Seaweed Extract as a Biostimulant Agent to Enhance the Fruit Growth, Yield, and Quality of Kiwifruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Materials
2.2. Experimental Design
2.3. Solution Preparation
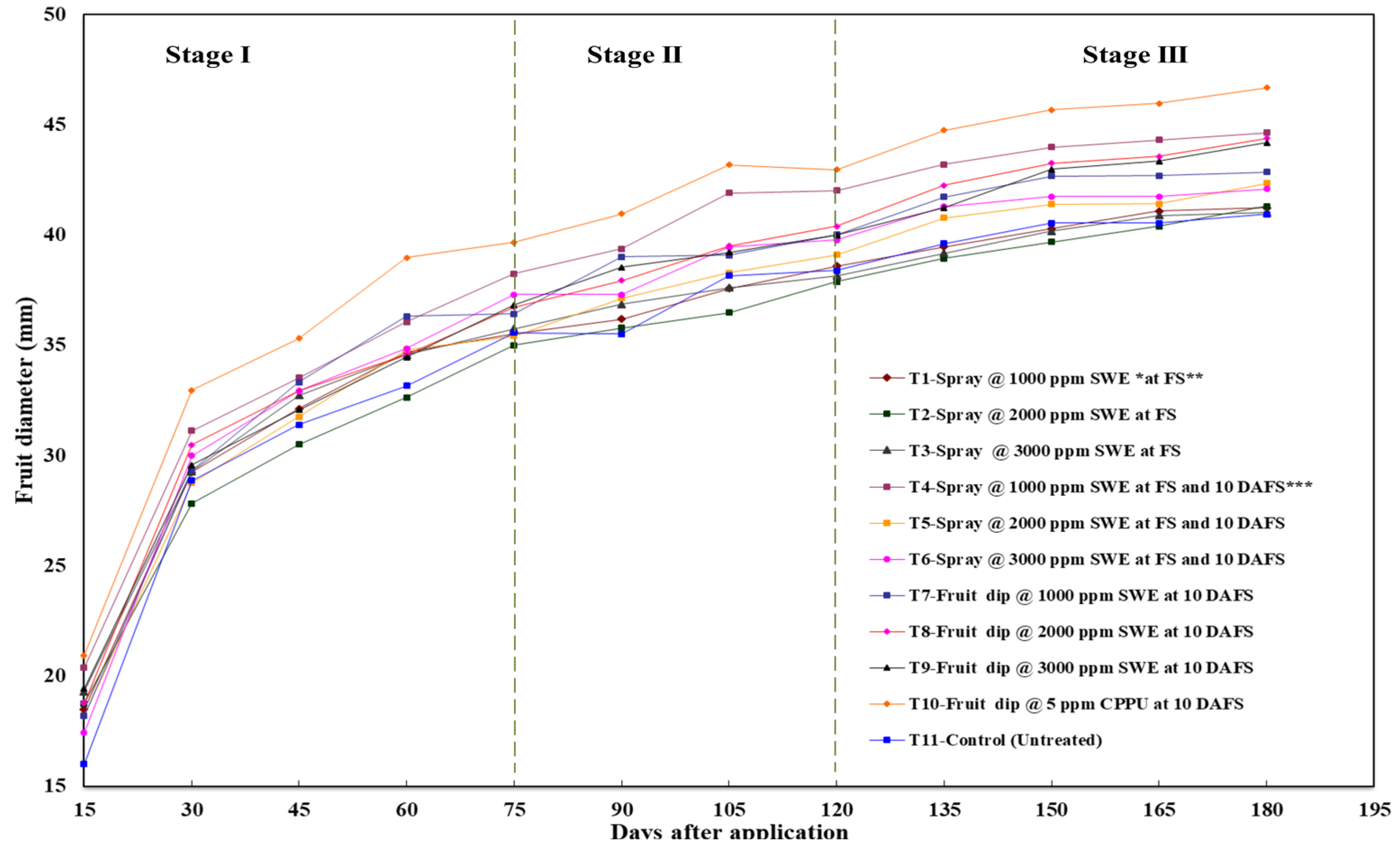
2.4. Pattern of Fruit Growth
2.5. Yield and Physical Parameters
2.6. Fruit Chemical Parameters
2.7. Physiological Loss in Weight (PLW)
2.8. Economic Analysis
2.9. Statistical Analysis
3. Results
3.1. Impact of Seaweed Extract on the Pattern of Fruit Growth
3.2. Impact of Seaweed Extract on the Fruit Yield Parameters of Kiwifruit
3.3. Effect of Seaweed Extract on Chemical Properties of Kiwifruit
3.4. Effect of Seaweed Extract on the Harvest Maturity
3.5. Effect of Seaweed Extract on Physiological Weight Loss (PLW)
3.6. Effect of Seaweed Extract on Economic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rana, V.S.; Kumar, G. Kiwifruit. In Temperate Fruits, 1st ed.; Mandal, D., Wermund, U., Phavaphutanon, L., Cronje, R., Eds.; Apple Academic Press: Waretown, NJ, USA, 2021; pp. 417–448. [Google Scholar]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.S.; Rana, N.S. Studies on Fruit Growth and Organic Metabolites in Developing Kiwifruit. Indian J. Plant Physiol. 2003, 8, 138–140. [Google Scholar]
- Garg, A.K.; Kaushal, R.; Rana, V.S. Impact of Vermicompost, Poultry Manure and Jeevaamrit on Growth Parameters of Kiwifruit (Actinidia deliciosa) cv. Allison. Int. J. Plant Soil Sci. 2020, 32, 31–40. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, V.S.; Kumari, M.; Mishra, P. Biofertilizers: Boon for Fruit Production. J. Pharmacogn. Phytochem. 2018, 7, 3244–3247. [Google Scholar]
- Hedden, P.; Hoad, G.V. Growth regulators and crop productivity. In Mechanisms of Plant Growth and Improved Productivity; CRC Press: Boca Raton, FL, USA, 2021; pp. 173–198. [Google Scholar]
- Babita; Rana, V.S. Effect of Manual Fruit Thinning and CPPU on the Fruit Yield and Changes in Physico-chemical Composition at Harvest and after Storage of Allison Kiwifruit. Int. J. Bio-Resour. Stress Manag. 2015, 6, 781–786. [Google Scholar] [CrossRef]
- Rana, V.S.; Sharma, S.; Rana, N. Sustainable production through biostimulants under fruit orchards. CABI Agric. Biosci. 2022, 3, 38. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, V.S. Energy efficiency and econometric analysis of organic kiwifruit (Actinidia deliciosa A. Chev.) Production. Bangladesh J. Bot. 2021, 50, 1051–1057. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, V.S.; Lakra, J.; Sharma, U. Appraisal of carbon capture, storage and utilization through Fruit Crops. Front. Environ. Sci. 2021, 9, 258. [Google Scholar] [CrossRef]
- Shaji, H.; Chandran, V.; Mathew, L. Organic fertilizers as a route to controlled release of nutrients. In Controlled Release Fertilizers for Sustainable Agriculture; Academic Press: New York, NY, USA, 2021; pp. 231–245. [Google Scholar]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Caedarelli, M.; Bonini, P.; Canaguier, R. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Hepworth, G.; McClintock, D.; McClinock, R. Effect of seaweed extract application on wine grape yield in Australia. J. Appl. Phycol. 2021, 33, 1883–1891. [Google Scholar] [CrossRef]
- Chapman, V.J.; Chapman, D.J. Seaweeds and Their Uses, 3rd ed.; Chapman and Hall: London, UK; New York, NY, USA, 1980; 334p. [Google Scholar]
- Mosa, W.F.; Sas-Paszt, L.; Górnik, K.; Ali, H.M.; Salem, M.Z. Vegetative growth, yield, and fruit quality of guava (Psidium guajava L.) cv. maamoura as affected by some biostimulants. Bioresources 2021, 16, 7379–7399. [Google Scholar] [CrossRef]
- Khan, A.S.; Ahmad, B.; Jaskani, M.J.; Ahmad, R.; Malik, A.U. Foliar application of mixture of amino acids and seaweed (Ascophylum nodosum) extract improve growth and physico-chemical properties of grapes. Int. J. Agric. Biol. 2012, 14, 383–388. [Google Scholar]
- Nedumaran, T.; Arulbalachandran, D. Seaweeds: A promising source for sustainable development. In Environmental Sustainability; Thangavel, P., Sridevi, G., Eds.; Springer: New Delhi, India, 2015; pp. 65–88. [Google Scholar]
- AOAC International. A.O.A.C. Official methods of analysis. In Association of Official Analytical Chemists, 20th ed.; Benjamin Franklin Station: Washington, DC, USA, 2016. [Google Scholar]
- Hayat, S. Studies on Physico-Chemical Changes during Fruit Growth, Maturity and Storage of Kiwifruit (Actinidia deliciosa Chev.) Grown in Kashmir Valley. Master’s Thesis, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir division Department of Pomology, Shalimar Campus, Shrinagar, India, 2007; 112p. [Google Scholar]
- Bangerth, F.; Schröder, M. Strong synergistic effects of gibberellins with the synthetic cytokinin N-(2-chloro-4-pyridyl)-N-phenylurea on parthenocarpic fruit set and some other fruit characteristics of apple. Plant Growth Regul. 1994, 15, 293–302. [Google Scholar] [CrossRef]
- Jao, J.P.; Chino, K.; Matsui, H.; Hirata, H. Physiological Studies of the Developing Fruit of Chinese Gooseberry (Actinidia chinensis Plank.); Technical Bulletin of the Faculty of Horticulture 43; Chiba University: Chiba, Japan, 1990; 190p. [Google Scholar]
- Lewis, D.H.; Burge, G.K.; Schmierer, D.M.; Jameson, P.E. Cytokinins and fruit development in the kiwifruit (Actinidia deliciosa) II. Effects of reduced pollination and CPPU application. Physiol. Plant. 1996, 98, 187–195. [Google Scholar] [CrossRef]
- Hameedawi, A.M.S.; Malikshah, Z.R.J. Influence of amino acids, bleed grape and seaweed extract on vegetative growth, yield and its quality of Fig. Int. J. Environ. Agric. Res. 2017, 3, 1–5. [Google Scholar]
- Pawar, R.; Rana, V.S. Manipulation of Source-Sink Relationship in Pertinence to Better Fruit Quality and Yield in Fruit Crops: A Review. Agric. Rev. 2019, 40, 200–207. [Google Scholar] [CrossRef]
- Norrie, J.; Keathley, J.P. Benefits of Ascophyllum nodosum marine plant extract marine-plant extract applications to ‘thompson seedless’ grape production. Acta Hortic. 2005, 727, 243–248. [Google Scholar]
- Miniawy, E.I.; Ragab, M.E.; Youssef, S.M.; Metwally, A.A. Influence of foliar spraying of seaweed extract on growth, yield and quality of strawberry plants. J. Appl. Sci. Res. 2014, 10, 88–94. [Google Scholar]
- Soppelsa, S.; Kelserer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production: Effects on tree growth, yield and fruit quality at harvest and during storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef]
- Ismail, M.; Wanden, M.T.; EI-Sheikh, M. Response of ‘Thompson seedless’ and ‘Roomy Red’ grape cultivars to foliar sprays with yeast extract and GA. J. Agric. Sci. 2003, 28, 6321–6334. [Google Scholar]
- Omar, A.E.D.K.; Ahmed, M.A.; Al-Saif, A.M. Influences of seaweed extract and potassium nitrate foliar application on yield and fruit quality of Date Palms (Phoenix dactylifera L.) cv. Sukary. Adv. Agric. Sci. 2017, 5, 16–22. [Google Scholar]
- Roshdy, K.A. Effect of spraying silicon and seaweed extract on growth and fruiting of grandnaine banana. Egypt. J. Agric. Res. 2014, 92, 979–991. [Google Scholar] [CrossRef]
- EI-Motty, E.Z.; Shahin, M.F.M.; EI-Shiekh, M.H.; Abd EI-Migeed, M.M.M. Effect of algae extract and yeast application on growth, nutritional status, yield and fruit quality of ‘Keitte’ mango trees. Agric. Biol. J. N. Am. 2010, 1, 421–429. [Google Scholar] [CrossRef]
- Melo, T.A.; Serra, I.M.R.S.; Sousa, A.A.; Sousa, T.Y.O.; Pascholati, S.F. Effect of Aschophyllum nodosum seaweed extract on post-harvest Tommy Atkins mangoes. Rev. Bras. Frutic. 2017, 40, 1–12. [Google Scholar]
- Patterson, K.J.; Mason, K.A.; Gould, K.S. Effects of CPPU (N-(2-chloro-4-pyridyl)-N′-phenylurea) on fruit growth, maturity, and storage quality of kiwifruit. N. Z. J. Crop Hortic. Sci. 1993, 21, 253–261. [Google Scholar] [CrossRef]
- Fornes, F.; Sanchez-Perales, M.; Guardiola, J.L. Effect of a seaweed extract on citrus fruit maturation. Acta Hortic. 2005, 379, 200–220. [Google Scholar] [CrossRef]

| Treatment Code | Treatment Details | Date of Initiation of Flowering | Date of Full Bloom | Duration of Flowering | |||
|---|---|---|---|---|---|---|---|
| 2018–19 | 2019–20 | 2018–19 | 2019–20 | 2018–19 | 2019–20 | ||
| T1 | 1000 ppm SWE spray at FS | 25/04 | 01/05 | 28/04 | 04/05 | 10 | 11 |
| T2 | 2000 ppm SWE spray at FS | 24/04 | 28/04 | 27/04 | 01/05 | 09 | 11 |
| T3 | 3000 ppm SWE spray at FS | 26/04 | 02/05 | 30/04 | 06/05 | 11 | 10 |
| T4 | 1000 ppm SWE spray at FS and 10 DAFS | 25/04 | 04/05 | 28/04 | 09/05 | 10 | 10 |
| T5 | 2000 ppm SWE spray at FS and 10 DAFS | 23/04 | 03/05 | 27/04 | 06/05 | 09 | 10 |
| T6 | 3000 ppm SWE spray at FS and 10 DAFS | 26/04 | 30/04 | 30/04 | 05/05 | 09 | 10 |
| T7 | 1000 ppm SWE dip at 10 DAFS | 24/04 | 29/04 | 26/04 | 03/05 | 11 | 08 |
| T8 | 2000 ppm SWE dip at 10 DAFS | 26/04 | 01/05 | 29/04 | 06/05 | 11 | 09 |
| T9 | 3000 ppm SWE dip at 10 DAFS | 26/04 | 04/05 | 28/04 | 06/05 | 11 | 09 |
| T10 | 5 ppm CPPU dip at 10 DAFS | 27/04 | 03/05 | 30/04 | 06/05 | 10 | 10 |
| T11 | Control (Untreated) | 23/04 | 30/04 | 26/04 | 08/05 | 09 | 10 |
| Code | Treatment Details | Total (kg/Vine) | Yield T/ha (%Increase in Yield over Control) | Graded Yield | Shape Index | Fruit Weight (g/Fruit) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| A (kg) | B (kg) | C (kg) | Fruit Length (cm) | Fruit Diameter (cm) | L/D Ratio | |||||
| T1 | 1000 ppm SWE spray at FS | 25.15 gh | 10.46 g (1.9) | 4.53 h | 8.94 fg | 11.68 b | 5.98 g | 4.10 ef | 1.45 | 52.06 h |
| T2 | 2000 ppm SWE spray at FS | 26.53 fg | 11.03 f (7.49) | 5.13 h | 16.70 bc | 4.70 e | 6.29 ef | 4.13 def | 1.52 | 58.10 f |
| T3 | 3000 ppm SWE spray at FS | 30.10 d | 12.55 d (22.32) | 8.04 f | 17.06 b | 5.09 cd | 6.09 fg | 4.07 f | 1.50 | 55.29 g |
| T4 | 1000 ppm SWE spray at FS and 10 DAFS | 27.39 ef | 11.36 ef (10.65) | 6.97 g | 15.70 d | 4.64 ef | 6.42 de | 4.30 bcd | 1.49 | 61.66 e |
| T5 | 2000 ppm SWE spray at FS and 10 DAFS | 28.14 e | 11.71 e (14.05) | 7.17 g | 15.63 d | 5.35 c | 6.54 cd | 4.26 cde | 1.49 | 63.75 de |
| T6 | 3000 ppm SWE spray at FS and 10 DAFS | 31.45 cd | 13.08 c (27.43) | 9.36 e | 18.75 a | 3.34 g | 6.35 de | 4.26 cde | 1.53 | 64.57 d |
| T7 | 1000 ppm SWE dip at 10 DAFS | 31.40 cd | 13.06 c (27.22) | 10.60 d | 15.90 cd | 4.90 de | 6.55 cd | 4.29 bcd | 1.52 | 62.66 de |
| T8 | 2000 ppm SWE dip at 10 DAFS | 32.50 bc | 13.52 bc (31.68) | 16.67 c | 11.47 e | 4.36 f | 6.70 c | 4.45 ab | 1.50 | 75.50 c |
| T9 | 3000 ppm SWE dip at 10 DAFS | 33.03 b | 13.86 b (35.01) | 19.11 b | 9.55 f | 4.66 ef | 7.05 b | 4.41 bc | 1.59 | 80.66 b |
| T10 | 5 ppm CPPU dip at10 DAFS | 35.46 a | 14.75 a (43.67) | 25.91 a | 6.04 h | 3.51 g | 7.41 a | 4.63 a | 1.60 | 84.50 a |
| T11 | Control (Untreated) | 24.68 h | 10.26 g | 2.52 i | 8.59 g | 13.57 a | 5.73 h | 4.05 f | 1.40 | 47.77 i |
| Significance | *** | *** | *** | *** | *** | *** | *** | NS | *** | |
| Code | Treatment Details | SSC (%) | TA (%) | SSC: Acid Ratio | Sugars Content (%) | AA (mg/100 g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A1 | A2 | A1 | A2 | Total (%) | Reducing (%) | Non-Reducing (%) | |||
| T1 | 1000 ppm SWE spray at FS | 11.82 cd | 16.10 b | 1.32 b | 0.88 d | 9.04 f | 18.23 c | 9.96 de | 7.86 d | 1.99 | 77.25 d |
| T2 | 2000 ppm SWE spray at FS | 11.72 d | 16.40 b | 1.26 c | 0.81 e | 9.30 ef | 20.28 ab | 10.07 de | 8.12 cd | 1.85 | 78.67 cd |
| T3 | 3000 ppm SWE spray at FS | 12.23 c | 16.20 b | 1.25 c | 0.88 d | 9.87 d | 18.28 c | 10.31 cd | 8.25 c | 1.95 | 79.15 bcd |
| T4 | 1000 ppm SWE spray at FS and 10 DAFS | 11.9 cd | 15.20 c | 1.24 cd | 0.95 c | 9.59 de | 16.01 e | 10.16 cde | 7.95 cd | 2.10 | 80.64 abc |
| T5 | 2000 ppm SWE spray at FS and 10 DAFS | 12.81 b | 15.17 c | 1.21 cde | 0.94 c | 10.58 c | 16.08 e | 10.29 cd | 8.18 cd | 1.99 | 81.31 abc |
| T6 | 3000 ppm SWE spray at FS and 10 DAFS | 13.37 a | 16.19 b | 1.13 fg | 0.79 e | 11.87 a | 20.60 a | 11.21 a | 9.15 a | 2.16 | 81.82 ab |
| T7 | 1000 ppm SWE dip at 10 DAFS | 12.96 ab | 15.96 b | 1.18 def | 0.81 e | 11.02 b | 19.64 b | 10.55 bc | 8.33 c | 2.11 | 80.62 abc |
| T8 | 2000 ppm SWE dip at 10 DAFS | 12.68 b | 16.30 b | 1.16 efg | 0.81 e | 10.97 bc | 20.05 ab | 10.84 ab | 8.75 b | 1.99 | 82.82 a |
| T9 | 3000 ppm SWE dip at 10 DAFS | 13.05 ab | 16.10 b | 1.12 g | 0.78 e | 11.94 a | 20.42 ab | 11.26 a | 9.24 a | 1.93 | 81.18 abc |
| T10 | 5 ppm CPPU dip at10 DAFS | 11.99 cd | 17.59 a | 1.27 bc | 1.02 b | 9.47 de | 17.31 d | 10.06 de | 8.04 cd | 1.91 | 80.41 abc |
| T11 | Control (Untreated) | 11.74 d | 16.63 b | 1.4 a | 1.13 a | 8.43 g | 14.72 f | 9.77 e | 7.44 e | 2.22 | 78.88 bcd |
| Significance | *** | *** | *** | *** | *** | *** | *** | *** | NS | *** | |
| Code | Treatment Details | Harvest Maturity | Physiological Loss in Weight | * Net Benefit (US $.) Percent Increase in Net Benefit over the Control |
|---|---|---|---|---|
| T1 | 1000 ppm SWE spray at FS | 195.00 a | 9.89 bc | 45.37 h (8.26) |
| T2 | 2000 ppm SWE spray at FS | 192.00 ab | 9.53 c | 53.17 g (26.86) |
| T3 | 3000 ppm SWE spray at FS | 190.00 abc | 8.97 d | 61.81 e (47.48) |
| T4 | 1000 ppm SWE spray at FS and 10 DAFS | 194.00 ab | 8.32 e | 55.96 fg (33.51) |
| T5 | 2000 ppm SWE spray at FS and 10 DAFS | 193.00 ab | 8.09 ef | 57.19 f (36.46) |
| T6 | 3000 ppm SWE spray at FS and 10 DAFS | 189.00 bc | 7.68 f | 66.49 d (58.62) |
| T7 | 1000 ppm SWE dip at 10 DAFS | 193.00 ab | 6.93 g | 66.44 d (58.53) |
| T8 | 2000 ppm SWE dip at 10 DAFS | 191.00 abc | 6.58 g | 72.99 c (74.14) |
| T9 | 3000 ppm SWE dip at 10 DAFS | 190.00 abc | 6.79 g | 76.01 b (81.34) |
| T10 | 5 ppm CPPU dip at10 DAFS | 186.00 c | 12.36 a | 85.24 a (103.37) |
| T11 | Control (Untreated) | 195.00 a | 10.32 b | 41.91 i |
| Significance | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, V.S.; Sharma, V.; Sharma, S.; Rana, N.; Kumar, V.; Sharma, U.; Almutairi, K.F.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Gudeta, K. Seaweed Extract as a Biostimulant Agent to Enhance the Fruit Growth, Yield, and Quality of Kiwifruit. Horticulturae 2023, 9, 432. https://doi.org/10.3390/horticulturae9040432
Rana VS, Sharma V, Sharma S, Rana N, Kumar V, Sharma U, Almutairi KF, Avila-Quezada GD, Abd_Allah EF, Gudeta K. Seaweed Extract as a Biostimulant Agent to Enhance the Fruit Growth, Yield, and Quality of Kiwifruit. Horticulturae. 2023; 9(4):432. https://doi.org/10.3390/horticulturae9040432
Chicago/Turabian StyleRana, Vishal Singh, Varsha Sharma, Sunny Sharma, Neerja Rana, Vijay Kumar, Umesh Sharma, Khalid F. Almutairi, Graciela Dolores Avila-Quezada, Elsayed Fathi Abd_Allah, and Kasahun Gudeta. 2023. "Seaweed Extract as a Biostimulant Agent to Enhance the Fruit Growth, Yield, and Quality of Kiwifruit" Horticulturae 9, no. 4: 432. https://doi.org/10.3390/horticulturae9040432
APA StyleRana, V. S., Sharma, V., Sharma, S., Rana, N., Kumar, V., Sharma, U., Almutairi, K. F., Avila-Quezada, G. D., Abd_Allah, E. F., & Gudeta, K. (2023). Seaweed Extract as a Biostimulant Agent to Enhance the Fruit Growth, Yield, and Quality of Kiwifruit. Horticulturae, 9(4), 432. https://doi.org/10.3390/horticulturae9040432








