Differential Responses of Cherry Tomatoes (Solanum lycopersicum) to Long-Term Heat Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Evaluation of Physiological Characteristics and Yields
2.3. Leaf Gas Exchange and Chlorophyll Fluorescence
2.4. Determination of Electrolyte Leakage Potential in Seedlings Leaves under Heat Stress
2.5. Calculation Nitrogen Use Efficiency
2.6. Statistical Analysis
3. Results
3.1. Physiological Characteristic Measurements and Analysis Simple Correlation Factors
3.2. Periodic Growth Responses in Morphological and Photoperiodic Parameters to Prolonged Heat Exposures
3.3. Effects of Long-Term Heat Stress on Leaf Heat Damage Levels and Nutrient Use Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Greenpeace. Extreme Temperature Rise in East Asia Cities. 2021. Available online: https://www.greenpeace.org/eastasia/press/6727/greenpeace-maps-growing-climate-risk-from-extreme-weather-in-chinas-major-cities/ (accessed on 7 September 2022).
- Nie, W.F.; Xing, E.; Wang, J.; Mao, Y.; Ding, X.; Guo, J. Emerging Strategies Mold Plasticity of Vegetable Plants in Response to High Temperature Stress. Plants 2022, 11, 959. [Google Scholar] [CrossRef]
- Formisano, L.; El-Nakhel, C.; Corrado, G.; Pascale, S.; Rouphael, Y. Biochemical, Physiological, and Productive Response of Greenhouse Vegetables to Suboptimal Growth Environment Induced by Insect Nets. Biology 2020, 9, 432. [Google Scholar] [CrossRef]
- Kostat. Tomato Reports. 2022. Available online: https://sri.kostat.go.kr/board.es?mid=a90102010100&bid=11918 (accessed on 7 September 2022).
- Sato, S.; Peet, M.; Thomas, J. Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress. Plant Cell Environ. 2000, 23, 719–726. [Google Scholar] [CrossRef]
- Charles, W.B.; Harris, R. Tomato fruit-set at high and low temperatures. Can. J. Plant Sci. 1972, 52, 497–506. [Google Scholar] [CrossRef]
- Dinar, M.; Rudich, J.; Zamski, E. Effects of heat stress on carbon transport from tomato leaves. Ann. Bot. 1983, 51, 97–103. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Saeed, A.; Hayat, K.; Khan, A.A.; Iqbal, S. Heat tolerance studies in tomato (Lycopersicon esculentum Mill.). Intl. J. Agr. Biol. 2007, 9, 649–652. [Google Scholar]
- Reddy, K.R.; Kakani, V.G. Screening Capsicum species of different origins for high temperature tolerance by in vitro pollen germination and pollen tube length. Sci. Hortic. 2007, 112, 130–135. [Google Scholar] [CrossRef]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat Stress Decreases Levels of Nutrient-Uptake and-Assimilation Proteins in Tomato Roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef]
- Bita, C.E.; Zenoni, S.; Vriezen, W.H.; Mariani, C.; Pezzotti, M.; Gerats, T. Temperature stress differentially modulates transcription in meiotic anthers of heat-tolerant and heat-sensitive tomato plants. BMC Genom. 2011, 12, 384. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Yang, E.Y.; Jeong, H.B.; Cho, M.C.; Chae, S.Y.; Paudel, N. Heat Treatment in Two Tomato Cultivars: A Study of the Effect on Physiological and Growth Recovery. Horticulturae 2021, 7, 119. [Google Scholar] [CrossRef]
- Qu, G.Q.; Liu, X.; Zhang, Y.L.; Yao, D.; Ma, Q.M.; Yang, M.Y.; Luo, Y.B. Evidence for programmed cell death and activation of specific caspa se-like enzymes in the tomato fruit heat stress response. Planta 2009, 229, 1269–1279. [Google Scholar] [CrossRef]
- Saidi, Y.; Peter, M.; Finka, A.; Cicekli, C.; Vigh, L.; Goloubinoff, P. Membrane lipid composition affects plant heat sensing and modulates Ca2+-dependent heat shock response. Plant Signal. Behav. 2010, 5, 1530–1533. [Google Scholar] [CrossRef]
- Fukuoka, N.; Enomoto, T. The occurrence of internal browning induced by high soil temperature treatment and its physiological function in Raphanus root. Plant Sci. 2001, 161, 117–124. [Google Scholar] [CrossRef]
- Wells, C.E.; Eissenstat, D.M. Beyond the roots of young seedlings: The influence of age and order on fine root physiology. J. Plant Growth Regul. 2002, 21, 324–334. [Google Scholar] [CrossRef]
- Guo, T.; Gull, S.; Ali, M.M.; Yousef, A.F.; Ercisli, S.; Kalaji, H.M.; Telesiński, A.; Auriga, A.; Wróbel, J.; Radwan, N.S.; et al. Heat stress mitigation in tomato (Solanum lycopersicum L.) through foliar application of gibberellic acid. Sci. Rep. 2022, 12, 11324. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving udner stress: How plants balance growht and the tresess resonse. Dev. Cell. 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Acevedo, E.; Fereres, E.; Henderson, D.W. Water stress, growth and osmotic adjustment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1976, 26, 479–500. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, L.; Wang, X.; Hou, Y.J.; Gao, J.; Wang, P.; Duan, C.G.; Zhu, X.; Zhu, J.K. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Sci. Signal. 2014, 3, 53. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Q.; Ci, D.; Shao, X.; Zhang, D. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 2014, 14, 111. [Google Scholar] [CrossRef]
- Elangbam, L.D.; Sudhir, K.; Basanta, S.; Susheel, K.S. Adaptation Strategies and Defense Mechanisms of Plants during Environmental Stress. Med. Plants 2017, 20, 359–413. [Google Scholar] [CrossRef]
- PanReacAplliChem. Nitrogen Determination by Kjeldahl Method. 2018. Available online: https://byjus.com/chemistry/kjeldahl-method/ (accessed on 21 January 2023).
- Sun, M.; Jiang, F.; Zhang, C.; Shen, M.; Wu, Z. A new comprehensive evaluation system for thermo-tolerance in tomato at different growth stage. J. Agric. Sci. Technol. B 2016, 6, 152–168. [Google Scholar] [CrossRef]
- Aldubai, A.A.; Alsadon, A.A.; Migdadi, H.H.; Alghamdi, S.S.; Al-Faifi, S.A.; Afzal, M. Response of Tomato (Solanum lycopersicum L.) Genotypes to Heat Stress Using Morphological and Expression Study. Plants 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Myster, J.; Moe, R. Effect of diurnal temperature alterations on plant morphology in some greenhouse crops—A mini review. Sci. Hort. 1995, 62, 205–215. [Google Scholar] [CrossRef]
- Gray, W.M.; Ostin, A.; Sandberg, G.; Romano, C.P.; Estelle, M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 7197–7202. [Google Scholar] [CrossRef] [PubMed]
- Scafaro, A.P.; Haynes, P.A.; Atwell, B.J. Physiological and molecular changes in Oryza meridionalis Ng, a heat-tolerant species of wild rice. J. Exp. Bot. 2010, 61, 191–202. [Google Scholar] [CrossRef]
- Nagarajan, S.; Jagadish, S.V.K.; Prasad, A.H.; Thomar, A.K.; Anand, A.; Pal, M.; Agarwal, P.K. Local climate affects growth, yield and grain quality of aromatic and non-aromatic rice in northwestern India. Agric. Ecosyst. Environ. 2010, 138, 274–281. [Google Scholar] [CrossRef]
- Craita, E.B.; Tom, G. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Xu, J.; Wolters-Arts, M.; Mariani, C.; Huber, H.; Rieu, I. Heat stress affects vegetative and reproductive performance and trait correlations in tomato (Solanum lycopersicum). Euphytica 2017, 213, 156. [Google Scholar] [CrossRef]
- Abdelmageed, A.H.A.; Gruda, N. Influence of heat shock pretreatment on growth and development of tomatoes under controlled heat stress conditions. J. Appl. Bot. Food Qual. 2007, 81, 26–28. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Mu, Q.; Cai, H.J.; Sun, S.K.; Wen, S.S.; Xu, J.T.; Dong, M.Q.; Saddique, Q. The physiological response of winter wheat under short-term drought conditions and the sensitivity of different indices to soil water changes. Agric. Water Manag. 2021, 243, 106475. [Google Scholar] [CrossRef]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef]
- Elio, E.T.M.; Chao, W.; Ashutus, S.; Elke, B.; Peiman, Z.; Beata, B.K.; Aminu, D.; Yaosheng, W. Reduced nitrogen proportion during the vegetative growth stage improved fruit yield and nitrogen uptake of cherry tomato plants under sufficient soil water regime. Acta Agric. Scand. B Soil Plant Sci. 2022, 72, 700–708. [Google Scholar] [CrossRef]
- Ashraf, M.; Iram, A.I. Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora 2005, 200, 535–546. [Google Scholar] [CrossRef]
- Hassanzadeh, M.; Asghari, A.; Jamaati-E-Somarin, S.H.; Saeidi, M.; Zabihi-E-Mahmoodabad, R.; Hokmalipour, S. Effects of water deficit on drought tolerance indices of sesame (Sesamum indicum L.) genotypes in Moghan region. J. Environ. Sci. 2009, 3, 116–121. [Google Scholar] [CrossRef]
- Kusaka, M.; Lalusin, A.G.; Fujimura, T. The maintenance of growth and turgor in pearl millet (Pennisetum glaucum [L.] Leeke) cultivars with different root structures and osmo-regulation under drought stress. Plant Sci. 2005, 168, 1–14. [Google Scholar] [CrossRef]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B.; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef]


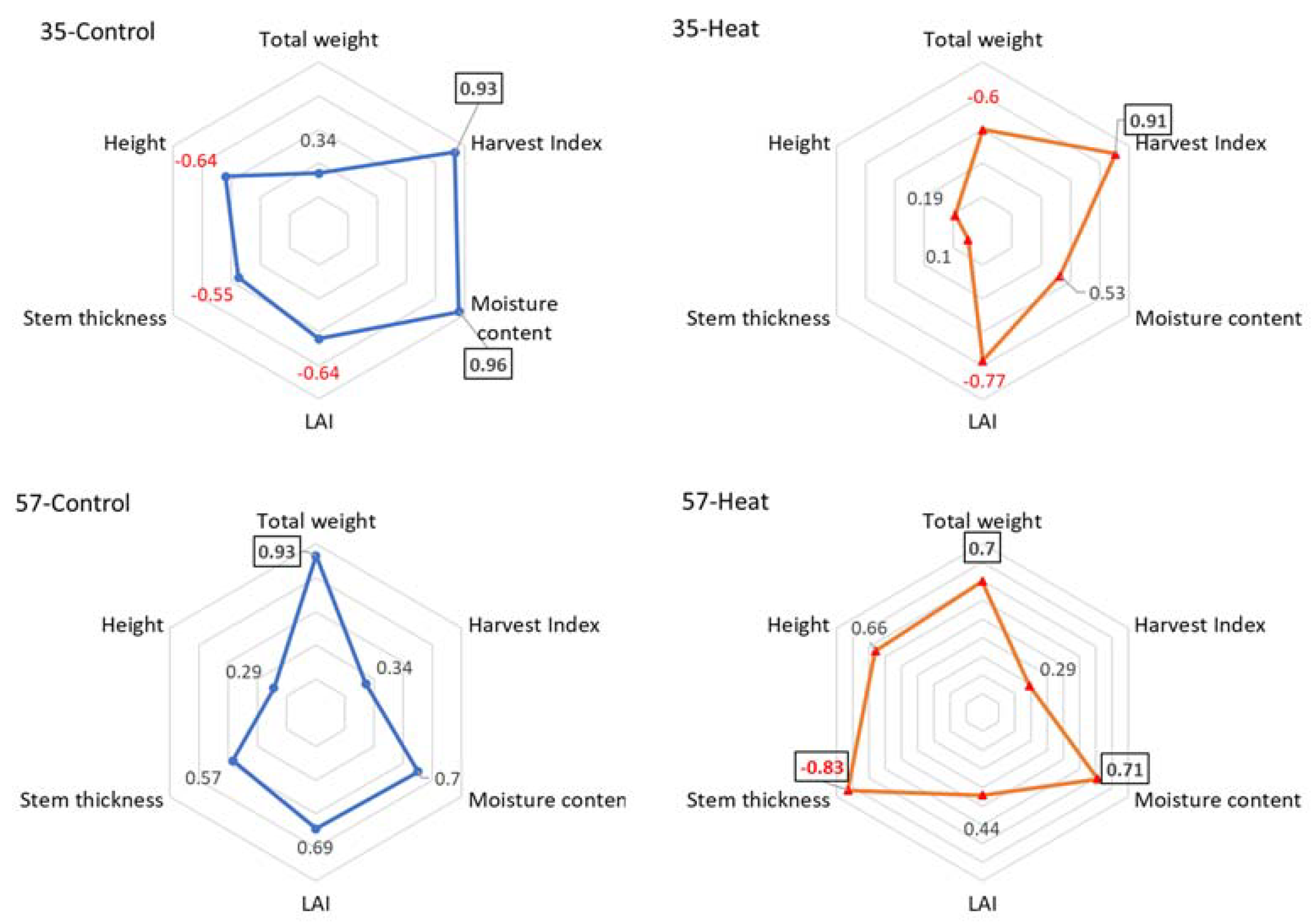
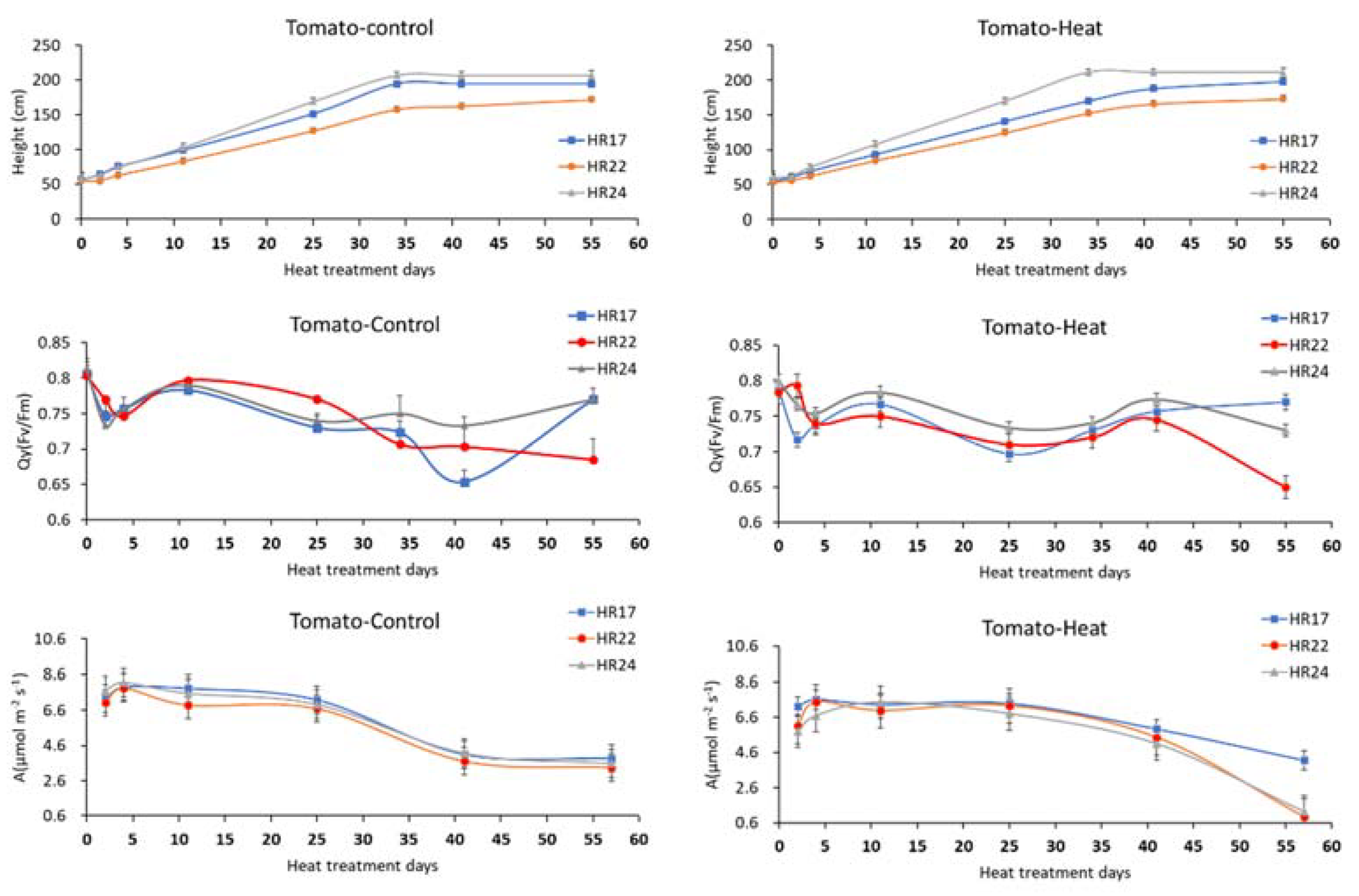
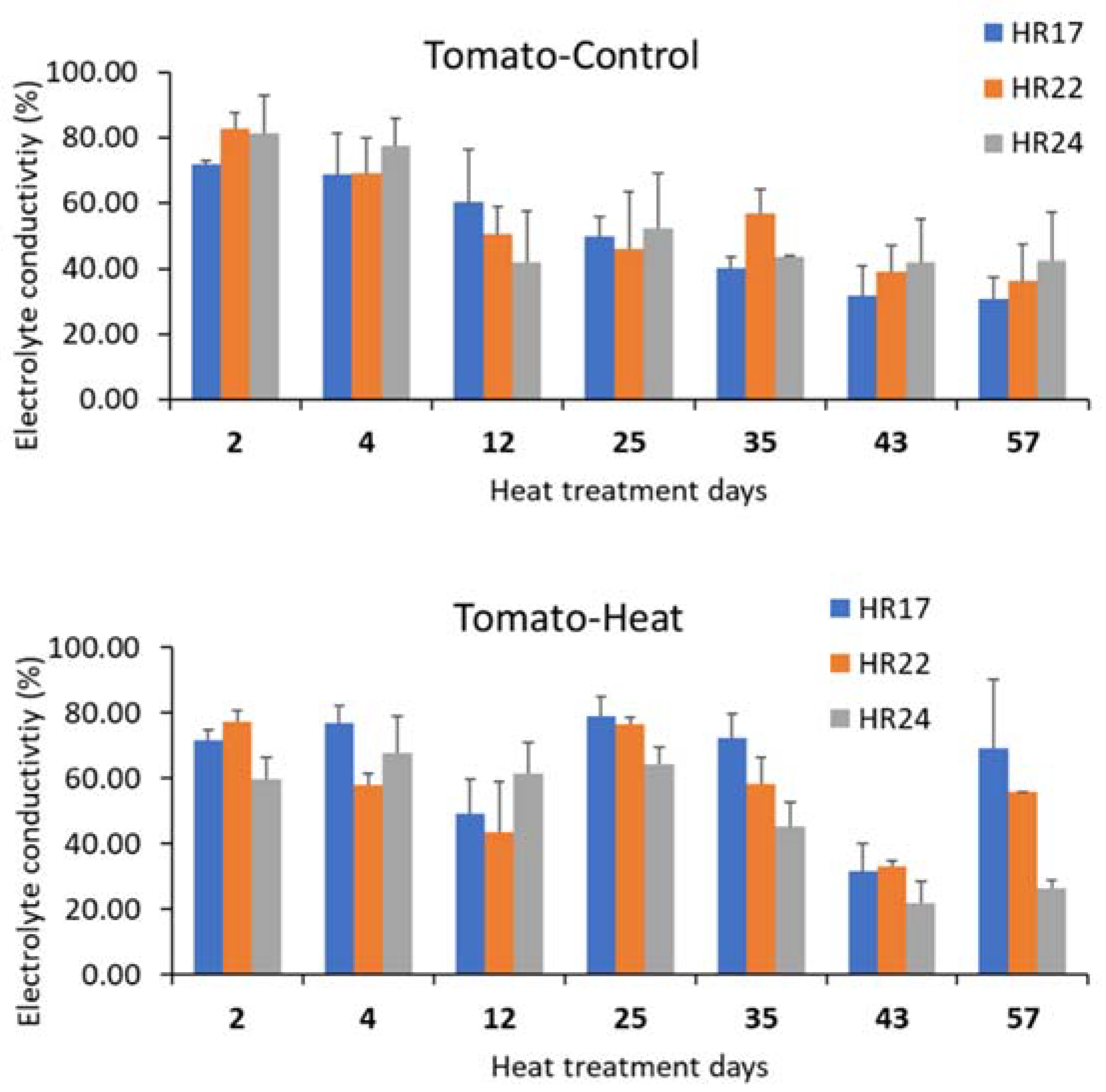
| Control | Heat Stress | Yield Difference (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Tomato | Total Fresh | Fresh Fruit | Harvest | Total Fresh | Fresh Fruit | Harvest | Total | |
| Varieties | Weight (g) | Weight (g) | Index | Weight (g) | Weight (g) | Index | Weight | Fruit |
| After 35 days | ||||||||
| HR17 | 1123 ± 192 | 687 ± 129 | 0.61 | 640 ± 14 | 395 ± 57 | 0.62 | −43 | −43 |
| HR22 | 1325 ± n.a. | 800 ± n.a. | 0.6 | 640 ± 14 | 348 ± 25 | 0.55 | −52 | −57 |
| HR24 | 1193 ± 131 | 293 ± 74 | 0.25 | 893 ± 272 | 300 ± 7 | 0.36 | −25 | 2 |
| After 57 days | ||||||||
| HR17 | 1594 ± 1071 | 1078 ± 721 | 0.50 | 742 ± 366 | 390 ± 227 | 0.50 | −53 | −64 |
| HR22 | 1453 ± 787 | 821 ± 463 | 0.56 | 617 ± 293 | 268 ± 118 | 0.44 | −58 | −67 |
| HR24 | 1559 ± 764 | 664 ± 299 | 0.43 | 1430 ± 97 | 438 ± 146 | 0.30 | −8 | −34 |
| Heat Stress Days | Factors | Fresh Weight | Fruit Weight | Harvest Index | Moisture Content | Leaf Area Index | Height | Stem |
|---|---|---|---|---|---|---|---|---|
| 35 | Trt. | 0.0032 | 0.004 | n.s. | 0.0002 | n.s. | n.s. | n.s. |
| AC | n.s. | 0.008 | 0.001 | 0.003 | 0.04 | 0.004 | n.s. | |
| Trt*AC | n.s. | 0.04 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| 57 | Trt. | n.s. | n.s. | 0.0005 | 0.04 | n.s. | n.s. | n.s. |
| AC | n.s. | n.s. | 0.0004 | n.s. | n.s. | 0.08 | n.s. | |
| Trt*AC | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| 35 Days | 57 Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Tomato Varieties | Moisture Content (%) | LAI | Plant Height (cm) | Stem Thickness (mm) | Moisture Content (%) | LAI | Plant Height (cm) | Stem Thickness (mm) |
| Control | HR17 | 89 ± 1.1 | 0.87 ± 0.05 | 180 ± 4 | 14.05 ± 0.33 | 87 ± 3.6 | 1.82 ± 1.40 | 186 ± 17 | 14.89 ± 1.55 |
| HR22 | 90 ± n.a. | 1.28 ± n.a. | 162 ± 10 | 13.95 ± 0.7 | 85 ± 4.6 | 1.62 ± 1.32 | 171 ± 50 | 14.54 ± 2.15 | |
| HR24 | 87 ± 0.1 | 1.69 ± 0.37 | 185 ± 5 | 14.72 ± 0.47 | 86 ± 4.3 | 2.69 ± 1.96 | 189 ± 21 | 15.91 ± 3.6 | |
| Heat | HR17 | 86 ± 0.8 | 0.62 ± 0.32 | 189 ± 6 | 15.25 ± 1.80 | 78 ± 6.4 | 1.16 ± 0.63 | 198 ± 7 | 15.11 ± 1.69 |
| HR22 | 87 ± 0.3 | 0.67 ± 0.09 | 166 ± 24 | 14.82 ± 1.47 | 73 ± 12.2 | 1.48 ± 0.30 | 173 ± 37 | 15.24 ± 1.88 | |
| HR24 | 83 ± 0.2 | 1.18 ± 0.54 | 188 ± 5 | 14.92 ± 0.86 | 84 ± 1.8 | 2.52 ± 0.19 | 190 ± 11 | 15.31 ± 2.33 | |
| Factors | Height | Qy | A | EC |
|---|---|---|---|---|
| Trt. Days (D) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatment (Trt) | <0.0001 | n.s. | 0.058 | n.s. |
| D*Trt | <0.0001 | 0.0049 | 0.006 | 0.0345 |
| Accession (AC) | n.s. | n.s. | n.s. | n.s. |
| D*AC | n.s. | 0.0234 | n.s. | n.s. |
| Trt*AC | n.s. | n.s. | n.s. | n.s. |
| D*Trt*AC | n.s. | n.s. | n.s. | n.s. |
| Treatment | Accessions | NUE (%) | Fruit/Biomass | ||||
|---|---|---|---|---|---|---|---|
| 35D | 57D | ||||||
| Fruit | Biomass | Fruit | Biomass | 35D | 57D | ||
| Control | HR17 | 49.2 | 49.9 | 14.1 | 33.1 | 1.0 | 0.4 |
| HR22 | 40.7 | 40.6 | 13.3 | 26.9 | 1.0 | 0.5 | |
| HR24 | 32.2 | 75.9 | 10.1 | 53.1 | 0.4 | 0.2 | |
| Heat | HR17 | 6.9 | 6.4 | 2.2 | 8.5 | 1.1 | 0.3 |
| HR22 | 3.0 | 7.3 | 1.1 | 9.2 | 0.4 | 0.1 | |
| HR24 | 8.0 | 15.9 | 2.8 | 18.0 | 0.5 | 0.2 | |
| Effect | p-value | Effect | p-value | ||||
| Days | <0.0001 | Organ | <0.0001 | ||||
| Treatment (Trt) | 0.045 | Days*Organ | <0.0001 | ||||
| Days*Trt | 0.016 | Trt*Organ | n.s. | ||||
| Accessions (AC) | <0.0001 | Days*Trt*Organ | 0.045 | ||||
| Days*AC | n.s. | AC*Organ | <0.0001 | ||||
| Trt*AC | n.s. | Days*AC*Organ | n.s. | ||||
| Days*Trt*AC | n.s. | Trt*AC*Organ | n.s. | ||||
| Days*Trt*AC*Organ | n.s. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.-M.; Jeong, H.-B.; Yang, E.-Y.; Kim, M.-K.; Kim, J.-W.; Chae, W.; Lee, O.-J.; Kim, S.G.; Kim, S. Differential Responses of Cherry Tomatoes (Solanum lycopersicum) to Long-Term Heat Stress. Horticulturae 2023, 9, 343. https://doi.org/10.3390/horticulturae9030343
Park B-M, Jeong H-B, Yang E-Y, Kim M-K, Kim J-W, Chae W, Lee O-J, Kim SG, Kim S. Differential Responses of Cherry Tomatoes (Solanum lycopersicum) to Long-Term Heat Stress. Horticulturae. 2023; 9(3):343. https://doi.org/10.3390/horticulturae9030343
Chicago/Turabian StylePark, Bo-Mi, Hyo-Bong Jeong, Eun-Young Yang, Min-Kyoung Kim, Ji-Won Kim, Wonbyoung Chae, Oak-Jin Lee, Sang Gyu Kim, and Sumin Kim. 2023. "Differential Responses of Cherry Tomatoes (Solanum lycopersicum) to Long-Term Heat Stress" Horticulturae 9, no. 3: 343. https://doi.org/10.3390/horticulturae9030343
APA StylePark, B.-M., Jeong, H.-B., Yang, E.-Y., Kim, M.-K., Kim, J.-W., Chae, W., Lee, O.-J., Kim, S. G., & Kim, S. (2023). Differential Responses of Cherry Tomatoes (Solanum lycopersicum) to Long-Term Heat Stress. Horticulturae, 9(3), 343. https://doi.org/10.3390/horticulturae9030343







