The Impact of Irrigation Intervals and NPK/Yeast on the Vegetative Growth Characteristics and Essential Oil Content of Lemongrass
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experimental Design
2.2. Plant Materials and Experimental Site
2.3. Vegetative Growth Characteristics
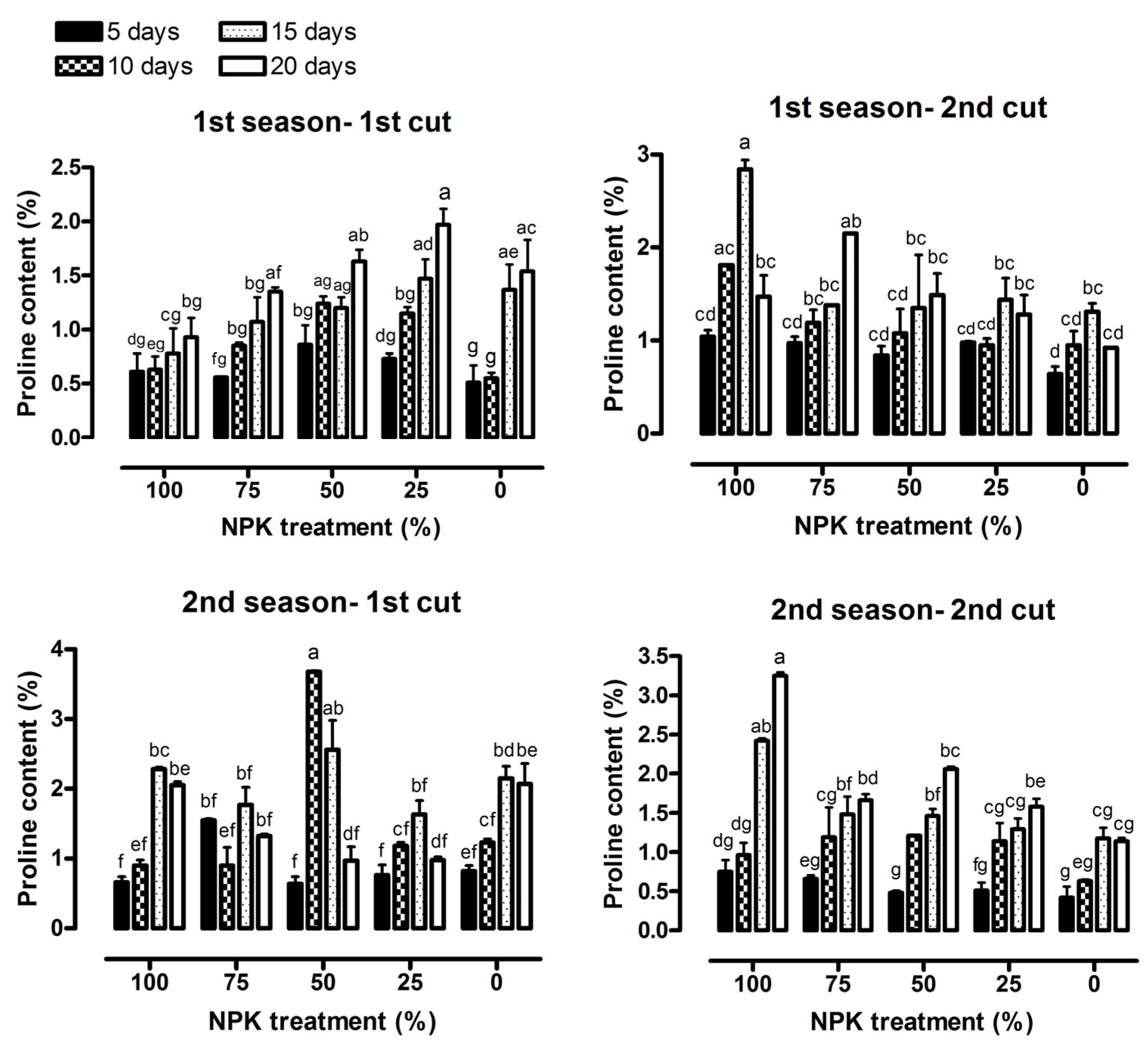
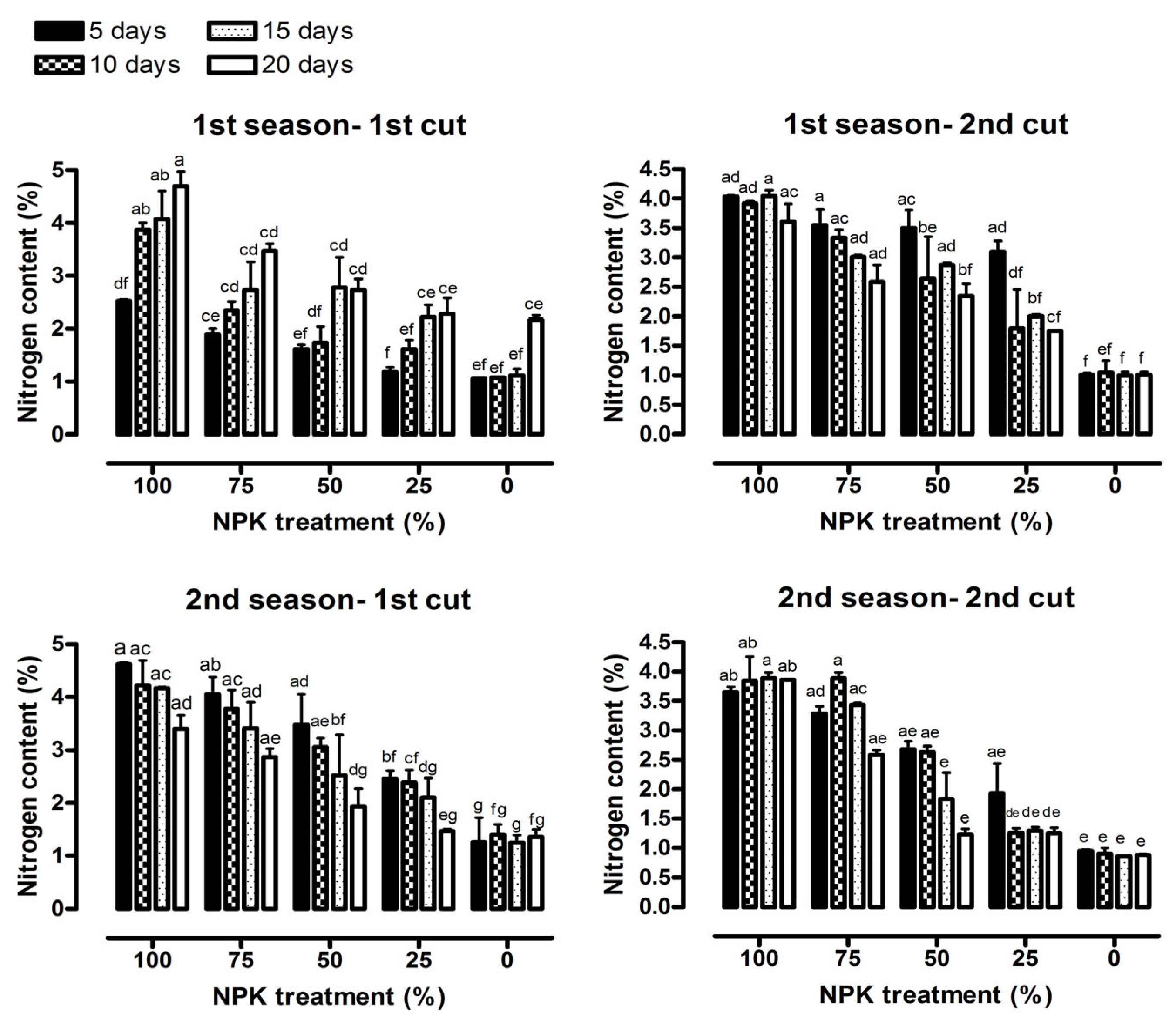
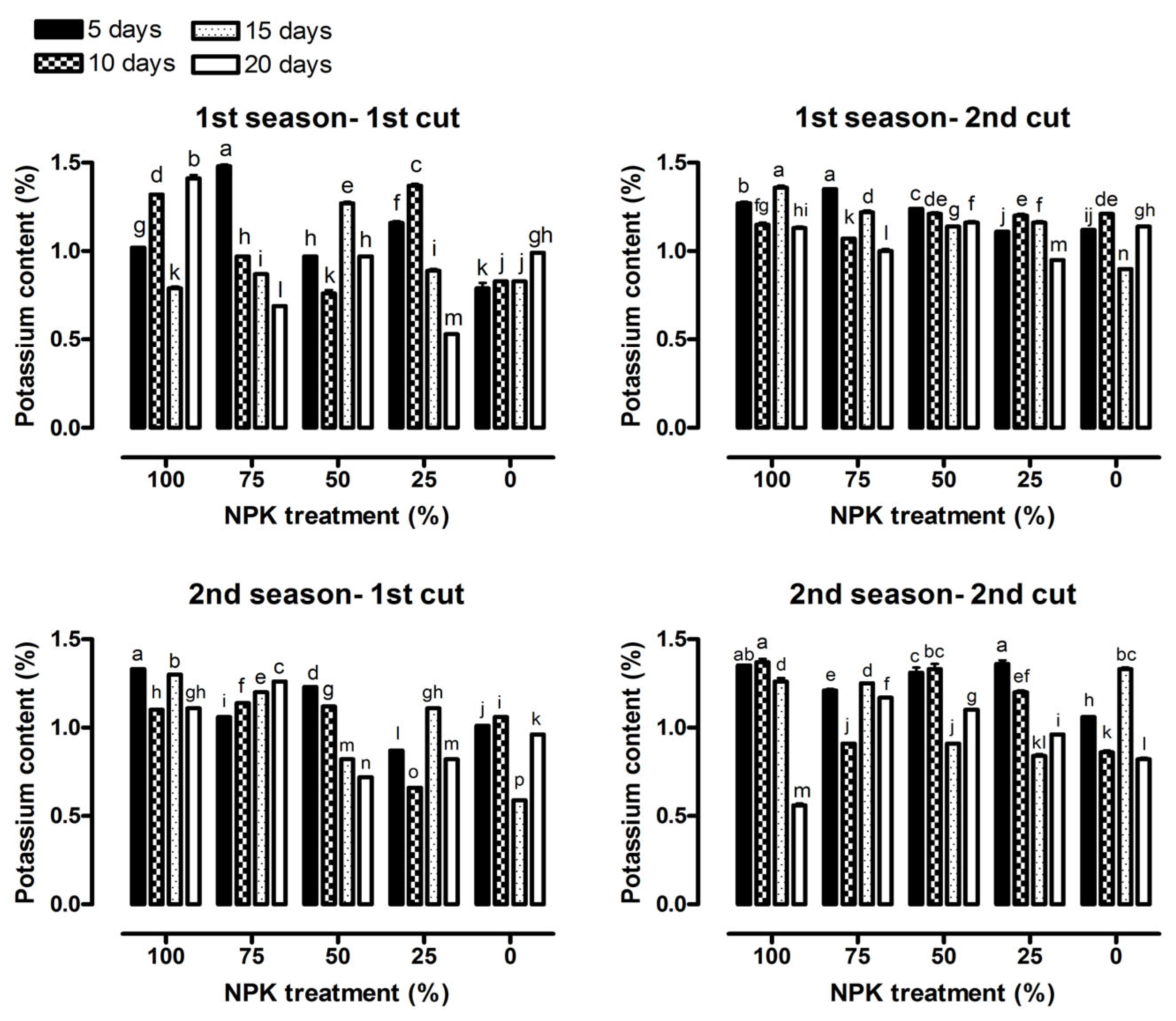
2.4. Chemical Constituents
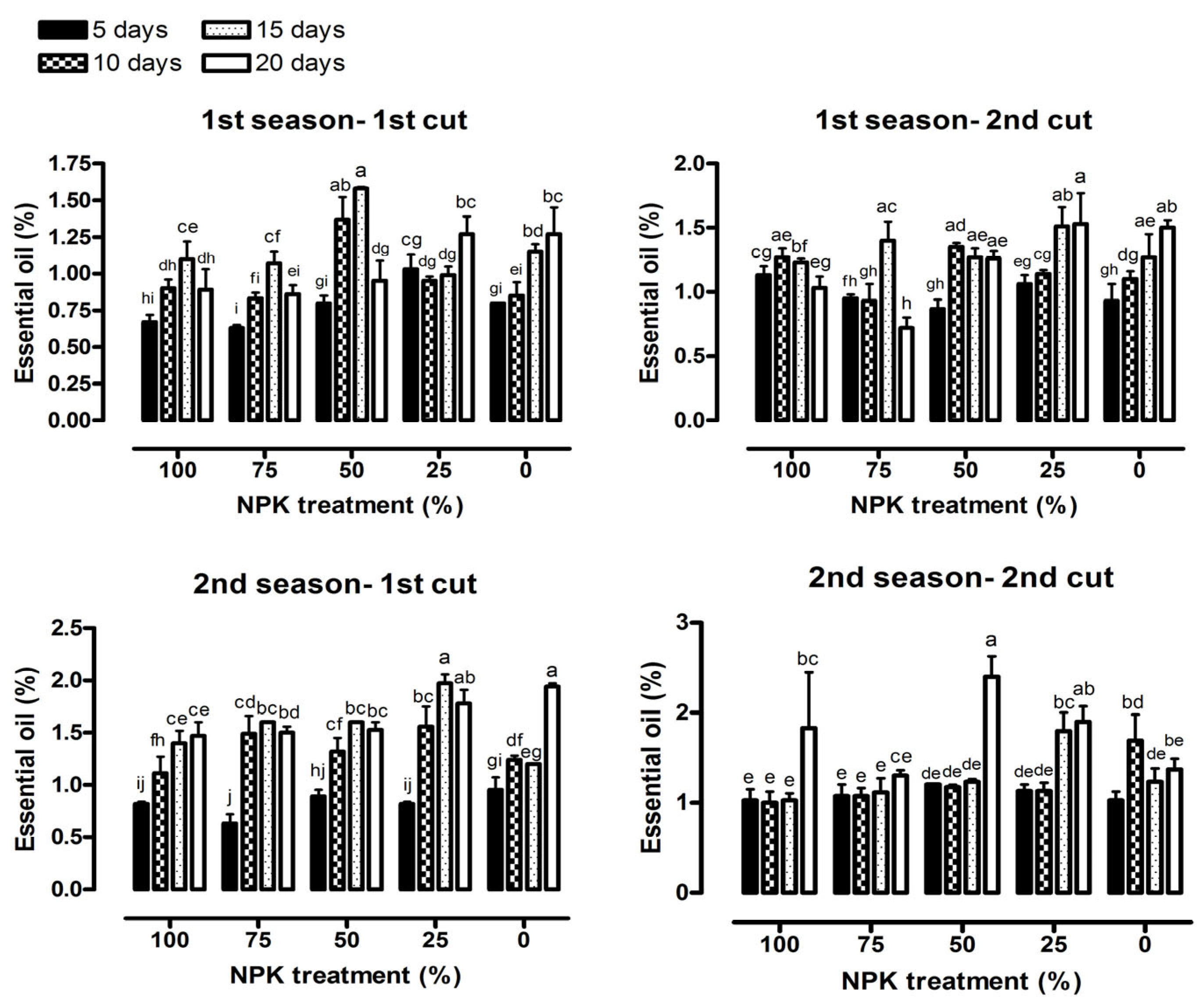
2.5. Essential Oil Percentage
2.6. Statistical Analysis
3. Results and Discussion
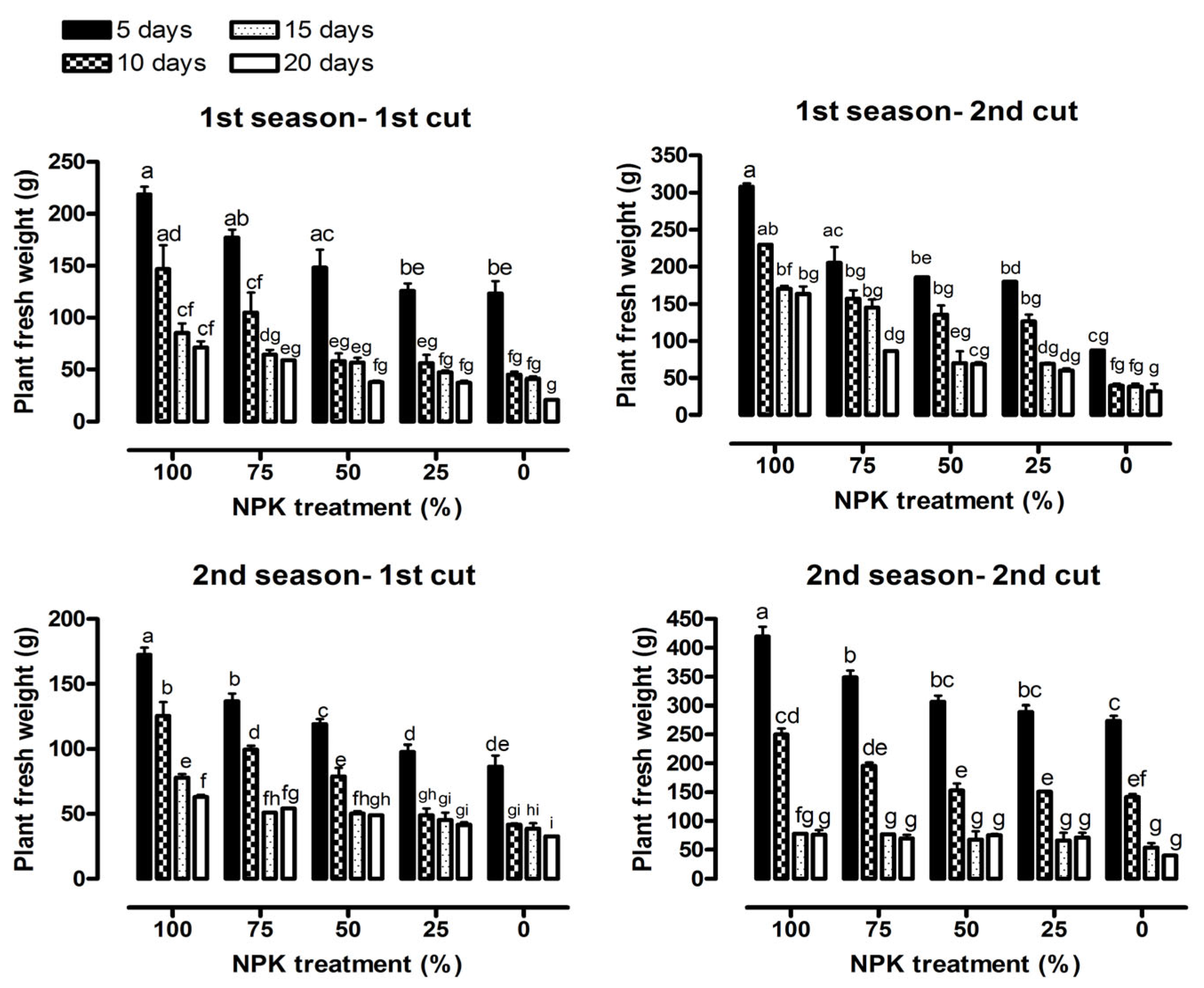
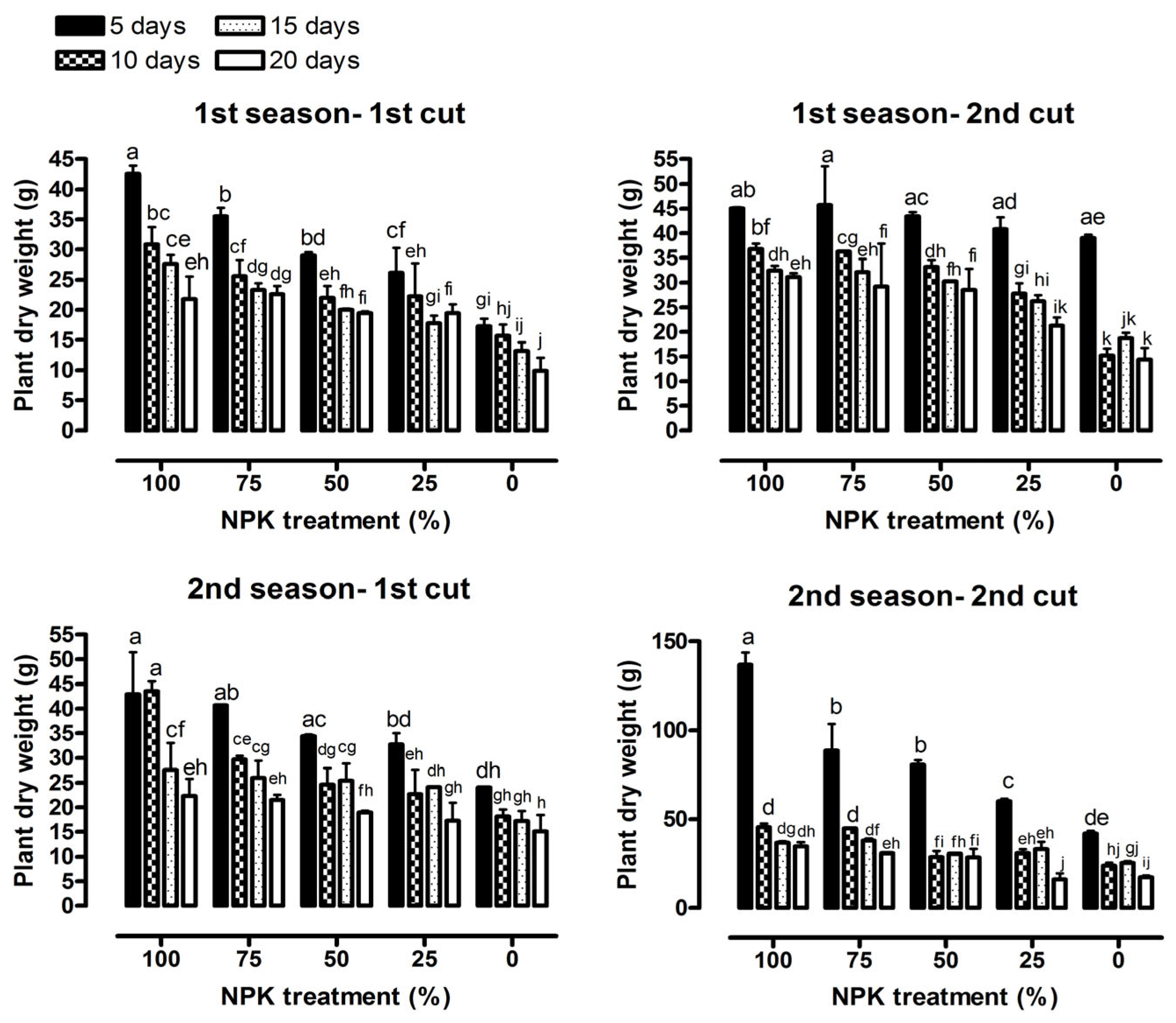
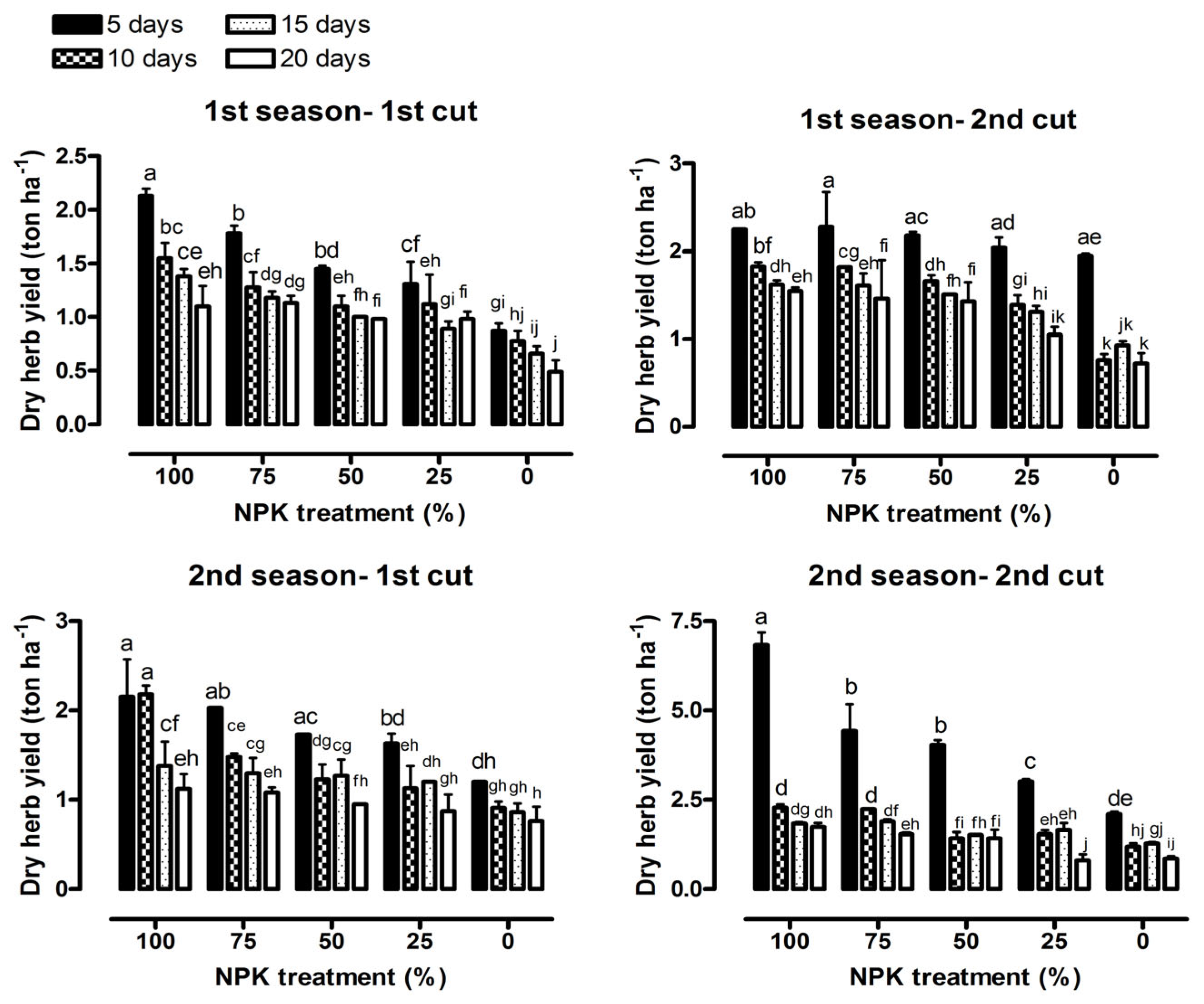
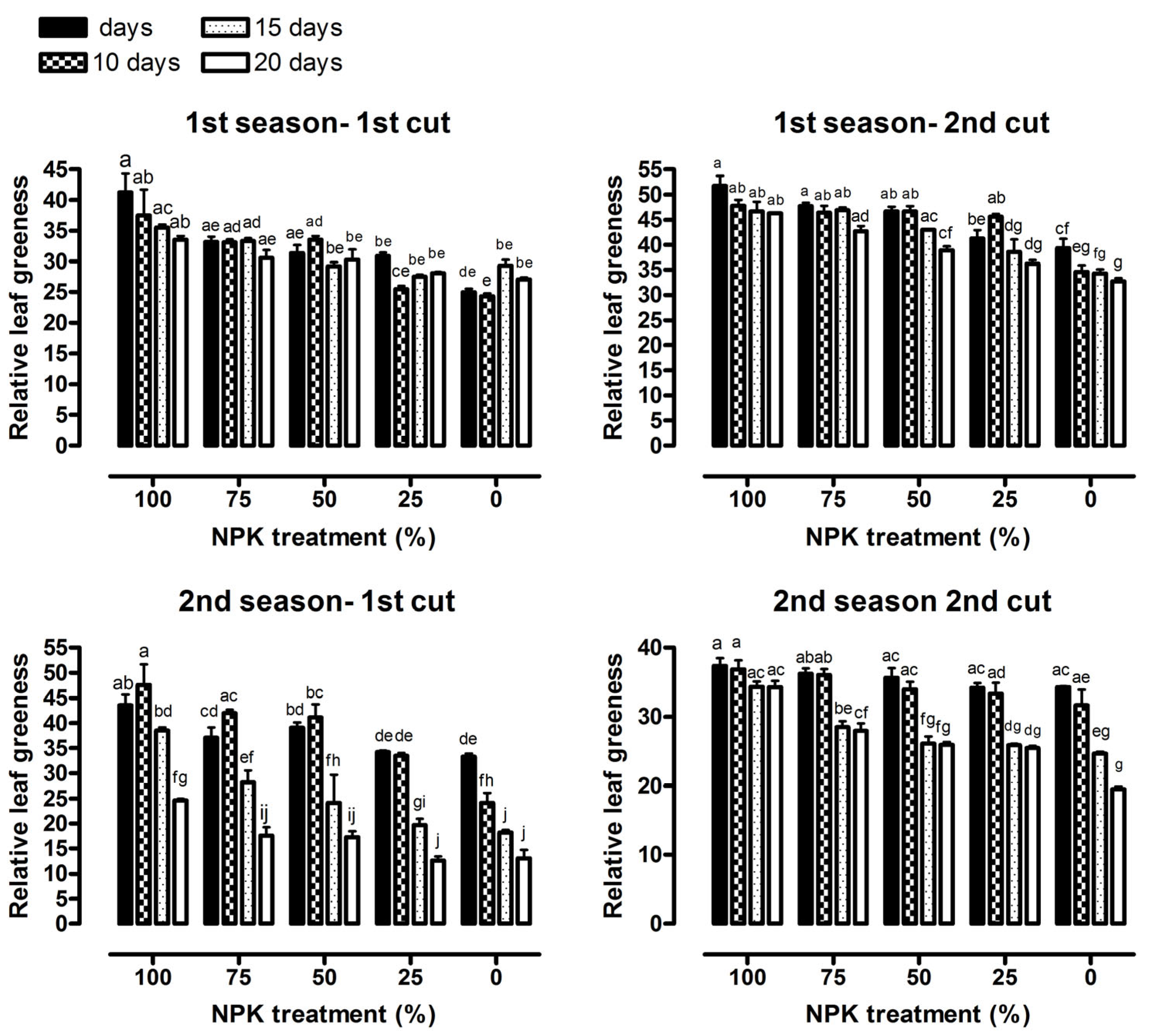
3.1. Vegetative Growth Characteristics
3.2. Chemical Constituents
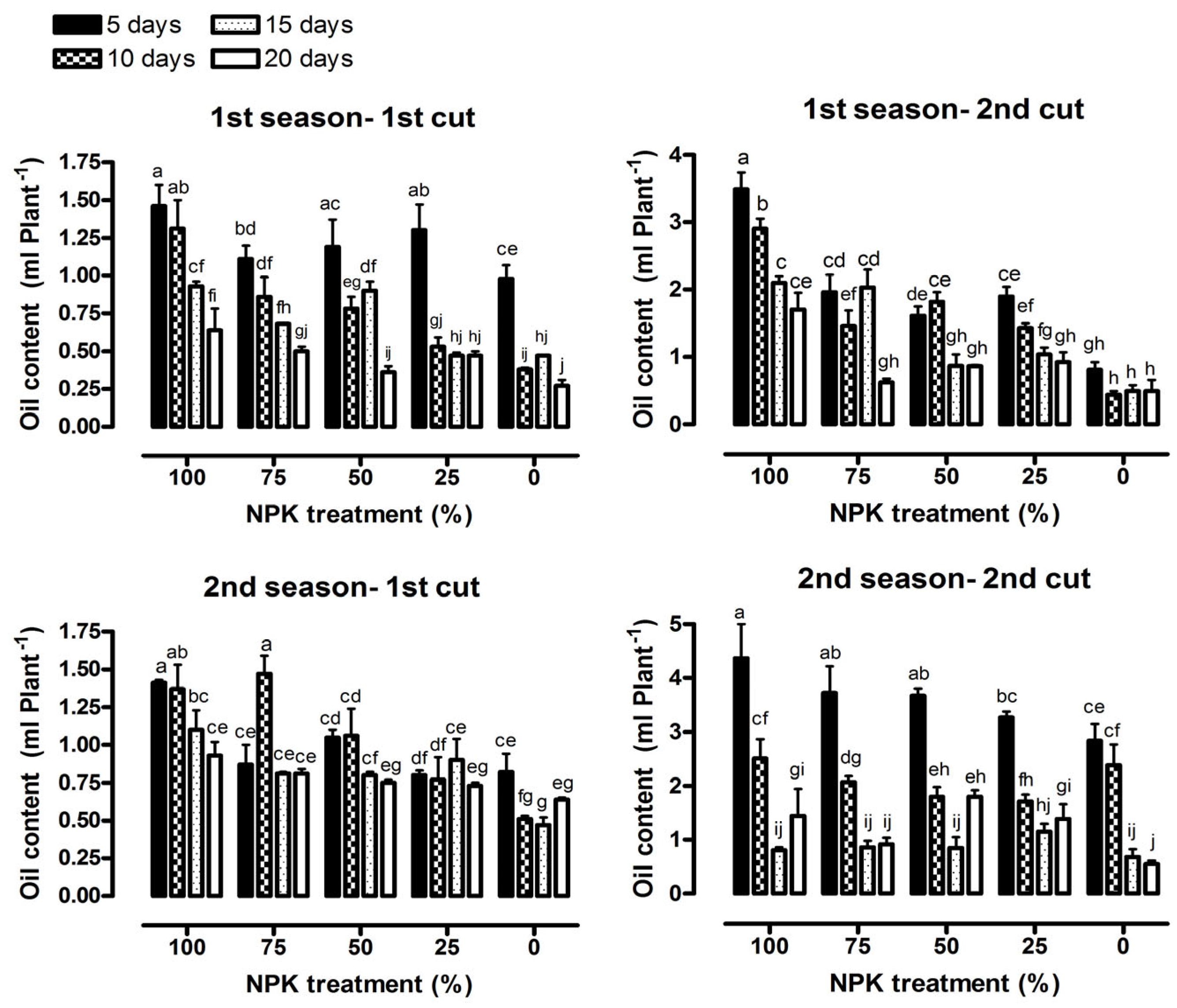
3.3. Essential Oil Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhila, A. Essential Oil-Bearing Grasses: The Genus Cymbopogon; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Chanthal, S.; Prachakoli, S.; Ruangviriyachai, C. Influence of extraction methodologies on the analysis of five major volatile aromatic compounds of citronella grass and lemongrass grown in Thailand. J. AOAC Int. 2012, 95, 763–772. [Google Scholar] [CrossRef]
- Francisco, V.; Figueirinha, A.; Neves, B.M.; García-Rodríguez, C.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Cymbopogon citratus as source of new and safe anti-inflammatory drugs: Bio-guided assay using lipo-polysaccharide-stimulated macrophages. J. Ethnopharmacol. 2011, 133, 818–827. [Google Scholar] [CrossRef]
- Soliman, W.S.; Salaheldin, S.; Amer, H.M. Chemical composition evaluation of Egyptian lemongrass, Cymbopogon citratus, essential oil. Int. J. Sci. Eng. Res. 2017, 8, 630–634. [Google Scholar]
- Carlson, L.H.C.; Machad, C.B.S.; Pereira, L.K.; Bolzan, A. Extraction of lemongrass essential oil with dense carbon dioxide. J. Supercrit. Fluids 2001, 21, 33–39. [Google Scholar] [CrossRef]
- Tavares, F.; Costa, G.; Francisco, V.; Liberal, J.; Figueirinha, A.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Cymbopogon citratus industrial waste as a potential source of bioactive compounds. J. Sci. Food and Agric. 2015, 95, 2652–2659. [Google Scholar] [CrossRef]
- Ganjewala, D. Cymbopogon essential oil composition and bio-actives. Int. J. Essent. Oil Therap. 2009, 3, 1–10. [Google Scholar]
- Alizadeh, A.; Khoshkhui, M.; Javidnia, K.; Firuzi, O.; Tafazoli, E.; Khalighi, A. Effects of fertilizer on yield, essential oil composition, total phenolic content and antioxidant activity in Satureja hortensis L. (Lamiaceae) cultivated in Iran. J. Med. Plants Res. 2010, 4, 33–40. [Google Scholar]
- Baj, T.; Kowalski, R.; ĝwiątek, À.; Modzelewska, M.; Wolski, T. Chemical composition and antioxidant activity of the essentials oil of hyssop (Hyssopus officinalis L. ssp. officinalis). Ann. Univ. Mariae Curie-Skłodowska 2010, 23, 55–61. [Google Scholar]
- Tajuddin, S.N.; Yusoff, M.M. Chemical composition of volatile oils of Aquilaria malaccensis (Thymelaeaceae) from Malaysia. Nat. Prod. Commun. 2010, 5, 1965–1968. [Google Scholar] [CrossRef]
- NurzyĔska-Wierdak, R. Sweet basil essential oil composition: Relationship between cultivar, foliar feeding with nitrogen and oil content. J. Essent. Oil Res. 2012, 24, 217–227. [Google Scholar] [CrossRef]
- Paviani, L.; Pergher, S.B.C.; Dariva, C. Application of molecular sieves in the fractionation of lemongrass oil from high-pressure carbon dioxide extraction. Braz. J. Chem. Eng. 2006, 23, 219–225. [Google Scholar] [CrossRef]
- Tajidin, N.E. Chemical composition and citral content in lemongrass (Cymbopogon citratus) essential oil at three maturity stages. Afr. J. Biotechnol. 2012, 11, 2685–2693. [Google Scholar] [CrossRef]
- Aziz, E.E.; El-Danasoury, M.M.; Craker, L.E. Impact of sulphur and ammonium sulphate on dragonhead plants grown in newly reclaimed soil. J. Herbs Spices Med. Plants 2010, 16, 126–135. [Google Scholar] [CrossRef]
- Jabbari, R.; Dehaghi, M.A.; Sanavi, A.M.M.; Agahi, K. Nitrogen and iron fertilization methods affecting essential oil and chemical composition of thyme (Thymus vulgaris L.) medical plant. Adv. Environ. Biol. 2011, 5, 433–438. [Google Scholar]
- Sharafzadeh, S.; Esmaeilli, M.; Mohammadi, A.H. Interaction effects of nitrogen, phosphorus and potassium on growth, essential oil and total phenolic content of sweet basil. Adv. Environ. Biol. 2011, 5, 1285–1289. [Google Scholar]
- Zheljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Cannon, J.B. Lemongrass productivity, oil content, and composition as a function of nitrogen, sulfur, and harvest time. Agron. J. 2011, 103, 805–812. [Google Scholar] [CrossRef]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Hirt, H.; Shinozaki, K. Plant Responses to Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Salehi-lisar, S.Y.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I.M.M. Water Stress in Plants: Causes, Effects and Responses, Water Stress; Rahman, I.M.M., Ed.; InTech: Vienna, Austria, 2012. [Google Scholar]
- Madhava Rao, K.V.; Raghavendra, A.S.; Janardhan Reddy, K. Physiology and Molecular Biology of Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Slaughter, A.; Daniel, X.; Flors, V.; Luna, E.; Hohn, B.; Mauch-Mani, B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 2012, 158, 835–843. [Google Scholar] [CrossRef]
- Cheng, L.; Han, M.; Yang, L.-m.; Li, Y.; Sun, Z.; Zhang, T. Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind. Crops Prod. 2018, 122, 473–482. [Google Scholar] [CrossRef]
- Khademian, R.; Yaghoubian, I. Growth of chick pea (Cicer arietinum) in response to salicylic acid under drought stress. J. Biodiv. Environ. Sci. 2018, 12, 255–263. [Google Scholar]
- Pintó-Marijuan, M.; Cotado, A.; Fleta-Soriano, E.; Munné-Bosch, S. Drought stress memory in the photosynthetic mechanisms of an invasive CAM species, Aptenia cordifolia. Photosynth. Res. 2017, 131, 241–253. [Google Scholar] [CrossRef]
- Kulak, M. Recurrent drought stress effects on essential oil profile of Lamiaceae plants: An approach regarding stress memory. Ind. Crops Prod. 2020, 154, 112695. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Bansal, M. Organic farming: Is it a solution to safe food? In Food Safety in the 21st Century; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Sharma, R.C.; Sarkar, S.; Das, D.; Banik, P. Impact assessment of arbuscular mycorrhiza Azospirillum and chemical fertilizer application on soil health and ecology. Commun. Soil Sci. Plant Anal. 2013, 44, 1116–1126. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Kuklinsky-Sobral, J.; Araujo, W.L.; Mendes, R.; Geraldi, I.O.; Pizzirani-Kleiner, A.A.; Azevedo, J.L. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 2004, 6, 1244–1251. [Google Scholar] [CrossRef]
- Lonhienne, T.; Mason, M.G.; Ragan, M.A.; Hugenholtz, P.; Schmidt, S.; Paungfoo-Lonhienne, C. Yeast as a biofertilizer alters plant growth and morphology. Crop Sci. 2014, 5, 785–790. [Google Scholar] [CrossRef]
- Xi, Q.; Lai, W.; Cui, Y.; Wu, H.; Zhao, T. Effect of yeast extract on seedling growth promotion and soil improvement in afforestation in a semiarid chestnut soil area. Forests 2019, 10, 76. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Bi, H.J. Research on yeast extract. China Condiment 2000, 2, 20–23. [Google Scholar]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.; Ferreira, I. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Fawzy, Z.F. Increasing productivity of head lettuce by foliar spraying of some bio- and organic compounds. J. Appl. Sci. 2007, 22, 237–240. [Google Scholar]
- El-Tohamy, W.A.; El-Abagy, H.M.; El-Greadly, N.H.M. Studies on the effect of putrescine, yeast and vitamin C on growth, yield and physiological responses of eggplant (Solanum melongena L.) under sandy soil conditions. Aust. J. Basic Appl. Sci. 2018, 2, 296–300. [Google Scholar]
- Ahmed, A.A.; El-Baky, M.M.H.A.; Zaki, M.F.; Abd El-Aal, F.S. Effect of foliar application of active yeast extract and zinc on growth, yield and quality of potato plant (Solanum tuberosum L.). J. Appl. Sci. Res. 2011, 7, 2479–2488. [Google Scholar]
- Shehata, S.A.; Fawzy, Z.F.; El-Ramady, H.R. Response of cucumber plants to foliar application of chitosan and yeast under greenhouse conditions. Aust. J. Basic Appl. Sci. 2012, 6, 63–71. [Google Scholar]
- Dawood, M.G.; El-Lethy, S.R.; Mervat, S. Role of methanol and yeast in improving growth, yield, nutritive value and antioxidants of soybean. World Appl. Sci. J. 2013, 26, 6–14. [Google Scholar]
- Shafeek, M.R.; Asmaa, R.M.; Aisha, H.A.; Magda, M.H.; Singer, S.M. Effect of different levels of potassium applied with foliar spraying of yeast on growth, yield and root quality of turnip under sandy soil conditions. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 868–877. [Google Scholar]
- Abdelaal, K.A.A.; Hafez, Y.M.; El Sabagh, A.; Saneoka, H. Ameliorative effects of abscisic acid and yeast on morpho-physiological and yield characteristics of maize plant (Zea mays L.) under water deficit conditions. Fresenius Environ. Bull. 2017, 26, 7372–7383. [Google Scholar]
- Shabbara, H.M.; Metwaly, Y.M.; El-Shafey, S.M. The productive economic efficiency of lemongrass in Fayoum governorate in Egypt. Int. J. Mech. Eng. 2022, 7, 841–847. [Google Scholar]
- Mahmoud, N.; Abdou, M.A.H.; Salaheldin, S.; Soliman, W.S. Lemongrass growth, essential oil, and active substances as affected by water deficit. Horticulturae 2022, 8, 250. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall of Indian Private: New Delhi, India, 1973; p. 498. [Google Scholar]
- Black, C.A.; Evans, D.O.; Ensminger, E.L.; White, J.L.; Clark, F.E.; Dinauer, R.C. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Bates, L.S.; Waldern, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Black, C.A.; Evans, D.D.; Ensminger, L.E. Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Chapman, H.D.; Parattm, P.F. Methods of Soil, Plants and Water Analysis; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1961; 314p. [Google Scholar]
- Clevenger, J.F. Apparatus for determination of volatile oil. J. Am. Pharm. Assoc. 1928, 17, 34. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. 2012, 24, 57–72. [Google Scholar]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Ding, Y.; Tao, Y.; Zhu, C. Emerging roles of MicroRNAs in the mediation of drought stress response in plants. J. Exp. Bot. 2013, 64, 3077–3086. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Bidwell, R.G.S. Plant Physiology (The Macmillan Biology Series); Plant Macmillan Publishing Go. Inc.: New York, NY, USA, 1974. [Google Scholar]
- Lambers, H.; Brundrett, M.C.; Raven, J.A.; Hopper, S.D. Plant mineral nutrition in ancient landscapes: High plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 2010, 334, 11–31. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; International Potash Institue: Berne, Switzerland, 1987. [Google Scholar]
- Mahdi, S.S.; Hassan, G.I.; Samoon, S.A.; Rather, H.A.; Showkat, A.D.; Zehra, B. Bio-fertilizers in organic agriculture. J. Phytol. 2010, 2, 42–54. [Google Scholar]
- Khalil, S.E.; Ismael, E.G. Growth, yield and seed quality of Lupinus termis as affected by different soil moisture levels and different ways of yeast application. J. Am. Sci. 2010, 6, 141–153. [Google Scholar]
- Nezhadahmadi, A.; Hossain Prodhan, Z.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef]
- Abou EL-Nasr, M.E.; EL-Shabrawy, R.A.; Abd EL-Rahman, M.M. Effect of bread yeast application and some nutrient elements on squash (Cucurbita pepo L.) plant growth, yield and fruit quality under conditions of the early summer planting. J. Agric. Sci. Mansoura Univ. 2001, 26, 4451–4464. [Google Scholar]
- Hussain, W.; Khalaf, L. Effect of Foliar Spraying with Yeast Solution on Growth and Yield of Potato Plant cv. Desiree. 2007. Available online: www.tropentage.de/2007/abstracts/links/khalaf.FPRAX90 (accessed on 10 January 2023).
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. Yeasts, Characteristics and Identification; Cambridge University Press: London, UK, 1990; p. 999. [Google Scholar]
- Fathy, S.L.; Farid, S. The possibility of using vitamin B and yeast to delay senescence and improve growth and yield of common beans (Phaseolus vulgaris L.). J. Agric. Sci. Mansoura Univ. 1996, 21, 1415–1423. [Google Scholar]
- Khedr, Z.M.A.; Farid, S. Response of naturally virus infected tomato plants to yeast extract and phosphoric acid application. Ann. Agric. Sci. Mashtohor Egypt 2002, 38, 927–939. [Google Scholar]
- Mahmoud, T.R. Botanical Studies on Growth and Germination of Magnolia (Magnolia grandiflora L.) Plants. Master’s Thesis, Zagazig University, Zagazig, Egypt, 2001; 103p. [Google Scholar]
- Castelfranco, P.A.; Beale, S.I. Chlorophyll Biosynthesis: Recent Advances and Areas of Current Interest. Annu. Rev. Plant Physiol. 1983, 34, 241–276. [Google Scholar] [CrossRef]
- Soliman, W.S.; El-Shaieny, A.A.H. Effect of saline water on germination and early growth stage of five Apiaceae species. Afr. J. Agric. Res. 2014, 9, 713–719. [Google Scholar] [CrossRef]
- Penka, M. Influence of irrigation on the contents of effective substances in official plants. Acta Hortic. 1978, 73, 181–189. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, R.P.; Singh, R.S. Effect of iron on herbage and oil yield of lemongrass (Cymbopogon flexuosus). Crop Res. 2003, 26, 185–187. [Google Scholar]
- Abdel-Wahab, M.A. Effect of some trace elements on growth, yield and chemical constituents of Trachysprmum ammi L. plants under Sinai conditions. Res. J. Agric. Biol. Sci. 2008, 4, 717–724. [Google Scholar]
- Mahmoud, N.; Abdou, M.A.; Salaheldin, S.; Soliman, W.S. Effect of partial substitution on mineral fertilizers with yeast on growth, yield and essential oil content of lemongrass (Cymbopogon citratus (DC.) Stapf). Aswan Univ. J. Sci. Technol. 2021, 1, 1–16. [Google Scholar] [CrossRef]




| 1-Physical Analysis | |||
| Sand % | 94.67 | ||
| Silt % | 2.27 | ||
| Clay % | 3.07 | ||
| Soil Texture | Sandy | ||
| 2-Chemical Analysis | |||
| pH | 8.25 | Electrical Conductivity (ds m−1) | 0.25 |
| Soluble Cations meq L−1 | Soluble Anions meq L−1 | ||
| Na+ | 17.74 | CO−3 | 0.00 |
| K+ | 7.51 | HCO−3 | 4.67 |
| Ca++ | 2.08 | Cl− | 2.33 |
| Mg++ | 0.53 | ||
| Variables | F Value | |||||
|---|---|---|---|---|---|---|
| A | B | A × B | A | B | A × B | |
| First season (1st cut) | First season (2nd cut) | |||||
| Number of tillers | 30.61 *** | 72.27 *** | 1.06 ns | 27.11 *** | 37.29 *** | 2.36 * |
| Plant fresh weight (g) | 143.47 *** | 39.50 *** | 2.28 * | 166.45 *** | 207.34 *** | 5.59 *** |
| Plant dry weight (g) | 23.45 *** | 29.00 *** | 1.30 ns | 32.10 *** | 15.17 *** | 0.75 ns |
| Fresh herb yield (ton ha−1) | 143.47 *** | 39.50 *** | 2.28 * | 166.45 *** | 207.34 *** | 5.59 *** |
| Dry herb yield (ton ha−1) | 23.45 *** | 29.00 *** | 1.30 ns | 32.10 *** | 15.17 *** | 0.75 ns |
| Relative leaf greenness (%) | 2.62 ns | 35.57 *** | 2.75 ** | 22.30 *** | 64.11 *** | 2.22* |
| Proline content (%) | 27.78 *** | 9.40 *** | 1.61 ns | 16.31 *** | 11.85 *** | 3.74 *** |
| Nitrogen content (%) | 26.41 *** | 49.10 *** | 1.44 ns | 7.19 *** | 65.48 *** | 1.19 ns |
| Phosphorus content (%) | 482.29 *** | 801.42 *** | 663.92 *** | 6.05** | 6.23 *** | 5.22 *** |
| Potassium content (%) | 293.74 *** | 321.07 *** | 715.38 *** | 515.45 *** | 367.40 *** | 366.52 *** |
| Essential oil percentage (%) | 16.44 *** | 8.48 *** | 4.80 *** | 9.45 *** | 4.55 *** | 3.55** |
| Essential oil content (mL plant−1) | 53.60 *** | 17.70 *** | 2.26* | 36.66 *** | 77.20 *** | 5.34 *** |
| Essential oil yield (litter ha−1) | 53.60 *** | 17.70 *** | 2.26* | 36.66 *** | 77.20 *** | 5.34 *** |
| Second season (1st cut) | Second season (2nd cut) | |||||
| Number of tillers | 38.07 *** | 11.48 *** | 0.63 ns | 173.79 *** | 85.15 *** | 5.55 *** |
| Plant fresh weight (g) | 264.78 *** | 101.07 *** | 7.91 *** | 896.28 *** | 43.56 *** | 8.35 *** |
| Plant dry weight (g) | 20.89 *** | 12.50 *** | 1.29 ns | 178.29 *** | 42.61 *** | 12.45 *** |
| Fresh herb yield (ton ha−1) | 264.78 *** | 101.07 *** | 7.91 *** | 896.28 *** | 43.56 *** | 8.35 *** |
| Dry herb yield (ton ha−1) | 20.89 *** | 12.50 *** | 1.29 ns | 178.29 *** | 42.61 *** | 12.45 *** |
| Relative leaf greenness (%) | 118.01 *** | 37.81 *** | 2.30 * | 98.89 *** | 36.51 *** | 3.80 *** |
| Proline content (%) | 42.19 *** | 12.74 *** | 19.05 *** | 92.04 *** | 27.33 *** | 7.28 *** |
| Nitrogen content (%) | 7.47 *** | 41.61 *** | 0.64 ns | 8.95 *** | 163.79 *** | 3.79 *** |
| Phosphorus content (%) | 0.87 ns | 0.82 ns | 2.41 * | 5.95 ** | 1.45 ns | 3.17 * |
| Potassium content (%) | 98.57 *** | 653.78 *** | 244.90 *** | 498.13 *** | 66.25 *** | 287.61 *** |
| Essential oil percentage (%) | 67.08 *** | 5.67 *** | 3.74 *** | 11.71 *** | 2.82 * | 2.45 * |
| Essential oil content (mL plant−1) | 9.30 *** | 21.22 *** | 3.52 *** | 93.17 *** | 3.05 * | 2.04 * |
| Essential oil yield (litter ha−1) | 9.30 *** | 21.22 *** | 3.52 ** | 93.17 *** | 3.05 * | 2.04 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, N.; Abdou, M.A.H.; Salaheldin, S.; Soliman, W.S.; Abbas, A.M. The Impact of Irrigation Intervals and NPK/Yeast on the Vegetative Growth Characteristics and Essential Oil Content of Lemongrass. Horticulturae 2023, 9, 365. https://doi.org/10.3390/horticulturae9030365
Mahmoud N, Abdou MAH, Salaheldin S, Soliman WS, Abbas AM. The Impact of Irrigation Intervals and NPK/Yeast on the Vegetative Growth Characteristics and Essential Oil Content of Lemongrass. Horticulturae. 2023; 9(3):365. https://doi.org/10.3390/horticulturae9030365
Chicago/Turabian StyleMahmoud, Nourhan, Mahmoud A. H. Abdou, Sabri Salaheldin, Wagdi S. Soliman, and Ahmed M. Abbas. 2023. "The Impact of Irrigation Intervals and NPK/Yeast on the Vegetative Growth Characteristics and Essential Oil Content of Lemongrass" Horticulturae 9, no. 3: 365. https://doi.org/10.3390/horticulturae9030365
APA StyleMahmoud, N., Abdou, M. A. H., Salaheldin, S., Soliman, W. S., & Abbas, A. M. (2023). The Impact of Irrigation Intervals and NPK/Yeast on the Vegetative Growth Characteristics and Essential Oil Content of Lemongrass. Horticulturae, 9(3), 365. https://doi.org/10.3390/horticulturae9030365






