Comparison of the Essential Oil Content, Constituents and Antioxidant Activity from Different Plant Parts during Development Stages of Wild Fennel (Foeniculum vulgare Mill.)
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Clevenger-Type Hydrodistillation
2.3. Gas Chromatography/Mass Spectrometry (GC/MS) and Gas Chromatography/Flame Ionization Detection (GC/FID) Analysis
2.4. DPPH Assay
2.5. Statistical Methods
3. Results and Discussion
3.1. Essential Oil Yield
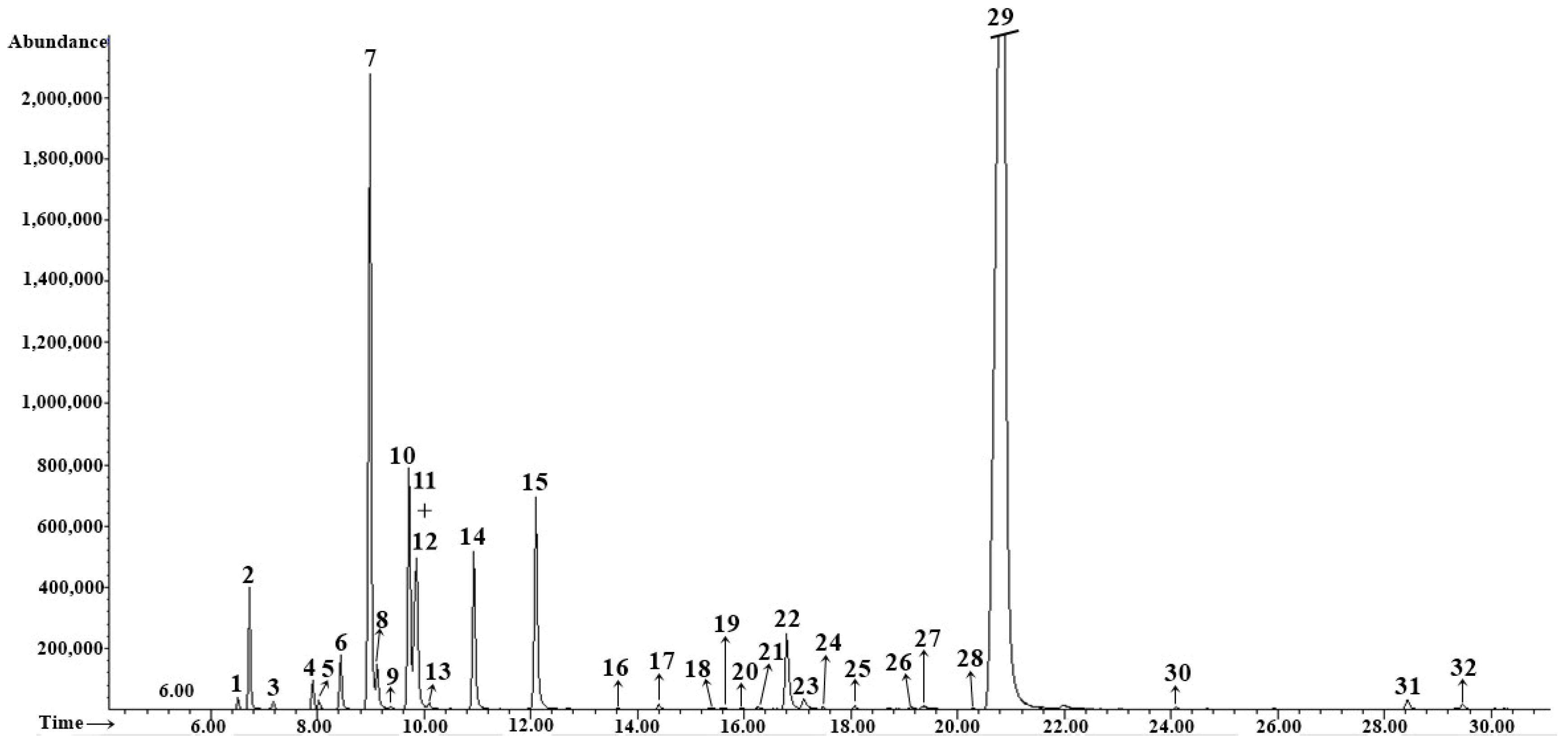
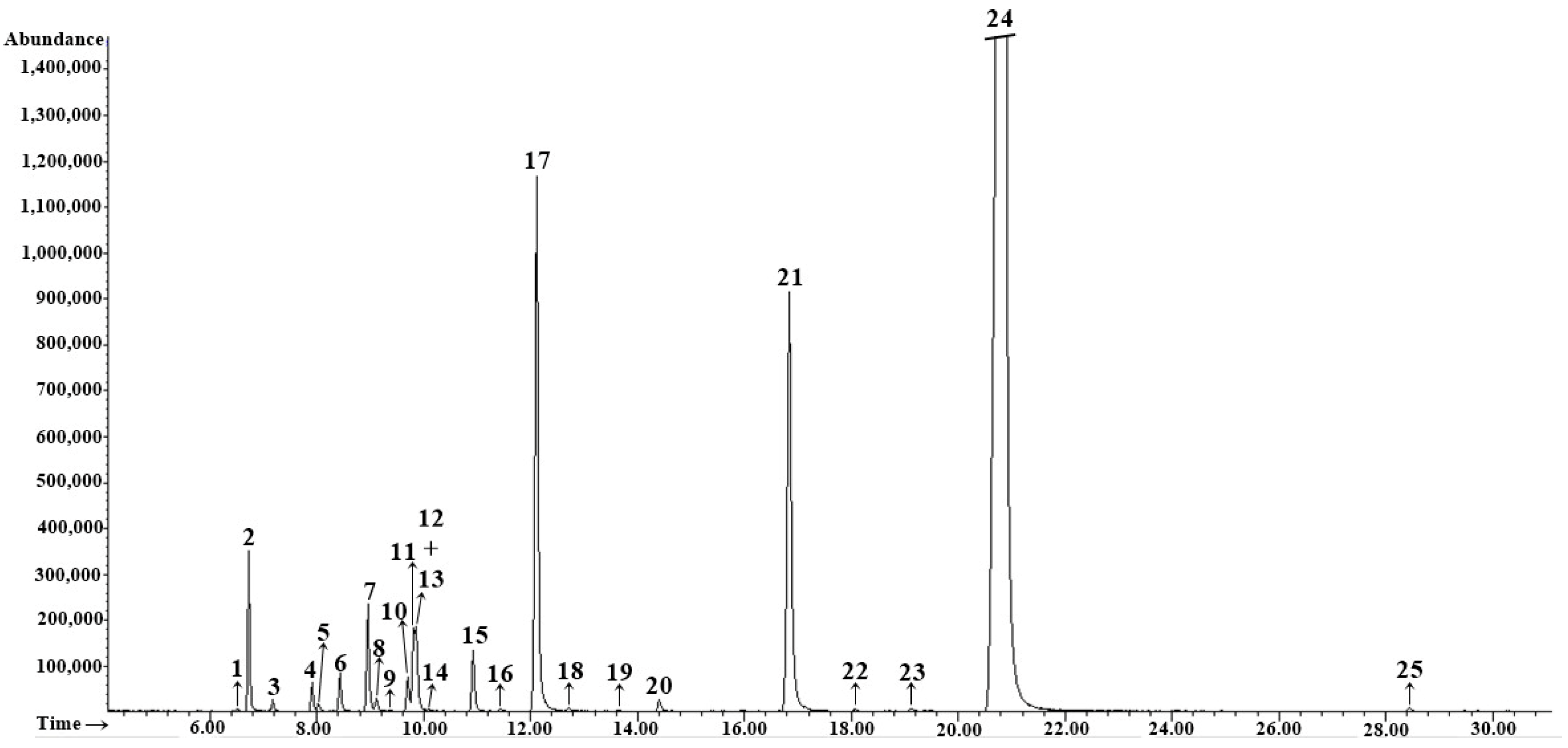
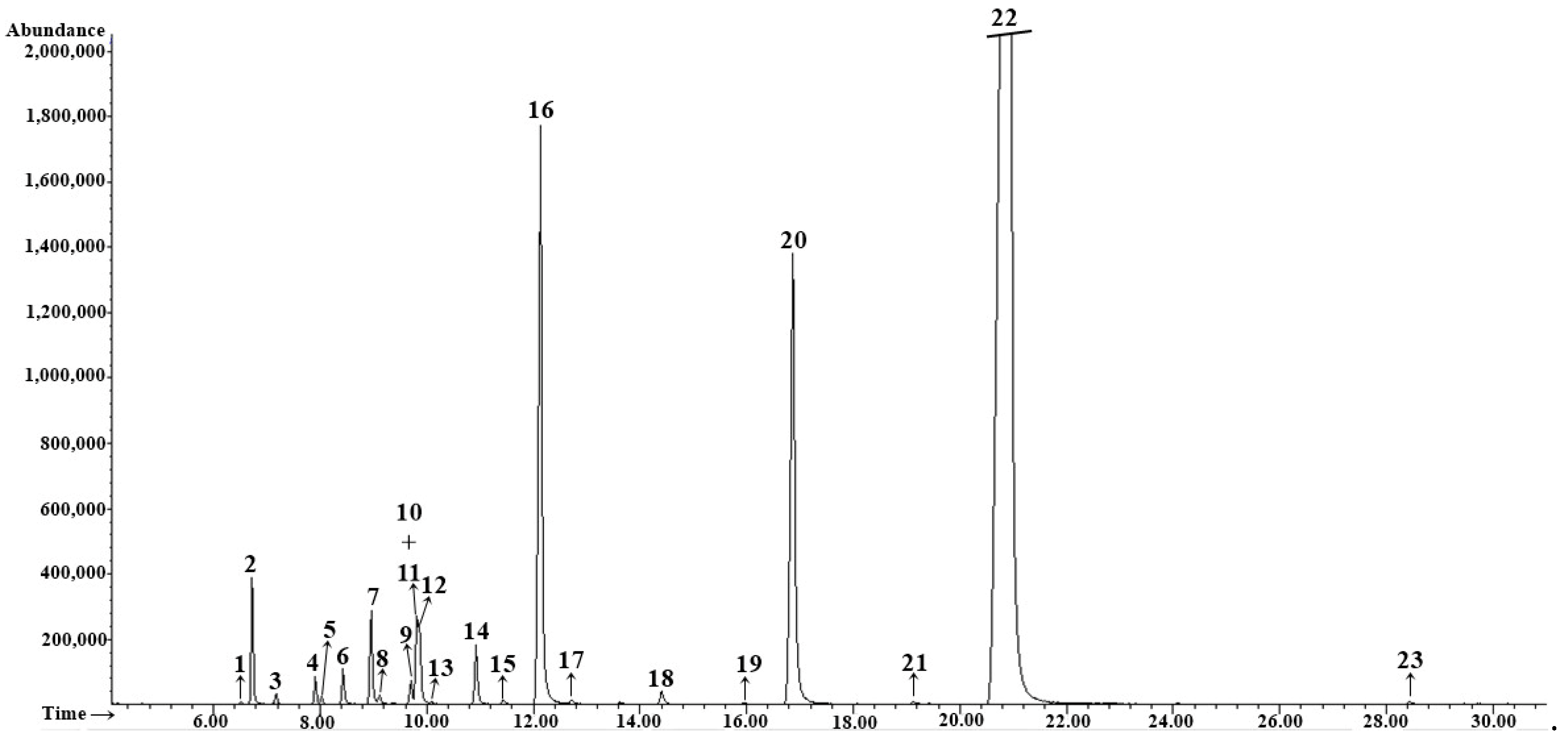
3.2. EOs Composition
3.3. Antioxidative Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Telci, I.; Dirican, A.; Elmastas, M.; Akşit, H.; Demirtas, I. Chemical diversity of wild fennel populations from Turkey. J. App. Res. Med. Arom. Plants. 2019, 13, 100201. [Google Scholar] [CrossRef]
- Gross, M.; Lewinsohn, E.; Dudai, N.; Cohen, Y.; Friedman, J. Flowering dynamics and crossability of different populations of bitter fennel (Foeniculumvulgare Mill. var. vulgare, Apiaceae).Israel J. Plant Sci. 2008, 56, 215–226. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Shi, M.; Liu, C.; Kang, W. Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculumvulgare Mill.) seeds from Egypt and China. Food Sci. Hum. Wellness 2019, 8, 67–72. [Google Scholar] [CrossRef]
- Khammassi, M.; Loupassaki, S.; Tazarki, H.; Mezni, F.; Slama, A.; Tlili, N.; Zaouali, Y.; Mighri, H.; Jamoussi, B.; Khaldi, A. Variation in essential oil composition and biological activities of Foeniculumvulgare Mill. populations growing widely in Tunisia. Food Biochem. 2018, 42, e12532. [Google Scholar] [CrossRef]
- Milenković, A.; Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Lalević, D.; Stanojević, J.; Danilović, B.; Cvetković, D. Essential oil yield, composition, antioxidant and microbial activity of wild fennel (Foeniculumvulgare Mill.) from Monte Negro coast. Horticulturae 2022, 8, 1015. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Palčić, I.; Marčac, N.; Dragović-Uzelac, V. Essential oils in wild Istrian fennel (Foeniculum vulgare Mill.): Variability in the content and composition. In Book of Abstracts of the 13th International Scientific and Professional Conference: With Food to Health; Babić, J., Šubarić, D., Jašić, M., Eds.; Josip Juraj Strossmayer University of Osijek, Faculty of Food Technology Osijek and Faculty of Pharmacy, University of Tuzla Osijek i Tuzla: Croatia, 2021; p. 134. [Google Scholar]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Antimicrobial, antibiofilm, and antioxidant properties of essential oil of Foeniculumvulgare Mill. leaves. Plants 2022, 11, 3573. [Google Scholar] [CrossRef]
- Cvetkovic, D.; Stanojevic, J.; Djordjevic, N.; Karabegović, I.; Stanojevic, L.; Pavlic, B.; Danilović, B. Effect of different extraction techniques on the composition of essential oil isolated from fennel (Foeniculumvulgare Mill.) rhizome. J. Essen. Oil. Res. 2023, 35, 24–34. [Google Scholar] [CrossRef]
- Shojaiefar, S.; Mirlohi., A.; Sabzalian, M.R.; Yaghini, H. Seed yield and essential oil content of fennel influenced by genetic variation and genotype × year interaction. Ind. Crops. Prod. 2015, 71, 97–105. [Google Scholar] [CrossRef]
- Tard, J.; Pardo-de-Santayana, M.; Morales, R. Ethnobotanical review of wildedible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Negahban, M.; Saeedfar, S.; Rowshan, V.; Najafian, S. Essential oil content and composition of fennel fruits (Foeniculumvulgare Mill.). Russ. J. Biol. Res. 2015, 4, 81–84. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.M.; Arak, E. Essential oil composition of Foeniculumvulgare Mill. fruits from pharmacies in different countries. Nat. Prod. Res. 2012, 26, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Datiles, M.J.; Popay, I. Foeniculumvulgare(Fennel); CABI: Wallingford, UK, 2015. [Google Scholar] [CrossRef]
- RahayuNingsih, R.S.; Asra, R.; Rivai, H. Overview of traditional use, phytochemical and pharmacological activities of fennel (Foeniculumvulgare). Int. J. Modern Pharm. Res. 2021, 5, 1–9. [Google Scholar]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculumvulgare Mill.). Food Control. 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculumvulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, 1574–1583. [Google Scholar] [CrossRef]
- Tuzlac, E. Türkiye Bitkileri Gelenekselİlaç Rehberi; Istanbul TıpKitabevleriİstanbul MedikalYayıncılık: Istanbul, Türkiye, 2016. [Google Scholar]
- Van Wyk, B.E.; Wink, M. Medicinal Plants of the World; Briza: Pretoria, South Africa, 2004; p. 480. [Google Scholar]
- Borotová, P.; Galovičová, L.; Valková, V.; Ďúranová, H.; Vuković, N.; Vukić, M.; Babošová, M.; Kačániová, M. Biological activity of essential oil from Foeniculumvulgare. Acta Hort. Regiotec. 2021, 24, 148–152. [Google Scholar] [CrossRef]
- Pedrotti, C.; Ribeiro, R.T.S.; Schwambach, J. Control of Postharvest Fungal Rots on Grapes Using Essential Oil of Foeniculumvulgare Mill. J. Agric. Sci. 2017, 9, 205–216. [Google Scholar]
- Bava, R.; Castagna, F.; Palma, E.; Musolino, V.; Carresi, C.; Cardamone, A.; Lupia, C.; Marrelli, M.; Conforti, F.; Roncada, P.; et al. Phytochemical profile of Foeniculumvulgare subsp. piperitum essential oils and evaluation of acaricidal efficacy against Varroa destructor in Apismellifera by in vitro and semi-field fumigation tests. Vet. Sci. 2022, 9, 684. [Google Scholar] [CrossRef]
- Koşar, M.; Özek, T.; Kürkçüoglu, M.; Başer, K.H.C. Comparison of microwave-assisted hydrodistillation and hydrodistillation methods for the fruit essential oils of Foeniculumvulgare. J. Essent. Oil Res. 2007, 19, 426–429. [Google Scholar] [CrossRef]
- Abdellaoui, M.; Bouhlali., E.T.; Kasrati, A.; El Rhaffari, L. The effect of domestication on seed yield, essential oil yield and antioxidant activities of fennel seed (Foeniculumvulgare Mill) grown in Moroccan oasis. J. Ass. Arab. Univ. Basic App. Sci. 2017, 24, 107–114. [Google Scholar]
- Özel, A.; Koşar, İ.; Demirbilek, T.; Erden, K. Changes in yields and volatile oil composition of fennel (Foeniculumvulgare Mill.) in high plant populations. Ital. J. Agron. 2019, 14, 147–152. [Google Scholar] [CrossRef]
- Hammouda, F.M.; Saleh, M.A.; Abdel-Azim, N.S.; Shams, K.A.; Ismail, S.I.; Shahat, A.A.; Saleh, I.A. Evaluation of the essential oil of Foeniculumvulgare Mill (fennel) fruits extracted by three different extraction methods by GC/MS. Afr. J. Tradit. Complement Altern. Med. 2014, 28, 277–279. [Google Scholar] [CrossRef]
- Servi, H.; Şen, A.; Yıldırım, S.E.; Doğan, A. Chemical composition and biological activities of essential oils of Foeniculumvulgare Mill. And Daucuscarota L. growing wild in Turkey. J. Res. Pharm. 2021, 25, 142–152. [Google Scholar]
- Cosge, B.; Ipek, A.; Gurbuz, B. Gas chromatography/mass spectrometry analysis of essential oil from different vegetative organs and fruits of FoeniculumvulgareMill. var. vulgare growing in Turkey. Asian J. Chem. 2009, 21, 4081–4087. [Google Scholar]
- Radulović, S.N.; Blagojević, D.P. A note on the volatile secondary metabolites of Foeniculumvulgare Mill. (Apiaceae). FactaUniversitatis. Ser. Phys. Chem. Technol. 2010, 8, 25–37. [Google Scholar] [CrossRef]
- Naves, Y.R.; Tucakov, J. Presence of anetholes in the essential oils of the fennel in Yugoslavia. Compt. Rend. 1959, 248, 843–854. [Google Scholar]
- Majid, P.; Ali, R.; Khalil, J. The effects of irrigation intervals and harvesting time on grain yield and essential oil of anise (Pimpinellaanisum L.). Iran J. Agric. Sci. 2014, 45, 453–460. [Google Scholar]
- El-Gamal, S.M.A.; Ahmed, H.M.I. Influence of different maturity stages on fruit yield and essential oil content of some Apiaceae family plants B: Fennel (Foeniculumvulgare Mill.). J. Plant Prod. Mansoura Univ. 2017, 8, 127–133. [Google Scholar] [CrossRef]
- Milenković, L.; Ilić, S.Z.; Šunić, L.J.; Tmušić, N.; Stanojević, L.J.; Cvetković, D. Modification of light intensity influence essential oils content, composition and antioxidant activity of thyme, marjoram and oregano. Saudi. J. Biol. Sci. 2021, 28, 6532–6543. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Tmušić, N.; Stanojević, L.J.; Cvetković, D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT Food Sci. Technol. 2022, 153, 112210. [Google Scholar] [CrossRef]
- Stanojević, J.S.; Stanojević, L.P.; Cvetković, D.J.; Danilović, B.R. Chemical composition, antioxidant and antimicrobial activity of the turmeric essential oil (Curcuma longa L.). Adv. Technol. 2015, 4, 19–25. [Google Scholar] [CrossRef]
- Stanojević, L.J.; Marjanović-Balaban, Z.; Kalaba, V.; Stanojević, J.; Cvetković, D.; Cakić, M. Chemical composition, antioxidant and antimicrobial activity of basil (Ocimumbasilicum L.) essential oil. J. Essent. Oil Bear. Plants 2017, 20, 1557–1569. [Google Scholar] [CrossRef]
- Peterson, L.E.; Clark, R.J.; Menary, R.C. Umbel initiation and stem elongation in fennel (Foeniculumvulgare) initiated by photoperiod. J. Ess. Oil Res. 1993, 5, 37–43. [Google Scholar] [CrossRef]
- Božović, M.; Garzoli, S.; Vujović, S.; Sapienza, F.; Ragno, R. Foeniculumvulgare Miller. a new chemotype from Montenegro. Plants 2022, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Székely, G.; Bernáth, J.; Németh, E. Floral-biological characteristics and fruit development of fennel. Acta Hortic. 2002, 576, 159–162. [Google Scholar] [CrossRef]
- Telci, I.; Demirtas, I.; Sahin, A. Variation in plant properties and essential oil composition of sweet fennel (Foeniculumvulgare Mill.) fruits during stages of maturity. Ind. Crops Prod. 2009, 30, 126–130. [Google Scholar] [CrossRef]
- Ravid, U.; Putievsky, E.; Snir, N. The volatile components of oleoresins and the essential oils of Foeniculumvulgare in Israel. J. Nat. Prod. 1983, 46, 848–851. [Google Scholar] [CrossRef]
- Pouryousef, M. Variation in the essential oil constituents in indigenous populations of Foeniculumvulgarevar. vulgare from different locations of Iran. J. Ess. Oil Res. 2014, 26, 441–445. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Tarakeme, A. Essential oil content and composition of fennel (Foeniculumvulgare L.) fruits at different stages of development. J. Essent. Oil Bear. Plants 2011, 14, 605–609. [Google Scholar] [CrossRef]
- Jugoslovenskafarmakopeja 2000. Beograd: SavremenaAdministracija, Ph.Jug.V, The European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2004. [Google Scholar]
- Telci, I.; Toncer, O.G.; Sahbaz, N. Yield, essential oil content and composition of Coriandrumsativum varieties (var. vulgareAlef and var. microcarpum DC.) grown in two different locations. J. Essent. Oil Res. 2006, 18, 189–193. [Google Scholar] [CrossRef]
- Callan, N.W.; Johnson, D.L.; Westcott, M.P.; Welty, L.E. Herb and oil composition of dill (Anethumgraveolens L.): Effects of crop maturity and plant density. Ind. Crop. Prod. 2007, 25, 282–287. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Maghsoudi, H.; Sabzalian, M.R.; Pirbalouti, A.G. Variability of essential oil content and composition of different Iranian Fennel (Foeniculumvulgare Mill.) Accessions in relation to some morphological and climatic factors. J. Agri. Sci. Technol. 2014, 16, 1365–3174. [Google Scholar]
- Khammassi, M.; Ben Ayed, R.; Loupasaki, S.; Amri, I.; Hanana, M.; Hamrouni, L.; Jamoussi, B.; Khaldi, A. Chemical diversity of wild fennel essential oils (Foeniculumvulgare Mill.): A source of antimicrobial and antioxidant activities. South Afr. J. Bot. 2023, 153, 136–146. [Google Scholar] [CrossRef]
- Wodnicka, A.; Huzar, E.; Dzięcioł, M.; Krawczyk, M. Comparison of the composition and fungicidal activity of essential oils from fennel fruits cultivated in Poland and Egypt. Pol. J. Chem. Tech. 2019, 21, 38–42. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Compounds by Gas Chromatography and Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2009. [Google Scholar]
- Telci, I.; Bayram, E.; Avci, B. Changes in yields, essential oil and linalool contents of Coriandrumsativum varieties (var. vulgareAlef. and var. microcarpumDC.) harvested at different development stages. Eur. J. Hortic. Sci. 2006, 71, 267–271. [Google Scholar]
- Msaada, K.; Hosni, K.; Taarit, M.B.; Chahed, T.; Kchouk, M.E.; Marzouk, M. Changes on essential oil composition of coriander (Coriandrumsativum L.) fruits during three stages of maturity. Food Chem. 2007, 102, 1131–1134. [Google Scholar] [CrossRef]
- Garzoli, S.; Božović, M.; Baldisserotto, A.; Sabatino, M.; Cesa, S.; Pepi, F.; Vicentini, C.B.; Manfredini, S.; Ragno, R. Essential oil extraction, chemical analysis and anti-Candida activity of Foeniculumvulgare Miller–New approaches. Nat. Prod. Res. 2017, 32, 1254–1259. [Google Scholar] [CrossRef]
- Barazani, O.; Cohen, Y.; Fait, A.; Diminshtein, S.; Dudai, N.; Ravid, U.; Putievsky, E.; Friedman, J. Chemotypic differentiation in indigenous populations of Foeniculumvulgare var. vulgare in Israel. Biochem. Syst. Ecol. 2002, 30, 721–731. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M. Characterization of some Italian types of wild fennel (Foeniculumvulgare Mill.). J. Agric. Food Chem. 2001, 49, 239–244. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Esteban, J.; Sanz, J. Comparison of the volatile composition of wild fennel samples (Foeniculumvulgare Mill.) from central Spain. J. Agric. Food Chem. 2006, 54, 6814–6818. [Google Scholar] [CrossRef]
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening the essential oil composition of wild Sicilian fennel. Biochem. Syst. Ecol. 2010, 38, 213–223. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The chemical constituents and pharmacological effects of Foeniculumvulgare-A review. IOSR J. Pharm. 2018, 8, 81–96. [Google Scholar]
- Abdullah, B.H.; Abbas, I.S.; Jasiem, T.M. Phytochemical study and evaluation of Iraqi fennel seed oil as antibacterial of urinary tract infection. Indian J. Forensic Med. Toxicol. 2020, 14, 842–846. [Google Scholar]
- Anubhuti, P.; Rahul, S.; Kant, K.C. Standardization of fennel (Foeniculumvulgare), its oleoresin and marketed ayurvedic dosage forms. Int. J. Pharm. Sci. Drug Res. 2011, 3, 265–269. [Google Scholar]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; Hammama, A.A.E.-A.; Sidky, M.M.; Mostafa, O.F.A. Distribution of transanethole and estragole in fennel (Foeniculumvulgare Mill) of callus induced from different seedling parts and fruits. Not. Sci. Biol. 2011, 3, 79–86. [Google Scholar] [CrossRef]
- Omer, E.A.; Said-Al Ahl, H.A.H.; El-Gendy, A.G. Productivity and essential oil of Foeniculumvulgare cultivated under soil salinity in Sinai comparing to non saline soil in Giza, Egypt. J. Plant Physiol. Photon. 2014, 115, 217–227. [Google Scholar]
- Abdossi, V.; Ghahremani, A.; Hadipanah, A.; Ardalani, H.; Aghaee, K. Quantitative and qualitative responses in chemical composition of three ecotypes of fennel (Foeniculumvulgare Mill.) cultivated in Iran climatic conditions. J. Biodivers. Environ. Sci. 2015, 6, 401–407. [Google Scholar]
- Gheorghita, D.; Robu, A.; Antoniac, A.; Antoniac, I.; Ditu, L.M.; Raiciu, A.D.; Tomescu, J.; Grosu, E.; Saceleanu, A. In Vitro antibacterial activity of some plant essential oils against four different microbial strains. Appl. Sci. 2022, 12, 9482. [Google Scholar] [CrossRef]
- Alvarado-García, P.A.; Soto-Vásquez, M.R.; Rosales-Cerquin, L.E.; Rodrigo-Villanueva, E.M.; Jara-Aguilar, D.R.; LurdesTuestaCollantes, L. Anxiolytic and antidepressant-like effects of Foeniculumvulgare Essential Oil. Pharmacogn. J. 2022, 14, 425–431. [Google Scholar] [CrossRef]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C. Systematic evaluation of the antioxidant potential of different parts of Foeniculumvulgare Mill. from Portugal. Food Chem. Toxicol. 2009, 47, 2458–2464. [Google Scholar] [CrossRef]
- Sharopov, F.; Valiev, A.; Satyal, P.; Gulmurodov, I.; Yusufi, S.; Setzer, W.N.; Wink, M. Cytotoxicity of the essential oil of fennel (Foeniculumvulgare) from Tajikistan. Foods 2017, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Senatore., F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; De Falco, E. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculumvulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell. Fitoterapia 2013, 90, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.; Uzunov, D.; Menichini, F. Comparative chemical composition and antioxidant activities of wild and cultivated Laurusnobilis L. leaves and Foeniculumvulgaresubsp. piperitum (Ucria) coutinho seeds. Biol. Pharm. Bull. 2006, 29, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
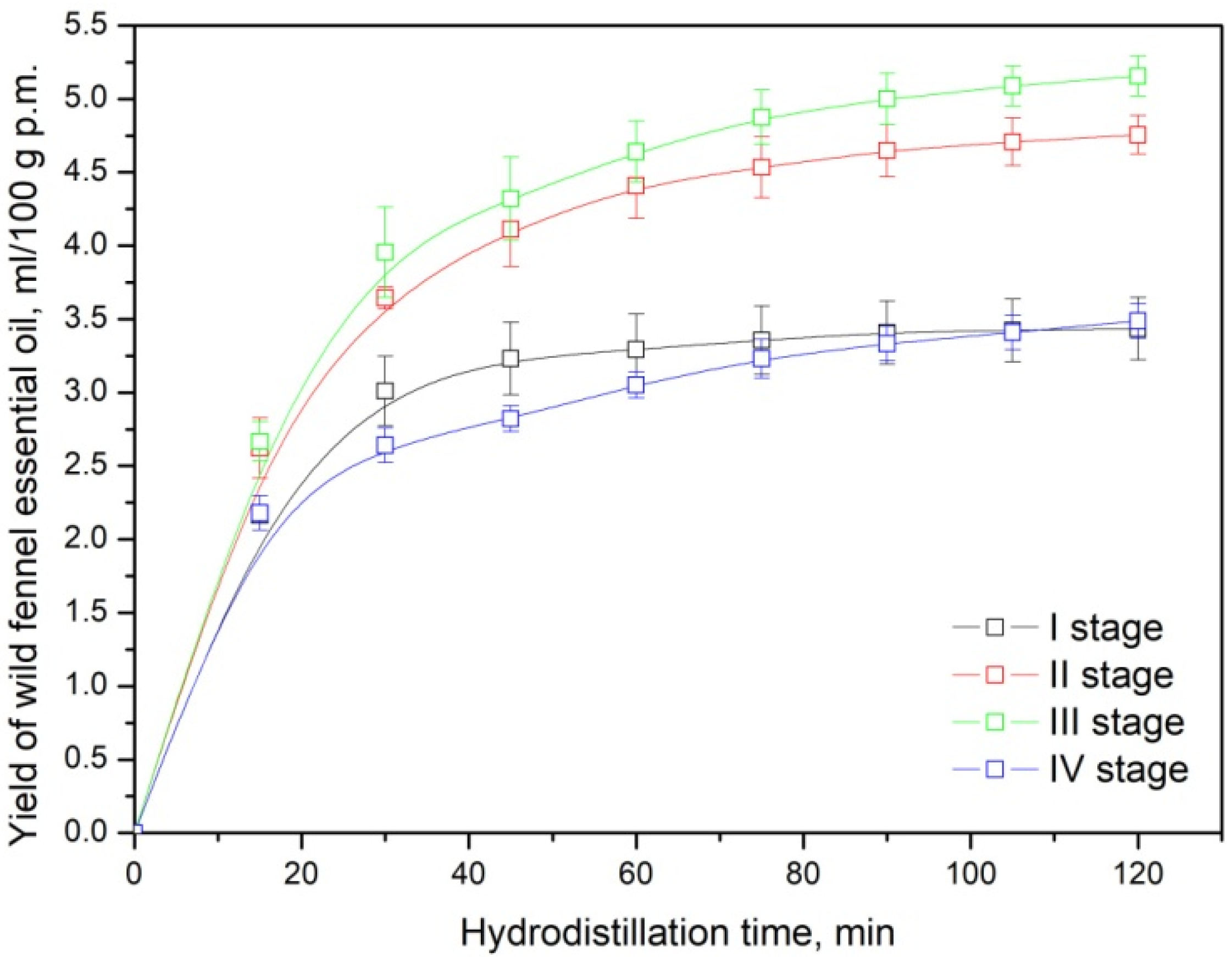
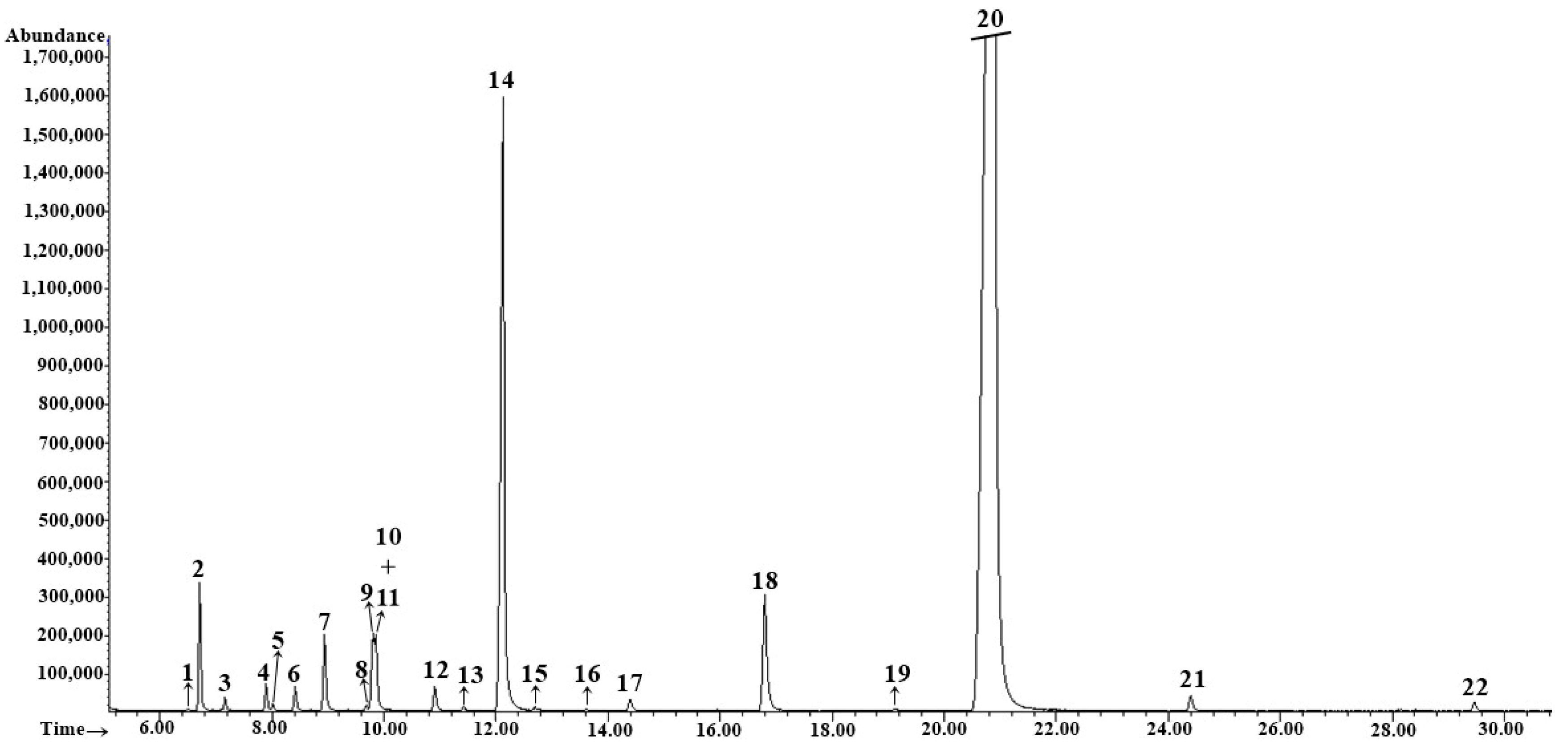
| Wild Fennel (Plant Part) | Essential Oil Yield, mL/100 g p.m. * |
|---|---|
| Leaves | 0.67 ± 0.03 a |
| Umbels-immature-pasty (1st stage) | 3.44 ± 0.21 b |
| Umbels-premature-waxy (2nd stage) | 4.76 ± 0.13 c |
| Umbels-mature-fully ripe (3rd stage) | 5.16 ± 0.14 d |
| Only fully ripe seeds (4th stage) | 3.49 ± 0.12 b |
| ** |
| No. | tret., min | Compound | RIexp | RIlit | Method of Identification | Essential Oil Content % | |||
|---|---|---|---|---|---|---|---|---|---|
| Stage of Umbel Maturity | Seed | ||||||||
| 1st | 2nd | 3rd | 4th | ||||||
| 1 | 6.52 | α-Thujene | 917 | 924 | RI, MS | 0.2 ± 0.01 | tr | tr | tr |
| 2 | 6.73 | α-Pinene | 924 | 932 | RI, MS | 1.8 ± 0.01 | 1.8 ± 0.01 | 1.3 ± 0.02 | 1.7 ± 0.01 |
| 3 | 7.18 | Camphene | 940 | 946 | RI, MS | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.02 | 0.2 ± 0.01 |
| 4 | 7.91 | Sabinene | 964 | 969 | RI, MS | 0.5 ± 0.02 | 0.4 ± 0.02 | 0.3 ± 0.02 | 0.4 ± 0.01 |
| 5 | 8.03 | β-Pinene | 968 | 974 | RI, MS, Co-I | 0.2 ± 0.01 | tr | 0.1 ± 0.01 | tr |
| 6 | 8.43 | Myrcene | 982 | 988 | RI, MS | 1.3 ± 0.01 | 0.7 ± 0.01 | 0.6 ± 0.01 | 0.5 ± 0.01 |
| 7 | 8.99 | α-Phellandrene | 1000 | 1002 | RI, MS | 11.0 ± 0.04 | 1.4 ± 0.01 | 1.2 ± 0.02 | 1.1 ± 0.01 |
| 8 | 9.13 | δ-3-Carene | 1004 | 1008 | RI, MS | 1.0 ± 0.01 | 0.2 ± 0.01 | 0.1 ± 0.01 | - |
| 9 | 9.38 | α-Terpinene | 1011 | 1014 | RI, MS | tr | tr | - | - |
| 10 | 9.72 | p-Cymene | 1020 | 1020 | RI, MS | 4.5 ± 0.02 | 0.5 ± 0.01 | 0.3 ± 0.01 | tr |
| 11 | 9.83 | Limonene | 1022 | 1024 | RI, MS, Co-I | tr | tr | 1.7 ± 0.76 | 1.3 ± 0.02 |
| 12 | 9.86 | β-Phellandrene | 1023 | 1025 | RI, MS, Co-I | 4.6 ± 0.02 | 1.3 ± 0.02 | 1.0 ± 0.02 | tr |
| 13 | 10.09 | (Z)-β-Ocimene | 1029 | 1032 | RI, MS | tr | tr | - | - |
| 14 | 10.94 | γ-Terpinene | 1052 | 1054 | RI, MS | 3.3 ± 0.01 | 1.0 ± 0.02 | 0.9 ± 0.01 | tr |
| 15 | 12.10 | Fenchone | 1082 | 1083 | RI, MS | 4.8 ± 0.02 | 9.6 ± 0.09 | 10.7 ± 0.11 | 13.7 ± 0.06 |
| 16 | 13.65 | cis-p-Menth-2-en-1-ol | 1120 | 1118 | RI, MS | tr | - | - | tr |
| 17 | 14.42 | Camphor | 1138 | 1141 | RI, MS, Co-I | tr | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.01 |
| 18 | 15.43 | Isoborneol | 1162 | 1155 | RI, MS | tr | - | - | - |
| 19 | 15.57 | Borneol | 1166 | 1165 | RI, MS, Co-I | tr | - | - | - |
| 20 | 15.95 | Terpinen-4-ol | 1175 | 1174 | RI, MS | tr | - | tr | - |
| 21 | 16.26 | 2-Methyl isoborneol | 1182 | 1178 | RI, MS | tr | - | - | - |
| 22 | 16.80 | Methyl chavicol | 1195 | 1195 | RI, MS | 2.1 ± 0.01 | 9.5 ± 0.01 | 10.3 ± 0.03 | 3.0 ± 0.01 |
| 23 | 17.11 | α-Phellandrene epoxide | 1202 | 1193 | RI, MS | 0.3 ± 0.01 | - | - | - |
| 24 | 17.49 | endo-Fenchyl acetate | 1211 | 1218 | RI, MS | tr | - | - | - |
| 25 | 18.09 | exo-Fenchyl acetate | 1225 | 1229 | RI, MS | tr | tr | - | - |
| 26 | 19.13 | (Z)-Anethole | 1249 | 1249 | RI, MS | tr | tr | tr | tr |
| 27 | 19.37 | p-Anis aldehyde | 1254 | 1247 | RI, MS | tr | - | tr | - |
| 28 | 20.29 | Isobornyl acetate | 1276 | 1283 | RI, MS | tr | - | - | - |
| 29 | 20.78 | (E)-Anethole | 1289 | 1282 | RI, MS | 64.0 ± 0.15 | 72.3 ± 0.05 | 71.6 ± 0.23 | 75.5 ± 0.26 |
| 30 | 24.10 | α-Copaene | 1365 | 1374 | RI, MS | tr | - | - | - |
| 31 | 28.44 | Germacrene D | 1474 | 1484 | RI, MS | 0.4 | tr | tr | - |
| 32 | 29.47 | β-Bisabolene | 1495 | 1505 | RI, MS | tr | - | - | 0.3 ± 0.01 |
| Total identified | 100.1 ± 0.3 | 99.0 ± 0.1 | 100 ± 1.19 | 100.1 ± 0.35 | |||||
| Grouped components (%) | |||||||||
| Monoterpene hydrocarbons (1–14) | 28.5 ± 0.12 | 7.4 ± 0.04 | 7.6 ± 0.82 | 5.6 ± 0.02 | |||||
| Oxygenated monoterpenes (15–21, 23–25, 28) | 5.1 ± 0.02 | 9.8 ± 0.08 | 10.9 ± 0.11 | 15.7 ± 0.06 | |||||
| Sesquiterpene hydrocarbons (30–32) | 0.4 ± 0.01 | tr | tr | 0.3 ± 0.01 | |||||
| Phenylpropanoids (22, 26, 27, 29) | 66.1 ± 0.16 | 81.8 ± 0.06 | 81.9 ± 0.26 | 78.5 ± 0.27 | |||||
| No. | tret., min | Compound | RIexp | RIlit | Method of Identification | Content % |
|---|---|---|---|---|---|---|
| 1 | 6.51 | α-Thujene | 917 | 924 | RI, MS | 0.5 ± 0.01 |
| 2 | 6.73 | α-Pinene | 924 | 932 | RI, MS | 2.3 ± 0.04 |
| 3 | 7.10 | α-Fenchene | 937 | 945 | RI, MS | tr |
| 4 | 7.18 | Camphene | 940 | 946 | RI, MS | 0.2 ± 0.02 |
| 5 | 7.91 | Sabinene | 964 | 969 | RI, MS | 0.5 ± 0.02 |
| 6 | 8.04 | β-Pinene | 968 | 974 | RI, MS, Co-I | 0.3 ± 0.01 |
| 7 | 8.44 | Myrcene | 982 | 988 | RI, MS | 3.1 ± 0.04 |
| 8 | 9.01 | α-Phellandrene | 1001 | 1002 | RI, MS | 18.8 ± 0.12 |
| 9 | 9.13 | δ-3-Carene | 1004 | 1008 | RI, MS | 2.4 ± 0.01 |
| 10 | 9.39 | α-Terpinene | 1011 | 1014 | RI, MS | tr |
| 11 | 9.77 | p-Cymene | 1021 | 1020 | RI, MS | 17.3 ± 0.04 |
| 12 | 9.86 | Limonene* | 1023 | 1024 | RI, MS, Co-I | tr |
| 13 | 9.90 | β-Phellandrene* | 1024 | 1025 | RI, MS, Co-I | 10.3 ± 0.15 |
| 14 | 10.10 | (Z)-β-Ocimene | 1030 | 1032 | RI, MS | tr |
| 15 | 10.93 | γ-Terpinene | 1051 | 1054 | RI, MS | tr |
| 16 | 12.09 | Fenchone | 1082 | 1083 | RI, MS | 2.8 ± 0.03 |
| 17 | 13.63 | cis-p-Menth-2-en-1-ol | 1120 | 1118 | RI, MS | tr |
| 18 | 14.41 | Camphor | 1138 | 1141 | RI, MS, Co-I | tr |
| 19 | 15.41 | Isoborneol | 1162 | 1155 | RI, MS | tr |
| 20 | 15.56 | Borneol | 1165 | 1165 | RI, MS, Co-I | tr |
| 21 | 15.94 | Terpinen-4-ol | 1174 | 1174 | RI, MS | tr |
| 22 | 16.26 | 2-Methyl isoborneol | 1182 | 1178 | RI, MS | tr |
| 23 | 16.80 | Methyl chavicol | 1195 | 1195 | RI, MS | 2.0 ± 0.02 |
| 24 | 17.12 | α-Phellandrene epoxide | 1202 | 1193 | RI, MS | 0.5 ± 0.01 |
| 25 | 17.33 | trans-Piperitol | 1207 | 1207 | RI, MS | tr |
| 26 | 17.48 | endo-Fenchyl acetate | 1211 | 1218 | RI, MS | tr |
| 27 | 18.07 | exo-Fenchyl acetate | 1224 | 1229 | RI, MS | tr |
| 28 | 18.59 | Cumin aldehyde | 1236 | 1238 | RI, MS | tr |
| 29 | 19.13 | (Z)-Anethole | 1249 | 1249 | RI, MS | tr |
| 30 | 20.78 | (E)-Anethole | 1287 | 1282 | RI, MS | 32.5 ± 0.40 |
| 31 | 21.98 | Carvacrol | 1308 | 1298 | RI, MS | 0.6 ± 0.02 |
| 32 | 23.04 | α-Longipipene | 1340 | 1350 | RI, MS | 0.3 ± 0.01 |
| 33 | 24.08 | α-Copaene | 1365 | 1374 | RI, MS | tr |
| 34 | 24.39 | Geranyl acetate | 1373 | 1379 | RI, MS | tr |
| 35 | 27.67 | Neryl propanoate | 1451 | 1452 | RI, MS | tr |
| 36 | 28.44 | Germacrene D | 1474 | 1484 | RI, MS | tr |
| 37 | 29.16 | 11-αH-Himachala-1,4-diene | 1488 | 1485 | RI, MS | tr |
| 38 | 29.49 | β-Bisabolene | 1496 | 1505 | RI, MS | 2.4 ± 0.04 |
| 39 | 30.79 | cis-α-Bisabolene | 1529 | 1529 | RI, MS | tr |
| 40 | 31.48 | Elemicin | 1547 | 1555 | RI, MS | 1.7 ± 0.01 |
| 41 | 34.84 | cis-Cadin-4-en-7-ol | 1635 | 1635 | RI, MS | 0.7 ± 0.02 |
| 42 | 36.21 | (E)-Asarone | 1672 | 1675 | RI, MS | 0.4 ± 0.01 |
| Total identified | 99.8 ± 0.85 | |||||
| Grouped components (%) | ||||||
| Monoterpene hydrocarbons (1–15) | 55.7 ± 0.34 | |||||
| Oxygenated monoterpenes (16–22, 24–28, 34, 35) | 3.5 ± 0.04 | |||||
| Sesquiterpene hydrocarbons (32, 33, 36–39) | 2.7 ± 0.04 | |||||
| Oxygenated sesquiterpenes (41) | tr | |||||
| Phenylpropanoids (23, 29–31, 40, 42) | 37.2 ± 0.43 | |||||
| Plant Part | EC50, mg/mL | ||
|---|---|---|---|
| 20 min Incubation | 40 min Incubation | 60 min Incubation | |
| Leaves | 20.22 ± 0.12 bc | 14.75 ± 0.12 ab | 12.37 ± 0.09 a |
| Umbels-immature-pasty (1st stage) | 32.99 ± 0.11 ef | 24.57 ± 0.12 cd | 20.52 ± 0.15 bc |
| Umbels-premature-waxy (2nd stage) | 49.07 ± 0.33 j | 34.12 ± 0.17 efg | 29.89 ± 0.15 de |
| Umbels-mature-fully ripe (3rd stage) | 47.46 ± 0.43 ij | 36.17 ± 0.21 fg | 31.97 ± 0.18 ef |
| Only fully ripe seed (4th stage) | 42.48 ± 0.32 hi | 39.46 ± 0.14 gh | 37.20 ± 0.31 fgh |
| Ripeness | ** | ||
| Incubation | ** | ||
| Ripeness × Incubation | * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šunić, L.; Ilić, Z.S.; Stanojević, L.; Milenković, L.; Stanojević, J.; Kovač, R.; Milenković, A.; Cvetković, D. Comparison of the Essential Oil Content, Constituents and Antioxidant Activity from Different Plant Parts during Development Stages of Wild Fennel (Foeniculum vulgare Mill.). Horticulturae 2023, 9, 364. https://doi.org/10.3390/horticulturae9030364
Šunić L, Ilić ZS, Stanojević L, Milenković L, Stanojević J, Kovač R, Milenković A, Cvetković D. Comparison of the Essential Oil Content, Constituents and Antioxidant Activity from Different Plant Parts during Development Stages of Wild Fennel (Foeniculum vulgare Mill.). Horticulturae. 2023; 9(3):364. https://doi.org/10.3390/horticulturae9030364
Chicago/Turabian StyleŠunić, Ljubomir, Zoran S. Ilić, Ljiljana Stanojević, Lidija Milenković, Jelena Stanojević, Renata Kovač, Aleksandra Milenković, and Dragan Cvetković. 2023. "Comparison of the Essential Oil Content, Constituents and Antioxidant Activity from Different Plant Parts during Development Stages of Wild Fennel (Foeniculum vulgare Mill.)" Horticulturae 9, no. 3: 364. https://doi.org/10.3390/horticulturae9030364
APA StyleŠunić, L., Ilić, Z. S., Stanojević, L., Milenković, L., Stanojević, J., Kovač, R., Milenković, A., & Cvetković, D. (2023). Comparison of the Essential Oil Content, Constituents and Antioxidant Activity from Different Plant Parts during Development Stages of Wild Fennel (Foeniculum vulgare Mill.). Horticulturae, 9(3), 364. https://doi.org/10.3390/horticulturae9030364








