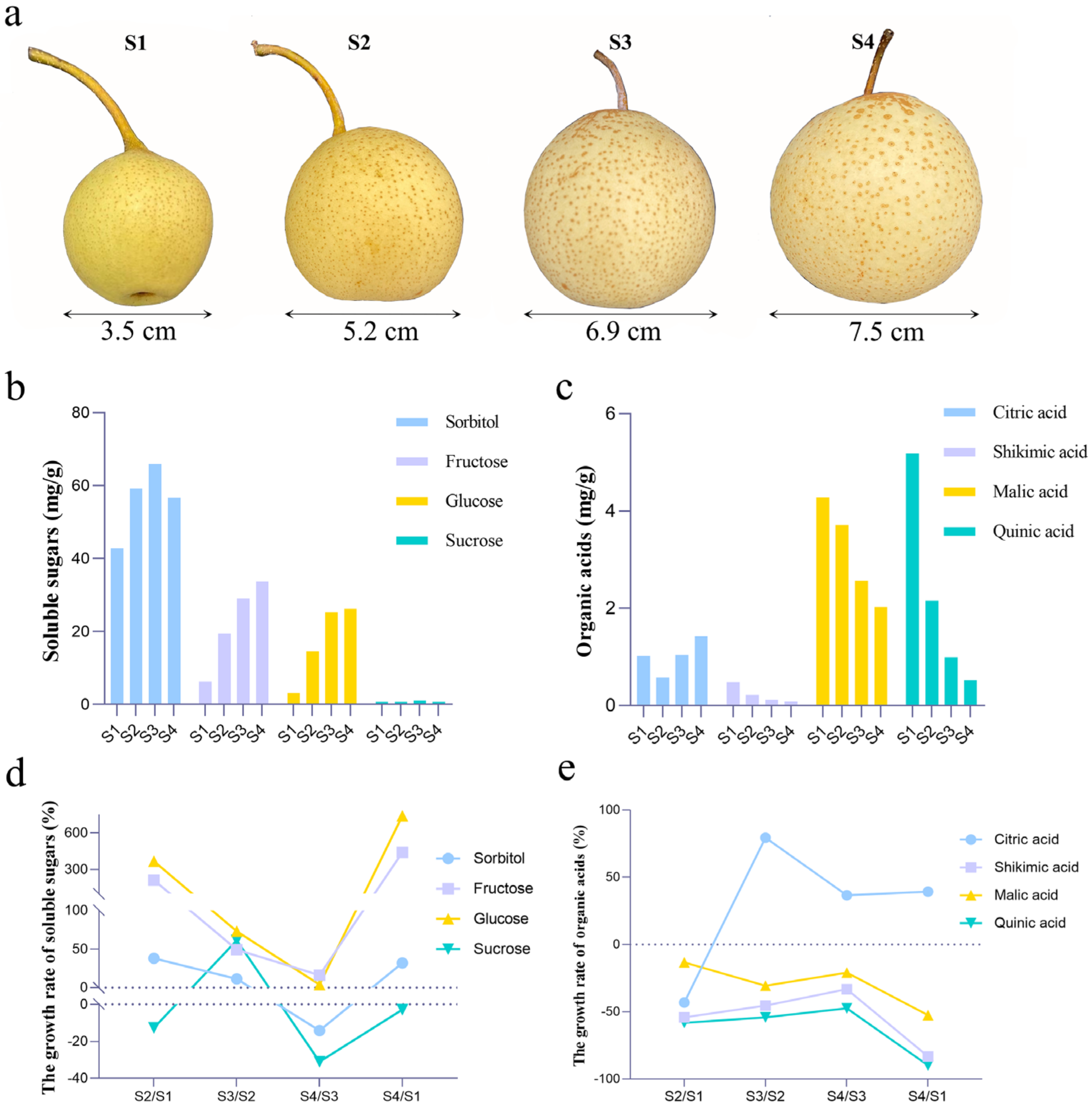
3.1. Changes in Physiological Indicators of Fruits
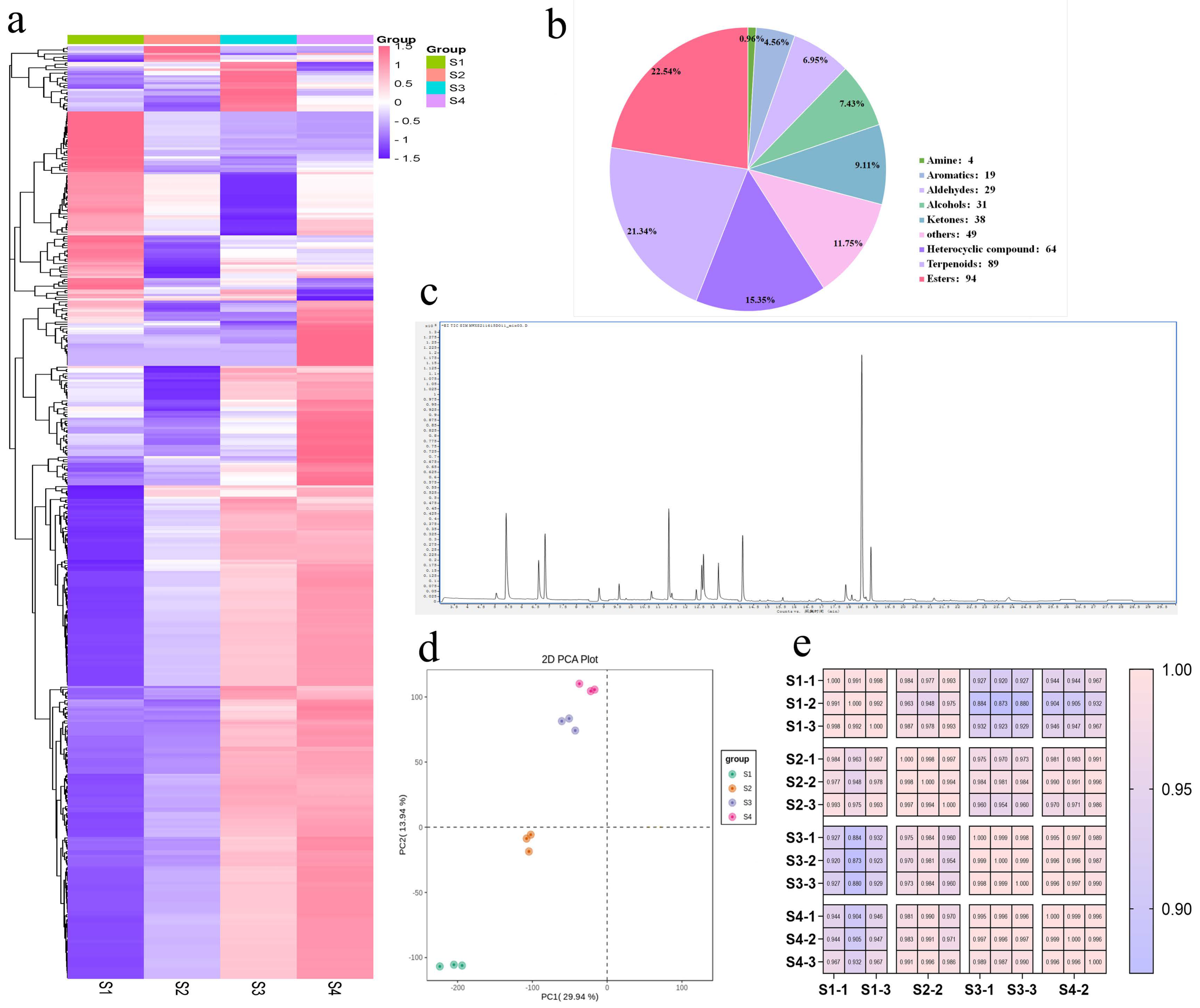
3.2. Metabolomic Analysis of Pear Fruits
3.3. Differences in VOCs of Pear Fruits
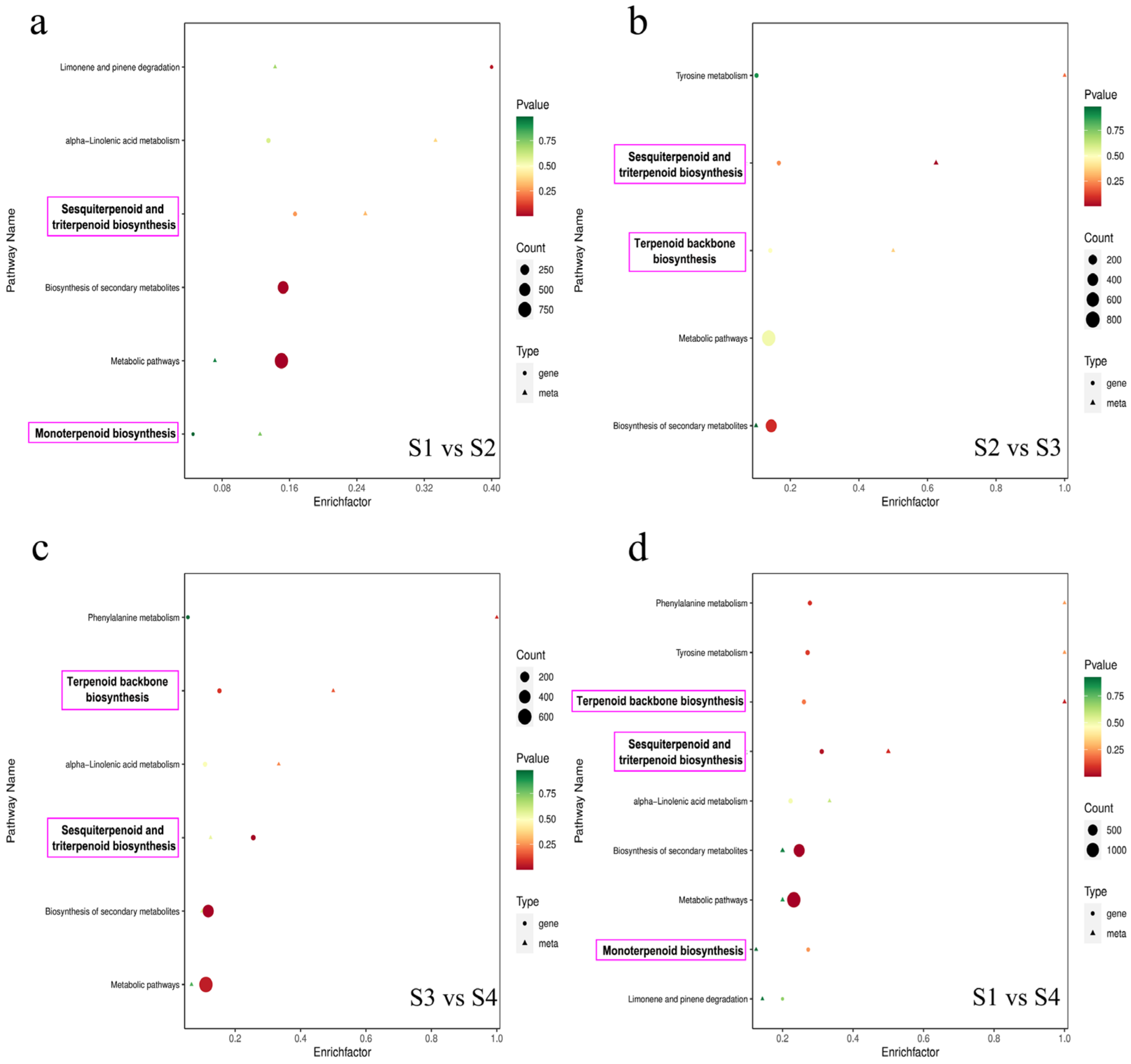
3.4. Correlation between Metabolites and Transcripts in Pear Fruits
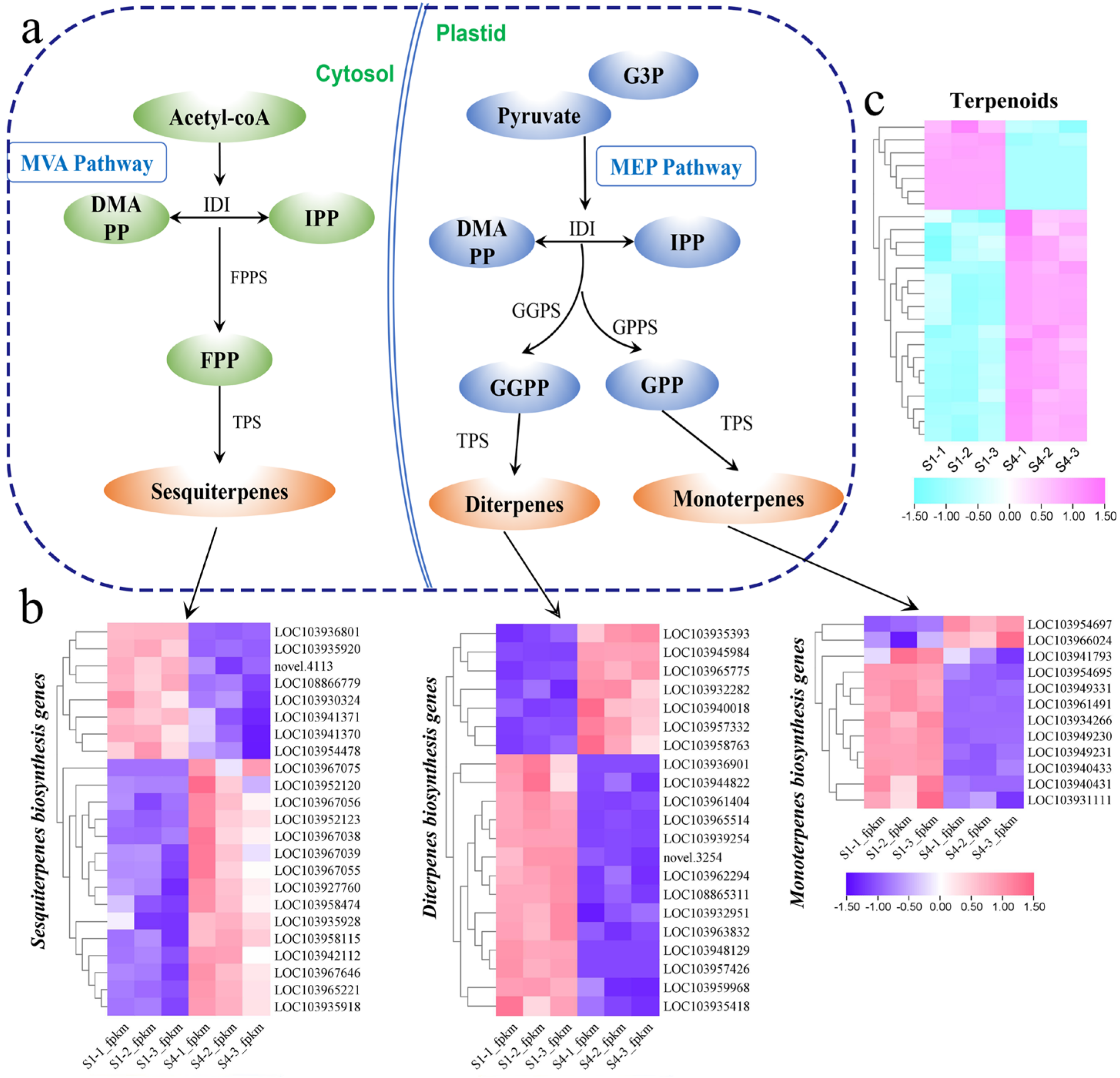
3.5. Gene Expression Profiling of Terpenoid Metabolic Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Available online: https://www.fao.org/faostat/zh/#data (accessed on 5 April 2023).
- Forster, B. Wild Crop Relatives: Genomic and Breeding Resources, Cereals; Experimental Agriculture; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 47, p. 736. [Google Scholar]
- Cui, T.; Nakamura, K.; Ma, L.; Li, J.-Z.; Kayahara, H. Analyses of Arbutin and Chlorogenic Acid, the Major Phenolic Constituents in Oriental Pear. J. Agric. Food Chem. 2005, 53, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Wang, Y.M.; Geng, X.D. Study on Grafting Seedling cultivation Technology of Pyrus spp. ‘Panguxiang’ pear in spring. J. Henan Agric. Univ. 2016, 50, 486–489. (In Chinese) [Google Scholar]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2014, 66, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Spaho, N.; Gaši, F.; Leitner, E.; Blesić, M.; Akagić, A.; Žuljević, S.O.; Kurtović, M.; Ratković, D.; Murtić, M.S.; Akšić, M.F.; et al. Characterization of Volatile Compounds and Flavor in Spirits of Old Apple and Pear Cultivars from the Balkan Region. Foods 2021, 10, 1258. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef]
- Chen, J.L.; Yan, S.; Feng, Z.; Xiao, L.; Hu, X.S. Changes in the volatile compounds and chemical and physical properties of Yali pear (Pyrus bertschneideri Reld) during storage. Food Chem. 2006, 97, 248–255. [Google Scholar] [CrossRef]
- Villatoro, C.; Altisent, R.; Echeverría, G.; Graell, J.; López, M.L.; Lara, I. Changes in biosynthesis of aroma volatile compounds during on-tree maturation of ‘Pink Lady®’ apples. Postharvest Biol. Technol. 2008, 47, 286–295. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Ibdah, M.; Meir, A.; Yosef, E.; Zamir, D.; Tadmor, Y. Not just colors—Carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends Food Sci. Technol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Lalel, H.J.D.; Singh, Z.; Tan, S.C. Aroma volatiles production during fruit ripening of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2003, 27, 323–336. [Google Scholar] [CrossRef]
- Obando-Ulloa, J.M.; Moreno, E.; García-Mas, J.; Nicolai, B.; Lammertyn, J.; Monforte, A.J.; Fernández-Trujillo, J.P. Climacteric or non-climacteric behavior in melon fruit: 1. Aroma volatiles. Postharvest Biol. Technol. 2008, 49, 27–37. [Google Scholar] [CrossRef]
- Van de Poel, B.; Vandendriessche, T.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Geeraerd, A. Detached ripening of non-climacteric strawberry impairs aroma profile and fruit quality. Postharvest Biol. Technol. 2014, 95, 70–80. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S.; Yang, L.; Wang, Y.; Zhao, J.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP–SPME with GC–MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Maul, F.; Sargent, S.A.; Sims, C.A.; Baldwin, E.A.; Balaban, M.O.; Huber, D.J. Tomato Flavor and Aroma Quality as Affected by Storage Temperature. J. Food Sci. 2000, 65, 1228–1237. [Google Scholar] [CrossRef]
- Rapparini, F.; Predieri, S. Pear Fruit Volatiles. Hortic. Rev. 2010, 28, 237–324. [Google Scholar]
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Ramya, M.; An, H.R.; Baek, Y.S.; Reddy, K.E.; Park, P.H. Orchid floral volatiles: Biosynthesis genes and transcriptional regulations. Sci. Hortic. 2018, 235, 62–69. [Google Scholar] [CrossRef]
- Gershenzon, J.; Kreis, W. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes, Diterpenes, Sterols, Cardiac Glycosides and Steroid Saponins. Annu. Plant Rev. Online 2018, 218–294. [Google Scholar]
- Newman, J.D.; Chappell, J. Isoprenoid biosynthesis in plants: Carbon partitioning within the cytoplasmic pathway. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 95–106. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, S.; Zhao, M.; Jing, T.; Zhang, N.; Wang, J.; Wu, B.; Song, C. Scenarios of Genes-to-Terpenoids Network Led to the Identification of a Novel α/β-Farnesene/β-Ocimene Synthase in Camellia sinensis. Int. J. Mol. Sci. 2020, 21, 655. [Google Scholar] [CrossRef]
- Yuan, H.; Cao, G.; Hou, X.; Huang, M.; Du, P.; Tan, T.; Zhang, Y.; Zhou, H.; Liu, X.; Liu, L.; et al. Development of a widely targeted volatilomics method for profiling volatilomes in plants. Mol. Plant 2022, 15, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Quan, J.; Rana, S.; Wang, Y.; Li, Z.; Cai, Q.; Ma, S.; Geng, X.; Liu, Z. The Molecular Network behind Volatile Aroma Formation in Pear (Pyrus spp. Panguxiang) Revealed by Transcriptome Profiling via Fatty Acid Metabolic Pathways. Life 2022, 12, 1494. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Li, P.; She, G.; Chen, D.; Wan, X.; Zhao, J. Transcriptome and metabolite analyses reveal the complex metabolic genes involved in volatile terpenoid biosynthesis in garden sage (Salvia officinalis). Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The Universal Protein knowledgebase. Nucleic Acids Res. 2004, 32, D115–D119. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Krylov, D.M.; Makarova, K.S.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; Rao, B.S.; et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004, 5, R7. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D2302. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- O’Donovan, C.; Martin, M.J.; Glemet, E.; Codani, J.J.; Apweiler, R. Removing redundancy in SWISS-PROT and TrEMBL. Bioinformatics 1999, 15, 258–259. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, C.; Sun, H.; Rosli, H.G.; Pombo, M.A.; Zhang, P.; Banf, M.; Dai, X.; Martin, G.B.; Giovannoni, J.J.; et al. iTAK: A Program for Genome-wide Prediction and Classification of Plant Transcription Factors, Transcriptional Regulators, and Protein Kinases. Mol. Plant 2016, 9, 1667–1670. [Google Scholar] [CrossRef]
- R-Project. Available online: http://www.r-project.org/ (accessed on 5 April 2023).
- Tang, N.; An, J.; Deng, W.; Gao, Y.; Chen, Z.; Li, Z. Metabolic and transcriptional regulatory mechanism associated with postharvest fruit ripening and senescence in cherry tomatoes. Postharvest Biol. Technol. 2020, 168, 111274. [Google Scholar] [CrossRef]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, F.; Huang, Y.; He, J.; Yang, S.; Zeng, J.; Deng, C.; Jiang, X.; Fang, Y.; Wen, S. Genome of wild mandarin and domestication history of mandarin. Mol. Plant 2018, 11, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shu, P.; Zhang, C.; Zhang, J.; Chen, Y.; Zhang, Y.; Du, K.; Xie, Y.; Li, M.; Ma, T.; et al. Integrative analyses of metabolome and genome-wide transcriptome reveal the regulatory network governing flavor formation in kiwifruit (Actinidia chinensis). New Phytol. 2022, 233, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Baietto, M.; Wilson, A.D. Electronic-nose applications for fruit identification, ripeness and quality grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef]
- Steingass, C.B.; Carle, R.; Schmarr, H.G. Ripening-dependent metabolic changes in the volatiles of pineapple (Ananas comosus (L.) Merr.) fruit: I. Characterization of pineapple aroma compounds by comprehensive two-dimensional gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2591–2608. [Google Scholar] [CrossRef]
- Li, X.J.; Qi, L.Y.; Zhang, N.N.; Zhao, L.H.; Sun, Y.Q.; Huang, X.T.; Wang, H.Y.; Yin, Z.P.; Wang, A.D. Integrated metabolome and transcriptome analysis of the regulatory network of volatile ester formation during fruit ripening in pear. Plant Physiol. Biochem. 2022, 185, 80–90. [Google Scholar] [CrossRef]
- Shi, F.; Zhou, X.; Zhou, Q.; Yao, M.M.; Wei, B.D.; Ji, S.J. Transcriptome analyses provide new possible mechanisms of aroma ester weakening of ‘Nanguo’ pear after cold storage. Sci. Hortic. 2018, 237, 247–256. [Google Scholar] [CrossRef]
- Mahalwal, V.S.; Ali, M. Volatile Constituents of the Fruits Peels of Citrus lemon (Linn) Burm. F. J. Essent. Oil Bear. Plants 2003, 6, 31–35. [Google Scholar] [CrossRef]
- Karabulut, I.; Gokbulut, I.; Bilenler, T.; Sislioglu, K.; Ozdemir, I.S.; Bahar, B.; Çelik, B.; Seyhan, F. Effect of fruit maturity level on quality, sensory properties and volatile composition of two common apricot (Prunus armeniaca L.) varieties. J. Food Sci. Technol. 2018, 55, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Liu, H.; Cao, X.; Zhang, M.; Li, X.; Chen, K.; Zhang, B. Synthesis of flavour-related linalool is regulated by PpbHLH1 and associated with changes in DNA methylation during peach fruit ripening. Plant Biotechnol. J. 2021, 19, 2082–2096. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R372–R392. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; D’Auria, J.C.; Gershenzon, J.; Pichersky, E. The Arabidopsis thaliana type I Isopentenyl Diphosphate Isomerases are targeted to multiple subcellular compartments and have overlapping functions in isoprenoid biosynthesis. Plant Cell 2008, 20, 677–696. [Google Scholar] [CrossRef]
- Monson, R.K. Metabolic and gene expression controls on the production of biogenic volatile organic compounds. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 153–179. [Google Scholar]
- Ding, Y.; Huffaker, A.; Köllner, T.G.; Weckwerth, P.; Robert, C.A.M.; Spencer, J.L.; Lipka, A.E.; Schmelz, E.A. Selinene volatiles are essential precursors for maize defense promoting fungal pathogen resistance. Plant Physiol. 2017, 175, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; Jongsma, M.A.; Bouwmeester, H.J. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 2005, 10, 594–602. [Google Scholar] [CrossRef]
- Wang, J.F.; Abbey, T.; Kozak, B.; Madilao, L.L.; Tindjau, R.; Nin, D.J.; Castellarin, S.D. Evolution over the growing season of volatile organic compounds in Viognier (Vitis vinifera L.) grapes under three irrigation regimes. Food Res. Int. 2019, 125, 108512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Quan, J.; Rana, S.; Yao, S.; Wang, Y.; Li, Z.; Cai, Q.; Ma, C.; Geng, X.; Liu, Z. Comprehensive Metabolomic and Transcriptomic Analysis of the Regulatory Network of Volatile Terpenoid Formation during the Growth and Development of Pears (Pyrus spp. ‘Panguxiang’). Horticulturae 2023, 9, 483. https://doi.org/10.3390/horticulturae9040483
Li H, Quan J, Rana S, Yao S, Wang Y, Li Z, Cai Q, Ma C, Geng X, Liu Z. Comprehensive Metabolomic and Transcriptomic Analysis of the Regulatory Network of Volatile Terpenoid Formation during the Growth and Development of Pears (Pyrus spp. ‘Panguxiang’). Horticulturae. 2023; 9(4):483. https://doi.org/10.3390/horticulturae9040483
Chicago/Turabian StyleLi, Huiyun, Jine Quan, Sohel Rana, Shunyang Yao, Yanmei Wang, Zhi Li, Qifei Cai, Chaowang Ma, Xiaodong Geng, and Zhen Liu. 2023. "Comprehensive Metabolomic and Transcriptomic Analysis of the Regulatory Network of Volatile Terpenoid Formation during the Growth and Development of Pears (Pyrus spp. ‘Panguxiang’)" Horticulturae 9, no. 4: 483. https://doi.org/10.3390/horticulturae9040483
APA StyleLi, H., Quan, J., Rana, S., Yao, S., Wang, Y., Li, Z., Cai, Q., Ma, C., Geng, X., & Liu, Z. (2023). Comprehensive Metabolomic and Transcriptomic Analysis of the Regulatory Network of Volatile Terpenoid Formation during the Growth and Development of Pears (Pyrus spp. ‘Panguxiang’). Horticulturae, 9(4), 483. https://doi.org/10.3390/horticulturae9040483






