Relationships between Phenotypes and Chemotypic Characteristics of Local Gymnema inodorum Plants in Northern Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Reagent and Chemicals
2.3. Plant Morphological Characteristics
2.4. Genetic Variation
2.4.1. DNA Extraction
2.4.2. DNA Quantification
2.4.3. RAPD-PCR
2.5. Phytochemical Analyses
2.5.1. Sample Preparation and Extraction
2.5.2. Proximate Analyses
2.5.3. Phenolic Content
2.5.4. Flavonoid Content
2.5.5. Determination of DPPH Radical Scavenging Activity
2.5.6. Determination of ABTS Radical Scavenging Activity
2.5.7. Gymnemic Acid and Saponin Contents
2.6. Statistical Analysis
3. Results
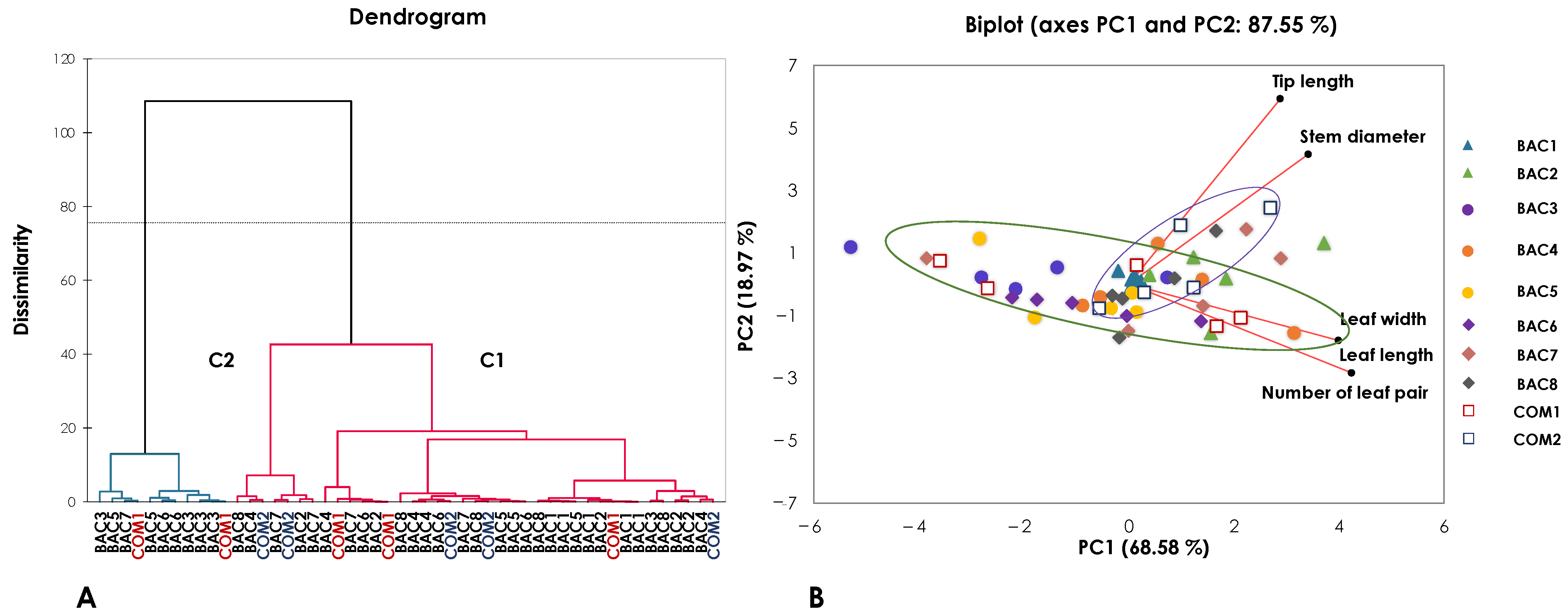
3.1. Morphology
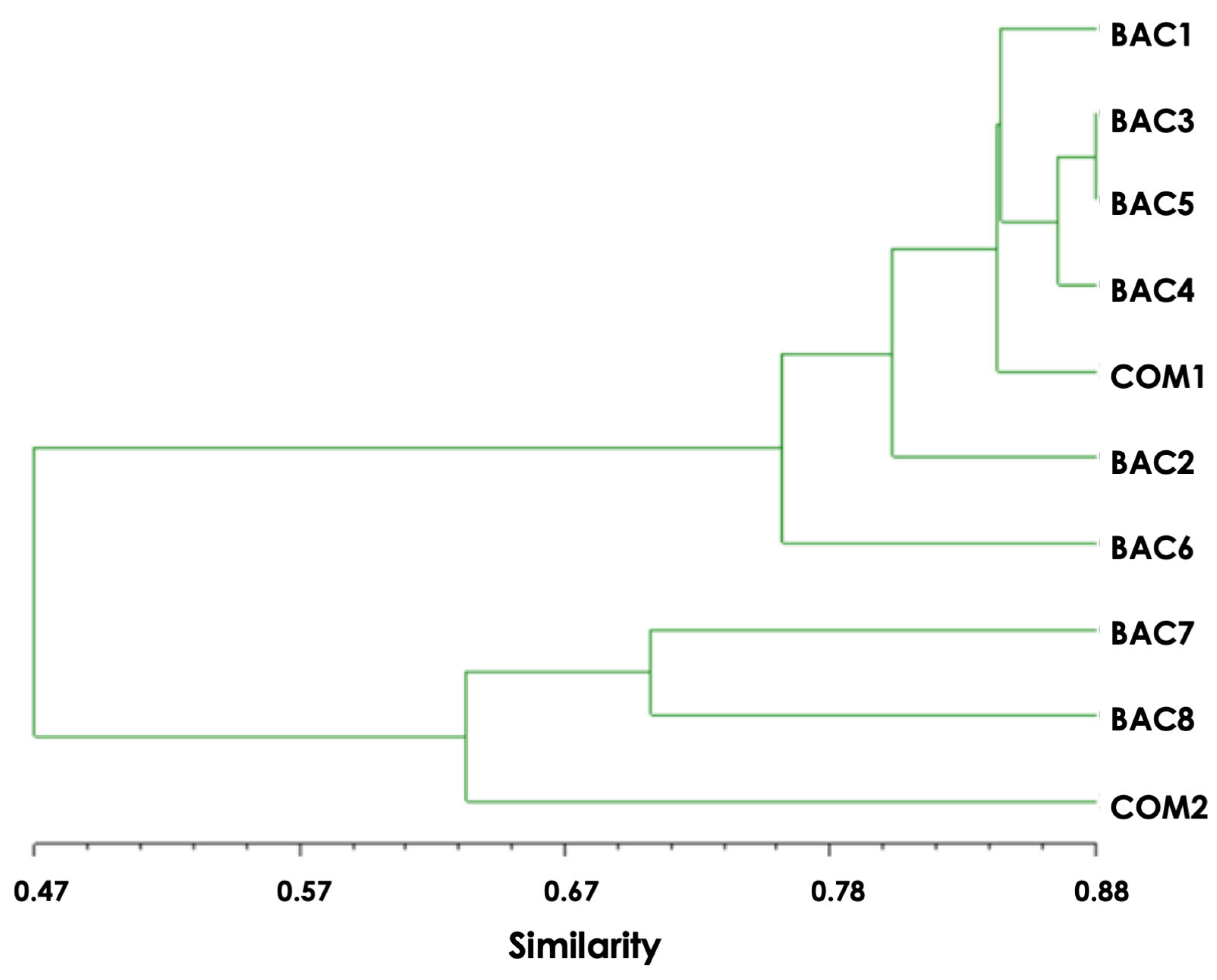
3.2. Genetic Variation of G. inodorum Samples
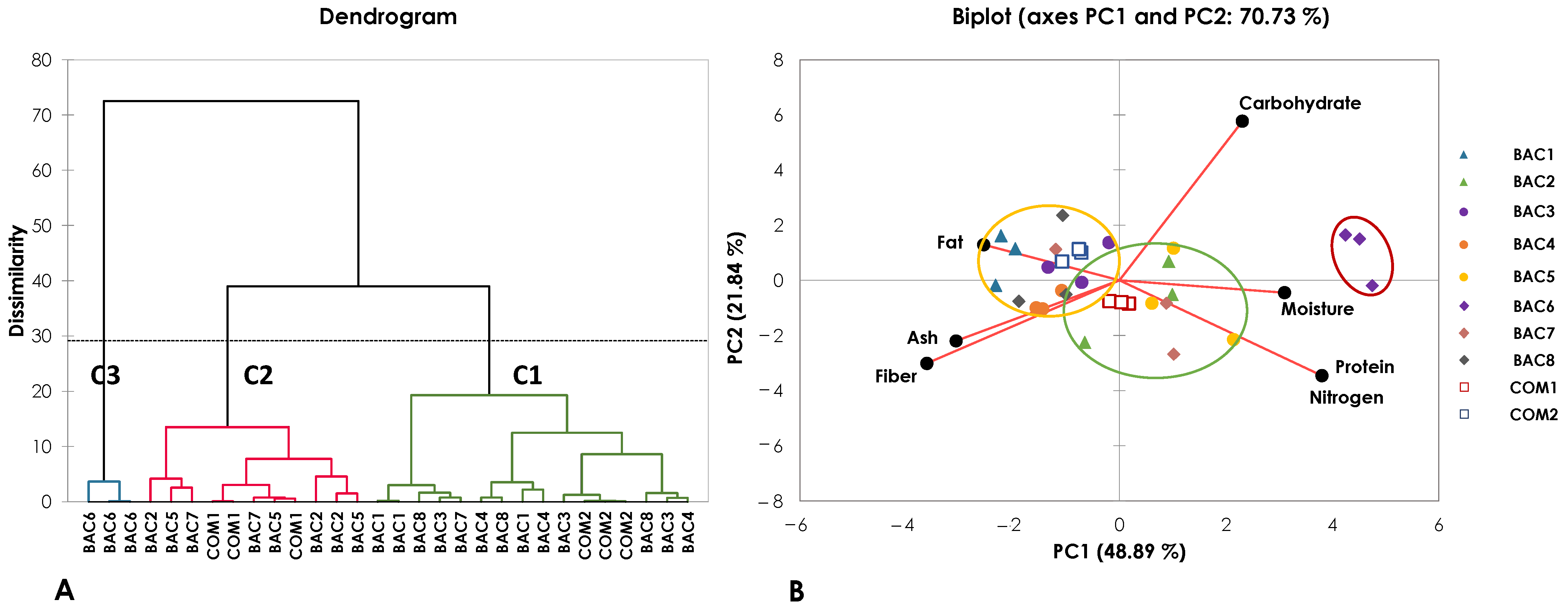
3.3. Proximate Compositions
3.4. Phytochemicals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, S.; Rashid, M.A.; Sarker, M.; Rahman, M.; Emon, N.U.; Arman, M.; Mohamed, I.N.; Haque, M.R. Antidiarrheal, antimicrobial and antioxidant potentials of methanol extract of Colocasia gigantea Hook. f. leaves: Evidenced from in vivo and in vitro studies along with computer-aided approaches. BMC Complement. Med. Ther. 2021, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. Biomed Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef] [PubMed]
- Endress, M.E.; Bruyns, P.V. A revised classification of the Apocynaceae sl. Bot. Rev. 2000, 66, 1–56. [Google Scholar] [CrossRef]
- Bhattacharyya, B.; Johri, B.M. Flowering Plants: Taxonomy and Phylogeny; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Chaikla, P. Characteristics and Varietal Phylogenetics of Peliosanthes teta Andr., Basella alba L. and Gymnema inodorum Decne. Collected from Some Areas in the Upper-North of Thailand Chiang Mai. Master’s Thesis, Graduate School, Chiang Mai University, Chiang Mai, Thailand, 2012. [Google Scholar]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Kesika, P.; Chaiyasut, K.; Sittiyuno, P.; Peerajan, S.; Sivamaruthi, B.S. Total phenolic content and free radical scavenging activity of representative medicinal plants of Thailand. Asian J. Pharm. Clin. Res. 2017, 10, 137–141. [Google Scholar] [CrossRef]
- Tiamyom, K.; Sirichaiwetchakoon, K.; Hengpratom, T.; Kupittayanant, S.; Srisawat, R.; Thaeomor, A.; Eumkeb, G. The effects of Cordyceps sinensis (Berk.) Sacc. and Gymnema inodorum (Lour.) Decne. Extracts on adipogenesis and lipase activity in vitro. Evid. -Based Complement. Altern. Med. 2019, 2019, 5370473. [Google Scholar] [CrossRef]
- Dunkhunthod, B.; Talabnin, C.; Murphy, M.; Thumanu, K.; Sittisart, P.; Eumkeb, G. Gymnema inodorum (Lour.) Decne. extract alleviates oxidative stress and inflammatory mediators produced by RAW264. 7 macrophages. Oxidative Med. Cell. Longev. 2021, 2021, 8658314. [Google Scholar] [CrossRef]
- Srinuanchai, W.; Nooin, R.; Pitchakarn, P.; Karinchai, J.; Suttisansanee, U.; Chansriniyom, C.; Jarussophon, S.; Temviriyanukul, P.; Nuchuchua, O. Inhibitory effects of Gymnema inodorum (Lour.) Decne leaf extracts and its triterpene saponin on carbohydrate digestion and intestinal glucose absorption. J. Ethnopharmacol. 2021, 266, 113398. [Google Scholar] [CrossRef]
- Sritontip, P.; Sritontip, C.; Khunsupa, N.; Thongdeepan, N.; Kantiwong, J. Study on Yield, Cost and Return of Gymnema inodorum (Lour.) Decne. Production in Model Community for Thailand Resources Conservation. Agric. Sci. J. 2018, 49 (Suppl. S1), 80–84. [Google Scholar]
- Pandey, A. Cultivation Technique of an Important Medicinal Plant Gymnema sylvestre R. Br. (Gurmar). Acad. J. Plant Sci. 2012, 05, 84–89. [Google Scholar] [CrossRef]
- Boonyapranai, K.; Surinkaew, S.; Somsak, V.; Rattanatham, R. Protective effects of Gymnema inodorum leaf extract on Plasmodium berghei-induced hypoglycemia, dyslipidemia, liver damage, and acute kidney injury in experimental mice. J. Parasitol. Res. 2021, 2021, 1896997. [Google Scholar] [CrossRef] [PubMed]
- Jeytawan, N.; Yadoung, S.; Jeeno, P.; Yana, P.; Sutan, K.; Naksen, W.; Wongkaew, M.; Sommano, S.R.; Hongsibsong, S. Antioxidant and Phytochemical Potential of and Phytochemicals in Gymnema inodorum (Lour.) Decne in Northern Thailand. Plants 2022, 11, 3498. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Li, Z.; Fu, J.; Wang, Y.; Xue, C. Hpyerglycemic effect of a mixture of sea cucumber and cordyceps sinensis in streptozotocin-induced diabetic rat. J. Ocean Univ. China 2014, 13, 271–277. [Google Scholar] [CrossRef]
- Rattanasombat, S.; Sangwichien, C. Saponin extraction from Gymnema inodorum Decne. using ultrasound extraction technique. In Proceedings of the TIChe International Conference, Hatyai, Thailand, 10–11 November 2011. [Google Scholar]
- Wankakeaw, K.; Khunsupa, N.; Sritontip, C.; Sritontip, P. Influence of various nitrogen on water consumption, growth and edible portion of Gymnema inodorum (Lour.) Decne. in lysimeter-grown. J. Agric. Res. Ext. 2014, 31, 41–51. [Google Scholar]
- Sekar, S.; Jayachandran, S.; Kodukkur, P.V. Idetification of gymnema species by random amplified polymorphic dna technique and chloroplast trnk gene. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 266–270. [Google Scholar]
- Onsa, N.E.; Prasad, S.K.; Chaiyaso, T.; Lumsangkul, C.; Sommano, S.R. Phenotypic and Chemotypic Relations among Local Andrographis paniculata (Burm. f.) Wall Landrace Collection. Horticulturae 2022, 8, 978. [Google Scholar] [CrossRef]
- Sriwichai, T.; Wisetkomolmat, J.; Pusadee, T.; Sringarm, K.; Duangmal, K.; Prasad, S.; Chuttong, B.; Sommano, S. Aromatic Profile Variation of Essential Oil from Dried Makwhaen Fruit and Related Species. Plants 2021, 10, 803. [Google Scholar] [CrossRef]
- Pandey, A.K.; Yadav, S. Variation in gymnemic acid content and non-destructive harvesting of Gymnema sylvestre (Gudmar). Pharmacogn. Res. 2010, 2, 309. [Google Scholar] [CrossRef]
- Watson, D.J. Comparative physiological studies on the growth of field crops: I. Variation in net assimilation rate and leaf area between species and varieties, and within and between years. Ann. Bot. 1947, 11, 41–76. [Google Scholar] [CrossRef]
- Kwanda, N.; Noikotr, K.; Sudmoon, R.; Tanee, T.; Chaveerach, A. Medicinal parasitic plants on diverse hosts with their usages and barcodes. J. Nat. Med. 2013, 67, 438–445. [Google Scholar] [CrossRef]
- Sunanta, P.; Chung, H.H.; Kunasakdakul, K.; Ruksiriwanich, W.; Jantrawut, P.; Hongsibsong, S.; Sommano, S.R. Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing. Food Sci. Nutr. 2020, 8, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Smita, N.; Keshavachandran, R. Molecular diversity in chakkarakolli (Gymnema sylvestre R. Br.) assessed through isozyme and RAPD analysis. J. Trop. Agric. 2006, 44, 31–36. [Google Scholar]
- Al-Khayri, J.M.; Mahdy, E.M.; Taha, H.S.; Eldomiaty, A.S.; Abd-Elfattah, M.A.; Abdel Latef, A.A.H.; Rezk, A.A.; Shehata, W.F.; Almaghasla, M.I.; Shalaby, T.A. Genetic and morphological diversity assessment of five kalanchoe genotypes by SCoT, ISSR and RAPD-PCR markers. Plants 2022, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.K.; Manvi, F.; Alagawadi, K.; Noolvi, M. Evaluation of anti-inflammatory activity of Gymnema sylvestre leaves extract in rats. Int. J. Green Pharm. (IJGP) 2008, 2, 114–115. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Inta, A.; Krongchai, C.; Kittiwachana, S.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R. Ethnochemometric of plants traditionally utilised as local detergents in the forest dependent culture. Saudi J. Biol. Sci. 2021, 28, 2858–2866. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists International: Rockville, MD, USA, 2000; Volume II, Chapter 39; pp. 1–27. [Google Scholar]
- Prabodhani, M.; Wansapala, J. Proximate analysis and bioactive compounds analysis of Gymnema lactiferum. Int. J. Food Sci. Nutr. 2018, 3, 191–294. [Google Scholar]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sezgin, A.C.; Artik, N. Determination of saponin content in Turkish tahini halvah by using HPLC. Adv. J. Food Sci. Technol. 2010, 2, 109–115. [Google Scholar]
- Manohar, S.H.; Naik, P.M.; Praveen, N.; Murthy, H.N. Distribution of gymnemic acid in various organs of Gymnema sylvestre. J. For. Res. 2009, 20, 268–270. [Google Scholar] [CrossRef]
- Animasaun, D. Selection of Elite Lines from Accessions of Gymnema sylvestre (Gudmar) Based on Characterization of Foliage And Gymnemic Acid Yield. Int. J. Med. Plants 2015, 108, 296–605. [Google Scholar]
- Nair, S.; Keshavachandran, R. Genetic variability of chakkarakolli (Gymnema sylvestre R. Br.) in Kerala assessed using morphological and biochemical markers. J. Trop. Agric. 2006, 44, 64–67. [Google Scholar]
- Armbruster, W.; Schwaegerle, K. Causes of covariation of phenotypic traits among populations. J. Evol. Biol. 1996, 9, 261–276. [Google Scholar] [CrossRef]
- Puttawarachai, P.; Chanrittisen, T.; Sritontip, P.; Khunsupa, N. Classification of Phak Chiang Da (Gymnema inodorum (Lour.) Decne) according to Leaf Morphology. Songklanakarin J. Plant Sci. 2016, 3, 23–29. [Google Scholar]
- Gichimu, B.M.; Omondi, C. Morphological characterization of five newly developed lines of Arabica coffee as compared to commercial cultivars in Kenya. 2010, 4, 238–246. Int. J. Plant Breed. Genet. 2010, 4, 238–247. [Google Scholar] [CrossRef]
- Bharathi, V.V.; Nalina, L.; Rajamani, K.; Ramakrishnan, P. Genetic variability, character association and path analysis in Gymnema (Gymnema sylvestre R. Br.). Med. Plants-Int. J. Phytomedicines Relat. Ind. 2018, 10, 252–255. [Google Scholar] [CrossRef]
- Mitchell-Olds, T.; Willis, J.H.; Goldstein, D.B. Which evolutionary processes influence natural genetic variation for phenotypic traits? Nat. Rev. Genet. 2007, 8, 845–856. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Zamani, Z.; Kashi, A. Genetic diversity evaluation of wild Persian shallot (Allium hirtifolium Boiss.) using morphological and RAPD markers. Sci. Hortic. 2009, 119, 345–351. [Google Scholar] [CrossRef]
- Tung, P.H.T.; Van on, T.; Huong, P.T. Assessment of Genetic Variation of the Genus Gymnema in Vietnam, Using RAPD and ITS-rDNA Markers. J. Herbs Spices Med. Plants 2022, 28, 281–292. [Google Scholar] [CrossRef]
- Shi, C.; He, C.; Zhu, J.; Chen, J. Genetic effects and genotype × environment interaction effects analysis for apparent quality traits of Indica rice. Chin. J. Rice Sci. 1999, 13, 179–182. [Google Scholar]
- Sharifi, P.; Dehghani, H.; Mumeni, A.; Moghaddam, M. Genetic and genotype × environment interaction effects for appearance quality of rice. Agric. Sci. China 2009, 8, 891–901. [Google Scholar] [CrossRef]
- Borràs-Gelonch, G.; Rebetzke, G.J.; Richards, R.A.; Romagosa, I. Genetic control of duration of pre-anthesis phases in wheat (Triticum aestivum L.) and relationships to leaf appearance, tillering, and dry matter accumulation. J. Exp. Bot. 2011, 63, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Hardy, O.J.; Maggia, L.; Bandou, E.; Breyne, P.; Caron, H.; Chevallier, M.H.; Doligez, A.; Dutech, C.; Kremer, A.; Latouche-Hallé, C. Fine-scale genetic structure and gene dispersal inferences in 10 Neotropical tree species. Mol. Ecol. 2006, 15, 559–571. [Google Scholar] [CrossRef]
- Lahbib, K.; Bnejdi, F.; El-Gazzah, M. Genetic diversity evaluation of pepper (Capsicum annuum L.) in Tunisia based on morphologic characters. Afr. J. Agric. Res. 2012, 7, 3413–3417. [Google Scholar]
- Shahnawaz, M.; Zanan, R.L.; Wakte, K.V.; Mathure, S.V.; Kad, T.D.; Deokule, S.S.; Nadaf, A.B. Genetic diversity assessment of Gymnema sylvestre (Retz.) R. Br. ex Sm. populations from Western Ghats of Maharashtra, India. Genet. Resour. Crop Evol. 2012, 59, 125–134. [Google Scholar] [CrossRef]
- Sharma, D.; Sawate, A.; Patil, B.; Kshirsagar, R. Studies on physico chemical characteristics of Gymnema sylvestre (Leaf, powder and extract). J. Pharmacogn. Phytochem. 2017, 6, 250–255. [Google Scholar]
- Indumathi, D.; Sujatha, R.; Shanmuga Sundaram, P. Evaluation of Nutrient, Mineral Analysis and Quality Characterization of Gymnema sylvestre Multi Grain Cookies for Diabetes. J. Pharm. Res. Int. 2021, 33, 638–648. [Google Scholar] [CrossRef]
- Ramiya, S.; Janarny, G.; Gunathilake, K. Development of Healthy Fiber-Rich Herbal Crackers from Whole Wheat, Finger Millet, Rice Bran, and Gymnema sylvestre Leaves. South Asian Res. J. Nat. Prod. 2022, 5, 71–89. [Google Scholar]
- Behera, S.K. Phytochemical Analysis and Antioxidant Activities of Gymnema sylvestre R. Br. Leaf Extracts. Free Radic. Antioxid. 2019, 9, 12–15. [Google Scholar] [CrossRef]
- Praveen, N.; Thiruvengadam, M.; Yang, Y.; Kim, S.; Murthy, H.; Chung, I. Production of gymnemic acid from hairy root cultures of Gymnema sylvestre R. Br. as influenced by polyunsaturated fatty acids (PUFAs) and their antioxidant activity. Ind. Crops Prod. 2014, 54, 54–61. [Google Scholar] [CrossRef]
- Sinha, S.N.; Saha, G.C.; Biswas, M. Screening of various solvent extracts of Gymnema sylvestre R. Br. leaf for antibacterial activity. Adv. Bioresearch125 2010, 28. [Google Scholar]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef]
- Ullrich, S.F.; Rothauer, A.; Hagels, H.; Kayser, O. Influence of light, temperature, and macronutrients on growth and scopolamine biosynthesis in Duboisia species. Planta Med. 2017, 83, 937–945. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Wang, D.; He, F.; Lv, Z.; Li, D. Phytochemical composition, antioxidant activity and HPLC fingerprinting profiles of three Pyrola species from different regions. PLoS ONE 2014, 9, e96329. [Google Scholar] [CrossRef]
- Verma, A.K.; Dhawan, S.S.; Singh, S.; Bharati, K.A. Genetic and chemical profiling of Gymnema sylvestre accessions from central India: Its implication for quality control and therapeutic potential of plant. Pharmacogn. Mag. 2016, 12, S407. [Google Scholar] [CrossRef]

| Primer Name | Sequence | Reference |
|---|---|---|
| OPA 11 | CAATCGCCGT | [25] |
| OPA 13 | CAGAACCCAC | [25] |
| OPA 14 | CTCGTGCTGG | [25] |
| OPA 15 | TTCCGAACCC | [25] |
| OPA 17 | GACCGCTTGT | [25] |
| OPA 18 | AGGTGACCGT | [25] |
| OPAH 12 | TCCAACGGCT | [25] |
| OPAH 17 | CAGTGGGGAG | [25] |
| OPE 14 | TGCGGCTGAG | [25] |
| OPE 15 | ACGCACAACC | [25] |
| OPE 17 | CTACTGCCGT | [25] |
| OPE 18 | GGACTGCAGA | [25] |
| OPF 13 | GGCTGCAGAA | [25] |
| OPF 14 | TGCTGCAGGT | [25] |
| OPF 19 | CCTCTACACC | [25] |
| Collection Number | Accession Number | Appearances | Description | General Characteristics | Picture | Tip Length (cm) | Stem Diameter (mm) | Leaf Width (cm) | Leaf Length (cm) | Number of Leaf Pair (Pair) |
|---|---|---|---|---|---|---|---|---|---|---|
| CM014 | BAC1 |  | Woody climbing shrub, dark green leaves, oval shape, cuspidate leaf apex, obtuse leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Ovate Leaf apex: Cuspidate Leaf base: Obtuse |  | 33.50 ± 6.07 ab | 4.52 ± 0.22 abc | 5.60 ± 0.15 abc | 10.70 ± 0.47 ab | 5.35 ± 0.24 ab |
| CM016 | BAC2 |  | Woody climbing shrub, light green leaves, elliptic shape, cuspidate leaf apex, rounded leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Elliptic Leaf apex: Cuspidate Leaf base: Rounded |  | 49.78 ± 33.35 ab | 5.06 ± 0.53 a | 6.26 ± 0.61 abc | 12.48 ± 1.44 a | 6.24 ± 0.72 a |
| CM025 | BAC3 |  | Woody climbing shrub, dark green leaves, oval shape, cuspidate leaf apex, obtuse leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Ovate Leaf apex: Cuspidate Leaf base: Obtuse |  | 17.30 ± 12.74 b | 3.79 ± 0.72 bc | 4.80 ± 1.82 c | 7.24 ± 2.36 b | 3.62 ± 1.18 b |
| CM058 | BAC4 |  | Woody climbing shrub, light green leaves, oval shape, cuspidate leaf apex, obtuse leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Ovate Leaf apex: Cuspidate Leaf base: Obtuse |  | 30.60 ± 20.36 ab | 4.49 ± 0.76 abc | 6.94 ± 1.32 a | 10.78 ± 1.95 a | 5.39 ± 0.98 a |
| CM064 | BAC5 |  | Woody climbing shrub, dark green leaves, oval shape, cuspidate leaf apex, rounded leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Ovate Leaf apex: Cuspidate Leaf base: Rounded |  | 18.20 ± 3.83 ab | 3.82 ± 0.77 bc | 5.42 ± 0.94 abc | 9.42 ± 2.17 ab | 4.71 ± 1.09 ab |
| PR049 | BAC6 |  | Woody climbing shrub, dark green leaves, elliptic shape, cuspidate leaf apex, obtuse leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Elliptic Leaf apex: Cuspidate Leaf base: Obtuse |  | 15.26 ± 2.76 b | 3.65 ± 0.49 c | 5.06 ± 1.17 bc | 10.48 ± 1.71 a | 5.24 ± 0.85 a |
| PY005 | BAC7 |  | Woody climbing shrub, dark green leaves, oval shape, cuspidate leaf apex, rounded leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Ovate Leaf apex: Cuspidate Leaf base: Rounded |  | 50.40 ± 40.84 ab | 4.28 ± 0.91 abc | 5.64 ± 1.52 abc | 11.16 ± 3.21 a | 5.58 ± 1.60 a |
| N018 | BAC8 |  | Woody climbing shrub, dark green leaves, oval shape, cuspidate leaf apex, rounded leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Ovate Leaf apex: Cuspidate Leaf base: Rounded |  | 42.48 ± 34.82 ab | 3.97 ± 0.75 bc | 6.68 ± 1.03 ab | 10.46 ± 0.23 a | 5.23 ± 0.12 a |
| LP004 | COM1 |  | Woody climbing shrub, light green leaves, oval shape, cuspidate leaf apex, obtuse leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Ovate Leaf apex: Cuspidate Leaf base: Obtuse |  | 18.80 ± 7.20 ab | 4.20 ± 0.93 abc | 5.58 ± 1.28 abc | 9.92 ± 3.75 ab | 4.96 ± 1.87 ab |
| LP006 | COM2 |  | Woody climbing shrub, dark green leaves, oval shape, cuspidate leaf apex, rounded leaf base, umbel inflorescence, green calyx, yellow petals, single, round, long fruit. | Leaf shape: Ovate Leaf apex: Cuspidate Leaf base: Rounded |  | 55.50 ± 42.59 a | 4.75 ± 0.91 ab | 5.98 ± 0.52 abc | 11.06 ± 0.68 a | 5.53 ± 0.34 a |
| Specimen Number | BAC1 | BAC2 | BAC3 | BAC4 | BAC5 | BAC6 | BAC7 | BAC8 | COM1 | COM2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Nitrogen (%) | 3.27 ± 0.11c | 3.84 ± 0.21ab | 3.39 ± 0.12bc | 3.48 ± 0.08bc | 3.86 ± 0.52ab | 4.27 ± 0.28a | 3.76 ± 0.39b | 3.43 ± 0.17bc | 3.72 ± 0.02bc | 3.27 ± 0.01c |
| Moisture (%) | 7.37 ± 0.22c | 8.41 ± 0.53ab | 7.95 ± 0.34bc | 8.25 ± 0.25ab | 8.89 ± 0.64a | 8.94 ± 0.24a | 8.55 ± 0.73ab | 7.39 ± 0.42c | 8.69 ± 0.03ab | 8.43 ± 0.06ab |
| Protein (%) | 20.42 ± 0.71c | 23.99 ± 1.29ab | 21.21 ± 0.76bc | 21.76 ± 0.50bc | 24.15 ± 3.24ab | 26.70 ± 1.73a | 23.48 ± 2.42b | 21.46 ± 1.04bc | 23.23 ± 0.11bc | 20.46 ± 0.09c |
| Fat (%) | 5.69 ± 0.37a | 3.43 ± 1.11ab | 2.87 ± 1.71b | 3.12 ± 1.17ab | 2.77 ± 1.11b | 1.49 ± 0.47b | 3.24 ± 2.05ab | 3.52 ± 2.63ab | 4.17 ± 1.13ab | 3.75 ± 0.22ab |
| Fiber (%) | 30.14 ± 0.11b | 28.05 ± 2.93b | 30.70 ± 2.99b | 31.94 ± 2.52b | 29.09 ± 1.85b | 19.75 ± 0.12a | 30.23 ± 1.87b | 30.66 ± 5.02b | 30.82 ± 0.13b | 30.53 ± 0.59b |
| Ash (%) | 2.82 ± 0.95ab | 2.97 ± 0.60ab | 2.49 ± 0.61b | 3.68 ± 1.08a | 1.93 ± 0.34bc | 1.278 ± 0.21c | 2.49 ± 0.19b | 2.86 ± 0.54ab | 2.23 ± 0.32bc | 2.33 ± 0.11bc |
| Carbohydrate (%) | 30.02 ± 1.68a | 29.31 ± 4.64a | 31.38 ± 1.34a | 27.76 ± 0.86a | 29.32 ± 4.16a | 37.57 ± 2.28b | 28.24 ± 3.43a | 30.68 ± 3.49a | 27.15 ± 0.91a | 31.23 ± 0.78a |
| Specimen Number | BAC1 | BAC2 | BAC3 | BAC4 | BAC5 | BAC6 | BAC7 | BAC8 | COM1 | COM2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Gymnemic Acid (mg/g DE) | 0.55 ± 0.03a | 0.55 ± 0.05a | 0.33 ± 0.02c | 0.57 ± 0.02a | 0.33 ± 0.02c | 0.49 ± 0.02b | 0.34 ± 0.01c | 0.55 ± 0.00a | 0.51 ± 0.01b | 0.33 ± 0.01c |
| Saponin Content (mg/g DE) | 47.58 ± 1.59bc | 55.15 ± 4.85b | 81.60 ± 1.30a | 34.50 ± 8.80d | 75.33 ± 5.04a | 51.07 ± 1.41bc | 76.91 ± 4.24a | 32.15 ± 3.44d | 43.08 ± 6.44c | 52.17 ± 4.28b |
| Total Phenolic Content (mg/g DE) | 20.19 ± 1.12ab | 18.80 ± 1.65ab | 21.16 ± 1.98a | 17.72 ± 1.37b | 18.72 ± 2.10ab | 19.34 ± 1.16ab | 18.75 ± 2.70ab | 18.44 ± 0.88ab | 20.12 ± 1.52ab | 18.86 ± 0.08ab |
| Total Flavonoid Content (mg/g DE) | 30.24 ± 2.52ab | 24.30 ± 3.20d | 27.50 ± 2.96ab | 16.54 ± 2.03e | 24.92 ± 1.56cd | 28.13 ± 2.43bc | 33.55 ± 1.12a | 16.48 ± 1.59e | 30.88 ± 0.85ab | 19.52 ± 0.71e |
| ABTS (%) | 90.82 ± 0.56ab | 90.03 ± 1.50ab | 90.25 ± 1.23ab | 88.67 ± 1.81b | 91.03 ± 0.70ab | 90.46 ± 1.28ab | 88.91 ± 1.59b | 89.74 ± 4.02ab | 92.02 ± 0.66a | 92.02 ± 0.42a |
| DPPH (%) | 88.02 ± 0.75a | 88.36 ± 0.91a | 88.65 ± 0.16a | 83.21 ± 3.74b | 85.23 ± 4.28ab | 88.56 ± 0.72a | 86.75 ± 0.83ab | 84.46 ± 2.97ab | 86.97 ± 0.75ab | 84.89 ± 2.46ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norkum ai, P.; Wongkaew, M.; Tangpao, T.; Sritontip, P.; Wongsiri, S.; Junmahasathien, T.; Lumsangkul, C.; Sommano, S.R. Relationships between Phenotypes and Chemotypic Characteristics of Local Gymnema inodorum Plants in Northern Thailand. Horticulturae 2023, 9, 484. https://doi.org/10.3390/horticulturae9040484
Norkum ai P, Wongkaew M, Tangpao T, Sritontip P, Wongsiri S, Junmahasathien T, Lumsangkul C, Sommano SR. Relationships between Phenotypes and Chemotypic Characteristics of Local Gymnema inodorum Plants in Northern Thailand. Horticulturae. 2023; 9(4):484. https://doi.org/10.3390/horticulturae9040484
Chicago/Turabian StyleNorkum ai, Pasin, Malaiporn Wongkaew, Tibet Tangpao, Parinyawadee Sritontip, Seksan Wongsiri, Taepin Junmahasathien, Chompunut Lumsangkul, and Sarana Rose Sommano. 2023. "Relationships between Phenotypes and Chemotypic Characteristics of Local Gymnema inodorum Plants in Northern Thailand" Horticulturae 9, no. 4: 484. https://doi.org/10.3390/horticulturae9040484
APA StyleNorkum ai, P., Wongkaew, M., Tangpao, T., Sritontip, P., Wongsiri, S., Junmahasathien, T., Lumsangkul, C., & Sommano, S. R. (2023). Relationships between Phenotypes and Chemotypic Characteristics of Local Gymnema inodorum Plants in Northern Thailand. Horticulturae, 9(4), 484. https://doi.org/10.3390/horticulturae9040484









