Micro-Tom Tomato Response to Fertilization Rates and the Effect of Cultivation Systems on Fruit Yield and Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site
2.2. Plant Material
2.3. Cultivation Systems
2.3.1. Conventional System
2.3.2. Organic System
2.3.3. Hydroponic System
2.4. Field Preparation and Crop Management
2.5. Fertilization Treatments
2.6. Growth Parameters
2.7. Fruit Quality Analysis
2.8. Metabolomic Analysis
2.9. Leaf Analysis
2.10. Experimental Design and Statistical Analysis
3. Results
3.1. Effect of Different Fertilization Rates on Plant Growth and Fruit Quality
3.1.1. Plant Growth
3.1.2. Yield and Fruit Quality Parameters
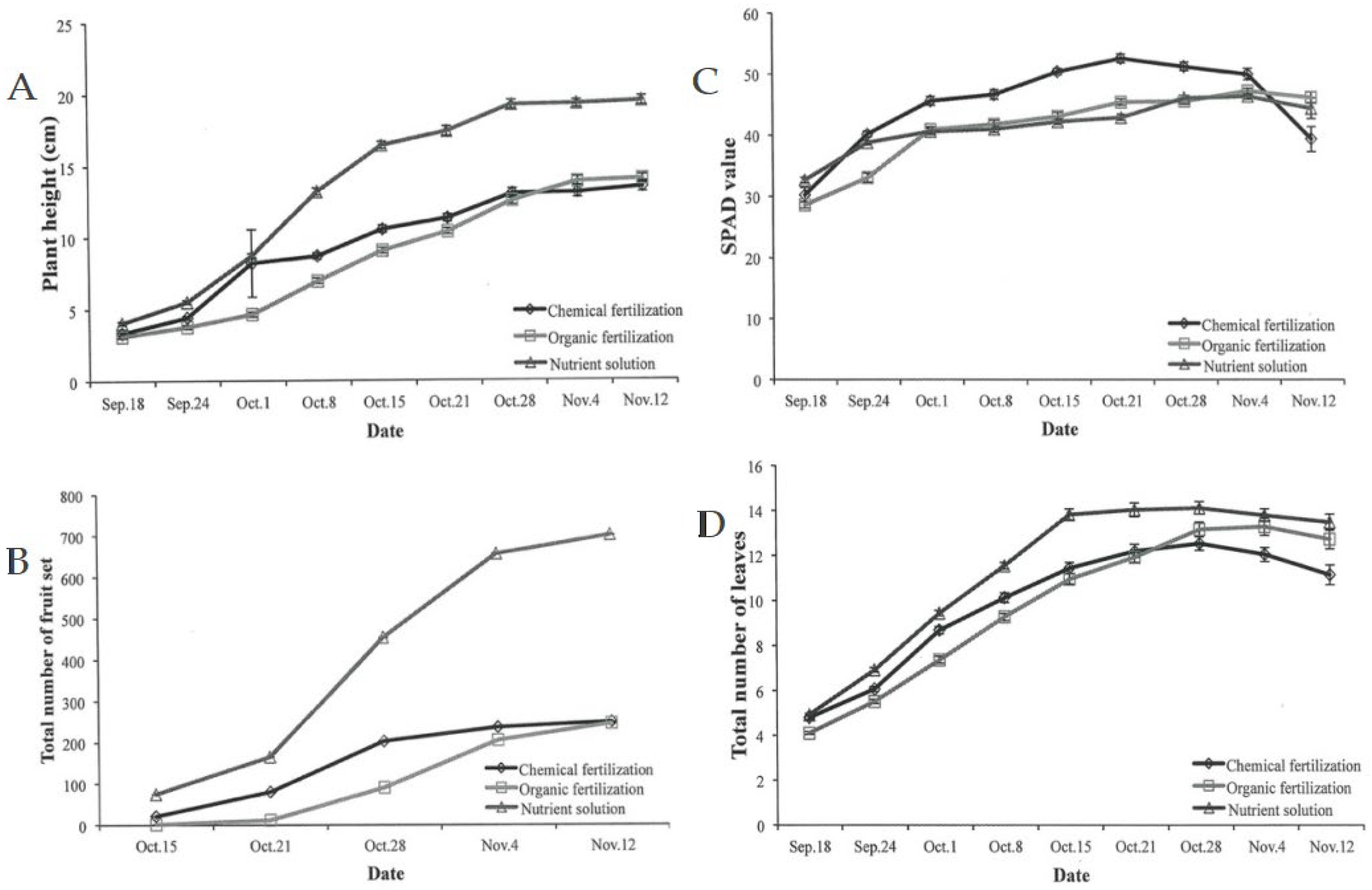
3.2. Effect of Cultivation System on Plant Growth and Fruit Quality
3.2.1. Leaf Parameters
3.2.2. Fruit Quality Parameters and The Number of Flowers Per Plant
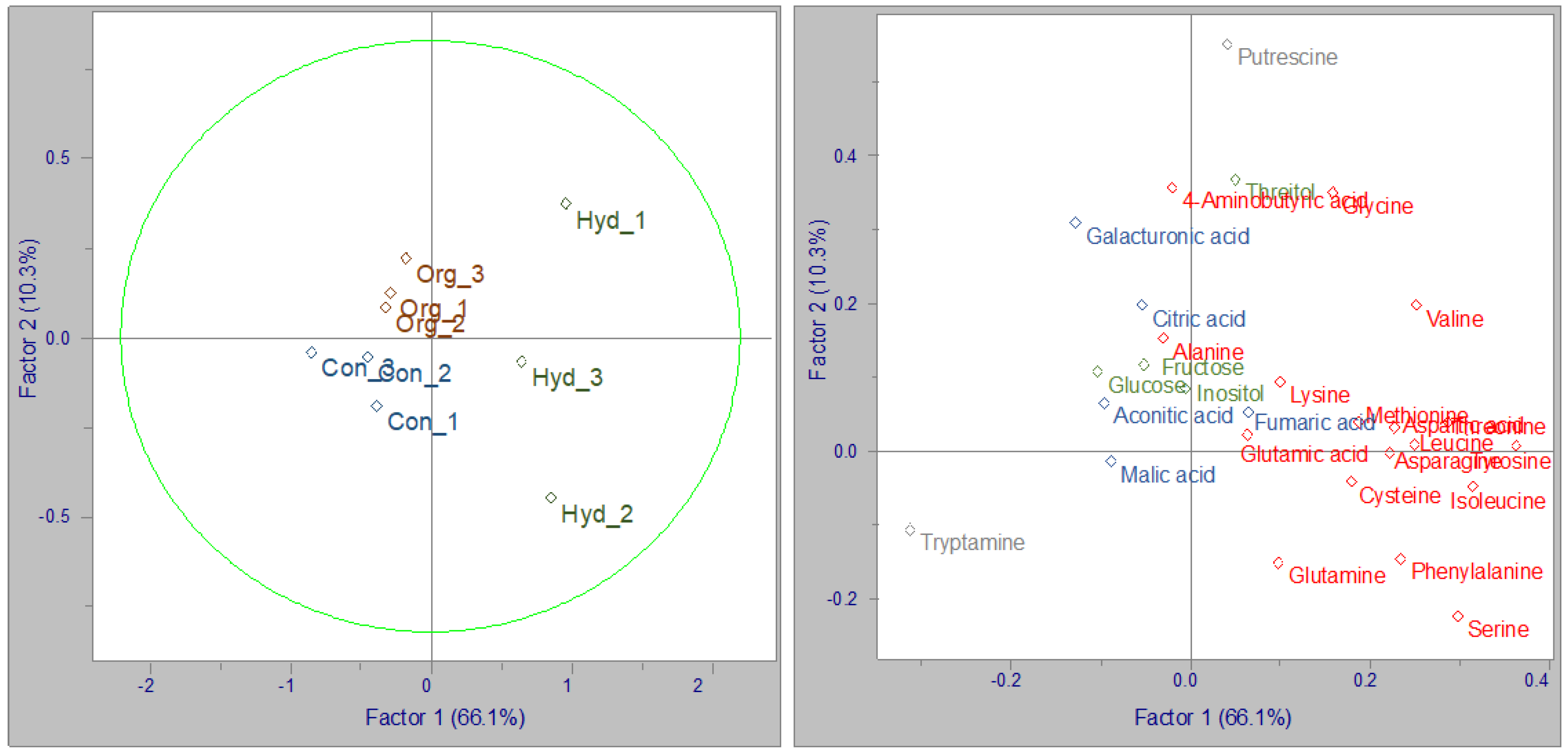
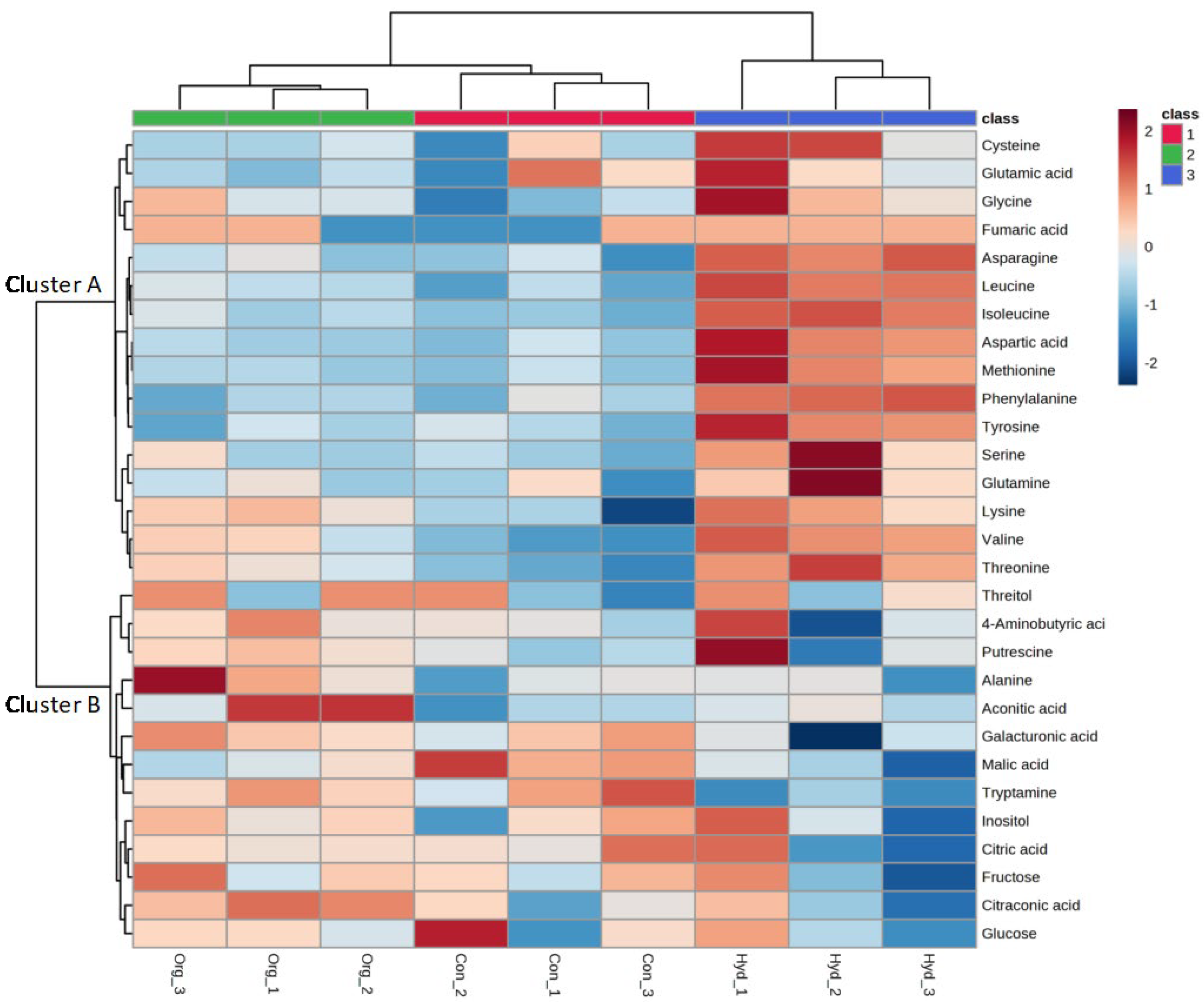
3.2.3. Principal Component Analysis (PCA)
4. Discussion
4.1. Effect of Different Fertilization Rates Plant Growth and Fruit Quality
4.2. Effect of Cultivation Systems on Plant Growth and Fruit Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Peace, Dignity, and Equality on a Healthy Planet. Available online: https://www.un.org/en/global-issues/population#:~:text=The%20world%20population%20is%20projected,surrounding%20these%20latest%20population%20projections (accessed on 12 July 2022).
- Tomlinson, I. Doubling food production to fed the 9 billion: A critical perspective on a key discourse of food security in UK. J. Rural Stud. 2013, 29, 81–89. [Google Scholar] [CrossRef]
- Nicola, S.; Tibaldi, G.; Fontana, E. Tomato production systems and their application to the tropics. Acta. Hort. 2009, 821, 27–34. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 July 2022).
- Dorais, M.; Ehret, D.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Albornoz, F. Crop responses to nitrogen overfertilization: A review. Sci. Hortic. 2016, 205, 79–83. [Google Scholar] [CrossRef]
- Weinbaum, S.A.; Johnson, R.S.; Dejong, T.M. Causes and consequences of overfertilization in orchards. Hort. Technol. 1992, 2, 112–120. [Google Scholar] [CrossRef]
- Locascio, S.J.; Wiltbank, W.J.; Gull, D.D.; Maynard, D.N. Nitrogen in Crop Production; Hauck, R.D., Ed.; American Society of Agronomy Inc.: Madison, WI, USA, 1984; pp. 617–625. [Google Scholar]
- Wang, Z.; Li, S.; Malhi, S. Effects of fertilization and other agronomic measures on nutritional quality of crops. J. Sci. Food Agric. 2008, 88, 7–23. [Google Scholar] [CrossRef]
- Benard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Genard, M. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef]
- Zhu, Q.; Ozofres-Hampton, M.; Li, Y.; Morgan, K.; Liu, G.; Mylavarapu, R.S. Effect of phosphorus rates on growth, yield, and postharvest quality of tomato in a calcareous soil. Hort. Sci. 2017, 52, 1406–1412. [Google Scholar] [CrossRef]
- Cao, F.; Guan, C.; Dai, H.; Li, X.; Zhang, Z. Soluble solids content is positively correlated with phosphorus content in ripening strawberry fruits. Sci. Hortic. 2015, 195, 183–187. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, Q.; Chen, Y.; Lin, D.; Huang, J.; Huang, T. Metabolite alteration in response to low phosphorus stress in developing tomato fruits. Plant Physiol. Biochem. 2021, 159, 234–243. [Google Scholar] [CrossRef]
- Kanai, S.; Ohkura, K.; Adu-Gyamfi, J.J.; Mohapatara, P.K.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Depression of sink activity precedes the inhibition of biomass production in tomato plants subjected to potassium deficiency stress. J. Exp. Bot. 2007, 58, 2917–2928. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Munoz, A.; Romero, I.; Osorio, P.; Manquez, S.; Arriola, R.; Reyes-Diaz, M.; Ribera-Fonseca, A. Impact of potassium pre-harvest application on fruit quality and condition of sweet cherry (Prunus avium L.) cultivated under plastic covers in southern Chile orchards. Plants 2007, 58, 2917–2928. [Google Scholar] [CrossRef] [PubMed]
- IFOAM (International Movement of Organic Agriculture Movements). Definition of Organic Agriculture, IFOAM. 2015. Available online: https://www.ifoam.bio/why-organic/organic-landmarks/definition-organic (accessed on 20 January 2023).
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. organic agriculture. CRC Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Chassy, A.W.; Bui, L.; Renaud EN, C.; Van Horn, M.; Mitchell, A.E. Three-year comparison of the content of antioxidant microconstituents and several quality characteristics in organic and conventionally managed tomatoes and bell papers. J. Agric. Food Chem. 2006, 54, 8244–8252. [Google Scholar] [CrossRef]
- Richa, A.; Touil, S.; Fizir, M.; Martinez, V. Recent advances and perspectives in the treatment of hydroponic wastewater: A review. Rev. Environ. Sci. Biotechnol. 2020, 19, 945–966. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Hydroponics A Practical Guide for the Soilless Grower, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–10. [Google Scholar]
- Asao, T.; Asaduzzaman, M. Hydroponics: A Standard Methodology for Plant Biological Researches; Asao, T., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 67–100. [Google Scholar]
- Verdoliva, S.G.; Jones, D.G.; Detheridge, A.; Robson, P. Controlled comparisons between soil and hydroponic systems reveal increased water use efficiency and higher lycopene and β-carotene contents in hydroponically grown tomatoes. Sci. Hortic. 2021, 279, 109896. [Google Scholar] [CrossRef]
- Kunsch, U.; Scharer, H.; Durr, P.; Hurter, A.; Martinoni, J.; Sulser, G.H.; Seeger, B. Do differences exist in the quality between soilless and conventionally produced tomatoes and head lettuce. Mitt. Geb. Lebensm. Hyg. 1994, 85, 18–21. [Google Scholar]
- Novaes, H.B.; Vaitsman, D.S.; Dutra, P.B. Determination of nitrate in lettuce by iron chromatography after microwave water extraction. Quim. Nova 2009, 32, 1647–1650. [Google Scholar] [CrossRef]
- Powlson, D.S.; Addiscott, T.M.; Benjamin, N.; Cassman, T.M.; Kok, D.; Grinsven, H.V.; L’hirondel, J.; Avery, A.A.; Kessel, C.V. When does nitrate become a risk for humans? J. Environ. Qual. 2008, 37, 291–295. [Google Scholar] [CrossRef]
- Ecarnot, M.; Baczyk, P.; Tessarotto, L.; Chervin, C. Rapid phenotyping of the tomato fruit model, Micro-Tom, with a portable VIS-NIR spectrometer. Plant Physiol. Biochem. 2013, 70, 159–163. [Google Scholar] [CrossRef]
- Kanagawa Prefectural Government. Available online: https://www.pref.kanagawa.jp/docs/f6k/cnt/f6802/index.html (accessed on 23 January 2023).
- Abdelhady, A.S.; El-Azm, N.A.I.A.; Ei-Kafafi, E.S.H. Effect of deficit irrigation levels and NPK fertilization rates on tomato growth, yield and fruit quality. Middle East J. Agric. Res. 2017, 6, 587–604. [Google Scholar]
- Ferreira, M.M.M.; Ferreira, G.B.; Fontes, P.C.R. Eficiência da adubação nitrogenada do tomateiro em duas épocas de cultivo. Rev. Ceres 2010, 57, 263–273. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, X.; Shi, D.; Li, Y.; Zhang, J.; Liu, P.; Zhao, B.; Dong, S. The mechanisms of low nitrogen induced weakened photosynthesis in summer maize (Zea mays L.) under field conditions. Plant Physiol. Biochem. 2016, 105, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Matsumaru, T.; Yoneyama, T.; Ishizuka, J.; Matsuoka, Y. Effect on growth of tomato and accumulation and assimilation of nitrogen. J. Jpn. Soc. Soil Sci. Plant Nutr. 1987, 58, 465–472. (In Japanese) [Google Scholar]
- Varis, S.; George, R.A.T. The influence of mineral nutrition on fruit yield, seed yield, and quality in tomato. J. Hort. Sci. 1985, 60, 373–376. [Google Scholar] [CrossRef]
- Rajasree, G.; Pillai, G.R. Effect of nitrogen nutrition on fruit quality and shelf life of cucurbitaceous vegetable bitter gourd. J. Plant Nutr. 2012, 35, 1139–1153. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. Improvement of nitrogen on flavonoids, glutathione, anthocyanin, ascorbic acid, and antioxidant activities of Malaysian medical plant Labisia pumila Blume (Kacip Fatimah). Int. J. Mol. Sci. 2012, 13, 393–408. [Google Scholar] [CrossRef]
- El-Nemar, M.A.; Abd El-Baky, M.M.H.; Salman, S.R.; El-Tohamy, W.A. Effect of different potassium levels on the growth, yield, and quality of tomato grown in sand-ponic culture. Aust. J. Basic Appl. Sci. 2012, 6, 779–784. [Google Scholar]
- Majid, F.; Jalal, M.; Munir, H. Effect of N, P, K concentrations on yield and fruit quality of tomato (Solanum lycopersicum L.) in tuff culture. J. Cent. Eur. Agric. 2010, 11, 179–184. [Google Scholar]
- Adebooye, O.C.; Adeoye, G.O.; Tijani-Eniola, H. Quality of fruits of three varieties of tomato (Lycopersicom esculentum (L.) Mill) as affected by phosphorus rates. J. Agron. 2006, 5, 396–400. [Google Scholar]
- Javaria, S.; Khan, M.Q.; Bakhsh, I. Effect of potassium on chemical and sensory attributes of tomato fruits. J. Anim. Plant Sci. 2012, 22, 1081–1085. [Google Scholar]
- Hao, X.; Papadopoulos, A.P. Growth, photosynthesis and productivity of greenhouse tomato cultivated in open or closed rockwool system. Can. J. Plant Sci. 2002, 82, 771–780. [Google Scholar] [CrossRef]
- Le, D.T.; Chu, H.D.; Le, N.Q. Improving nutritional quality of plant proteins through genetic engineering. Curr. Genom. 2016, 17, 220–229. [Google Scholar]
- Li, S.; Wang, Z.; Stewart, B.A. Five—Responses of crop plants to ammonium and nitrate N. Adv. Agron. 2013, 118, 205–397. [Google Scholar]
- Gravel, V.; Blok, W.; Hallmann, E.; Carmona-Torres, C.; Wang, H.; Van De Peppel, A.; Condor Golec, A.F.; Dorais, M.; Van Meeteren, U.; Heuvelink, E.; et al. Differences in N uptake and fruit quality between organically and conventionally grown greenhouse tomatoes. Agron. Sustain. Dev. 2010, 30, 797–806. [Google Scholar] [CrossRef]
- Nakano, A.; Uehara, Y. An effect of fertilization and cultivation on yield, quality, and environment of tomato. Agric. Hortic. 2006, 81, 291–301. (In Japanese) [Google Scholar]
- Heeb, A.; Lundegardh, B.; Ericsson, T.; Savage, G.P. Nitrogen form affects yield and taste of tomatoes. J. Sci. Food Agric. 2005, 85, 1405–1414. [Google Scholar] [CrossRef]
- Gad, N.; Kandil, H. Influence of cobalt on phosphorus uptake, growth and yield of tomato. Agric. Biol. J. N. Am. 2010, 5, 1069–1075. [Google Scholar] [CrossRef]
- Pieper, J.R.; Barrett, D.M. Effects of organic and conventional production systems on quality and nutritional parameters of processing tomatoes. J. Sci. Food Agric. 2009, 89, 177–194. [Google Scholar] [CrossRef]
- Juroszek, P.; Lumpkin, H.M.; Yang, R.Y.; Ledesma, D.R.; Ma, C.H. Fruit quality and bioactive compounds with antioxidant activity of tomatoes grown on-farm: Comparison of organic and conventional management systems. J. Agric. Food Chem. 2009, 57, 1188–1194. [Google Scholar] [CrossRef]
- Almeselmani, M.; Pant, R.C.; Singh, B. Potassium level and physiological response and fruit quality in hydroponically grown tomato. Int. J. Veg. Sci. 2009, 16, 85–99. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Review: Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Agric. Food Chem. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Worthington, V. Nutritional quality of organic versus conventional fruits, vegetables, and grains. J. Altern. Complement. Med. 2001, 7, 161–173. [Google Scholar] [CrossRef]
- Bourn, D.; Prescott, J. A comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods. Crit. Rev. Food Sci. 2002, 42, 1–34. [Google Scholar] [CrossRef]
- Colpan, E.; Zengin, M.; Ozbahce, A. Effects of potassium on the yield and fruit quality components of stick tomato. Hort. Envi-ron. Biotechnol. 2013, 54, 20–28. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, T.Q.; Tan, C.S.; Astatkie, T. Responses of fruit yield and quality of processing tomato to drip-irrigation and fertilizers phosphorus and potassium. Agron J. 2011, 103, 339–345. [Google Scholar] [CrossRef]
- Ali, S.; Javed, H.U.; Rehman, R.N.U.; Sabir, I.A.; Naeem, M.S.; Siddiqui, M.Z.; Saeed, D.A.; Nawaz, M.A. Foliar application of some macro and micro nutrients improves tomato growth, flowering and yield. Int. J. Biosci. 2013, 3, 280–287. [Google Scholar]
- Haleem, B.; Rab, A.; Hussain, S.A. Effect of calcium, boron and zinc foliar application on growth and fruit production of to-mato. Sarhad J. Agric. 2017, 34, 19–30. [Google Scholar]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Yamane, Y.; Shikanai, T.; Kashino, Y.; Koike, H.; Satoh, K. Reduction of QA in the dark: Another cause of fluorescence FO increases by high temperature in higher plants. Photosynth. Res. 2000, 63, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y.; Berninger, F.; Li, C.Y. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 2006, 44, 62–68. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids. 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, E. Effect of nitrogen sources on the nitrogenous forms and accumulations of amino acid in head cabbage. Plant Soil. Environ. 2008, 54, 66–71. [Google Scholar] [CrossRef]
- Kopple, J.D. Phenylalanine and tyrosine metabolism in chronic kidney failure. J. Nutr. 2007, 137, 1586S–1590S. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Quinn, P.J. Production of L- valine from metabolically engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2018, 102, 4319–4330. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Agius, C.; Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef]
- Sawant, O.; Mahale, S.; Ramachandran, V.; Nagaraj, G.; Bankar, A. Fungal citric acid production using waste materials: A mini-review. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 821–828. [Google Scholar] [CrossRef]


| Treatment | Number of Flowers | Plant Height (cm) | Root Length (cm) | Dry Root Weight (g) |
|---|---|---|---|---|
| A | 0 c | 1.7 c | 4.8 de | 0.02 c |
| B | 0 c | 3.1 bc | 9.2 cd | 0.06 c |
| C | 1.4 c | 5.2 b | 14.9 bc | 0.18 c |
| D | 11.2 bc | 9.4 a | 23.5 a | 0.28 bc |
| E | 18.8 b | 11.3 a | 20.7 ab | 0.52 b |
| F | 35.4 a | 11.7 a | 16.7 abc | 1.11 a |
| G | 1.3 c | 1.8 c | 2.5 de | 0.03 c |
| Treatment | Yield (g plant−1) | Weight Per Fruit (g) | a* Value | Ascorbic Acid (mg 100 g−1) | Brix (%) |
|---|---|---|---|---|---|
| D | 377.8 b | 2.6 b | 41.2 a | 28.7 a | 5.3 b |
| E | 447 b | 3.7 a | 42.4 a | 25.9 a | 5.7 a |
| F | 2144.5 a | 3.3 ab | 43.0 a | 25.9 a | 5.9 a |
| Cultivation System | Number of Leaves | Leaf Area (cm2) | Length of Fully Developed Leaf (cm) | NO3−-N (mg 100 g−1) |
|---|---|---|---|---|
| Conventional | 7.2 ab | 129.2 b | 6.1 b | 59.6 b |
| Organic | 6.8 b | 87.1 c | 5.9 b | 42.2 b |
| Hydroponic | 8.6 a | 175.5 a | 8.8 a | 98.4 a |
| Cultivation System | Number of Flowers (Per Plant) | Yield (g plant−1) | Weight Per Fruit (g) | a* Value | Ascorbic Acid (mg 100 g−1) | Brix (%) | Organic Acids (nmol g−1) | Amino Acids (nmol g−1) | Total Sugar (nmol g−1) |
|---|---|---|---|---|---|---|---|---|---|
| Conventional | 27.5 b | 194.0 b | 2.4 b | 39.4 a | 30 a | 5.5 a | 15 a | 90.5 b | 55.1 a |
| Organic | 22.0 c | 182.0 b | 2.9 b | 42.6 a | 30.6 a | 5.7 a | 14.2 a | 88.1 b | 56.2 a |
| Hydroponic | 36.4 a | 432.0 a | 3.6 a | 42.2 a | 23.7 b | 5.2 b | 12.1 b | 131.1 a | 49.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terada, N.; Dissanayake, K.; Okada, C.; Sanada, A.; Koshio, K. Micro-Tom Tomato Response to Fertilization Rates and the Effect of Cultivation Systems on Fruit Yield and Quality. Horticulturae 2023, 9, 367. https://doi.org/10.3390/horticulturae9030367
Terada N, Dissanayake K, Okada C, Sanada A, Koshio K. Micro-Tom Tomato Response to Fertilization Rates and the Effect of Cultivation Systems on Fruit Yield and Quality. Horticulturae. 2023; 9(3):367. https://doi.org/10.3390/horticulturae9030367
Chicago/Turabian StyleTerada, Naoki, Kalara Dissanayake, Chiharu Okada, Atsushi Sanada, and Kaihei Koshio. 2023. "Micro-Tom Tomato Response to Fertilization Rates and the Effect of Cultivation Systems on Fruit Yield and Quality" Horticulturae 9, no. 3: 367. https://doi.org/10.3390/horticulturae9030367
APA StyleTerada, N., Dissanayake, K., Okada, C., Sanada, A., & Koshio, K. (2023). Micro-Tom Tomato Response to Fertilization Rates and the Effect of Cultivation Systems on Fruit Yield and Quality. Horticulturae, 9(3), 367. https://doi.org/10.3390/horticulturae9030367







