Quality Improvement of Tomato Fruits by Preharvest Application of Chitosan Oligosaccharide
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Treatments and Sampling
2.3. Fruit Quality Index Determination
2.4. Volatile Profiles Analysis
2.5. Ethylene Production Analysis
2.6. Gene Expression Analysis
2.7. Statistics
3. Results
3.1. Effect of COS Treatment on Fruit Coloring and Firmness
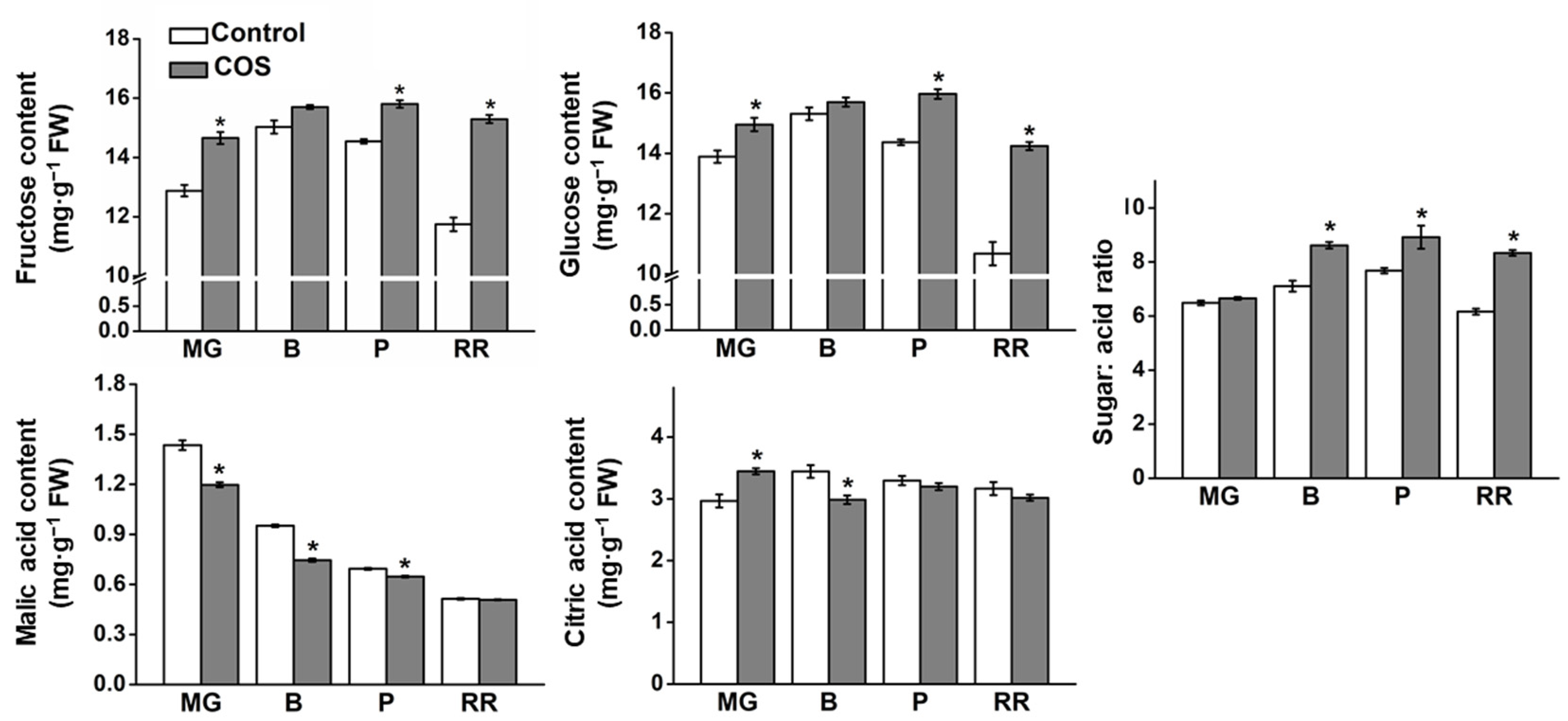
3.2. Effect of COS Treatment on Sugar and Organic Acid Content
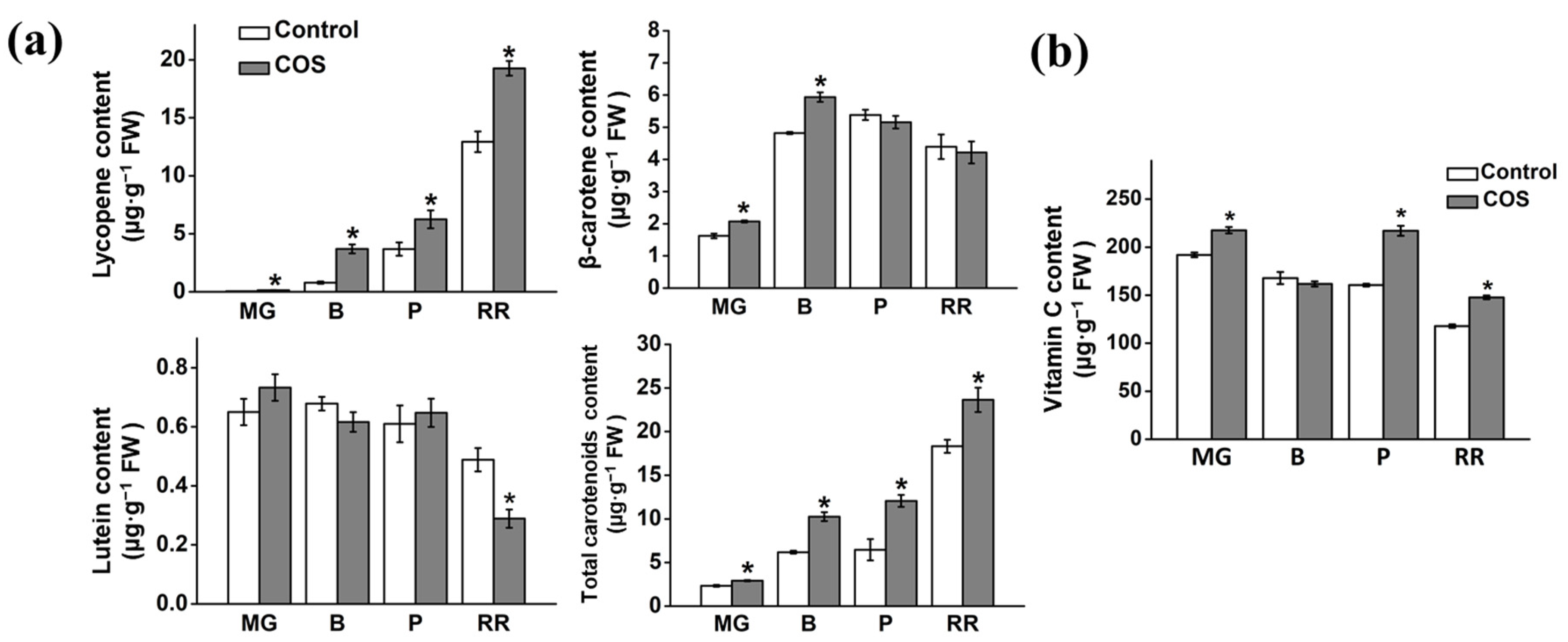
3.3. Effect of COS Treatment on Carotenoid Composition and Content
3.4. Effect of COS Treatment on Vitamin C Content
3.5. Effect of COS Treatment on Volatile Profiles
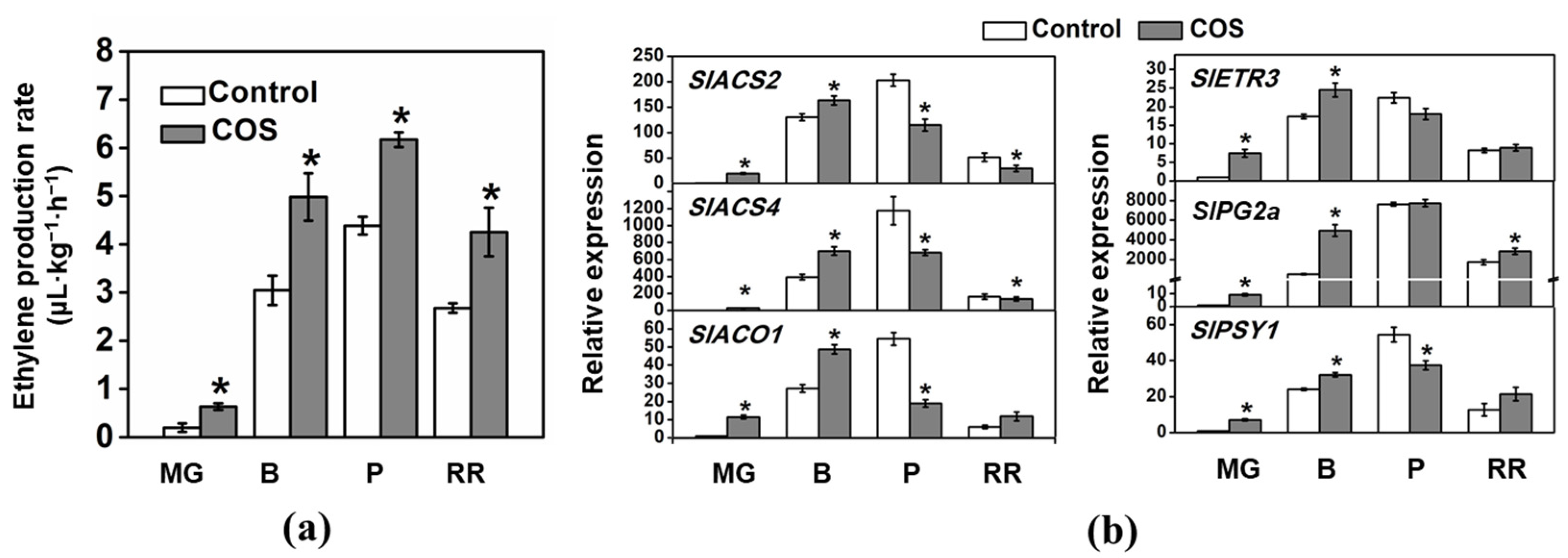
3.6. Effect of COS on Ethylene Production and Signaling in Tomato Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Meng, F.; Miao, H.; Chen, S.; Yin, T.; Hu, S.; Shao, Z.; Liu, Y.; Gao, L.; Zhu, C.; et al. Effects of postharvest methyl jasmonate treatment on main health-promoting components and volatile organic compounds in cherry tomato fruits. Food Chem. 2018, 263, 194–200. [Google Scholar] [CrossRef]
- Liu, H.; Meng, F.; Chen, S.; Yin, T.; Hu, S.; Shao, Z.; Liu, Y.; Zhu, C.; Ye, H.; Wang, Q. Ethanol treatment improves the sensory quality of cherry tomatoes stored at room temperature. Food Chem. 2019, 298, 125069. [Google Scholar] [CrossRef]
- Thole, V.; Vain, P.; Yang, R.-Y.; Almeida Barros da Silva, J.; Enfissi, E.M.A.; Nogueira, M.; Price, E.J.; Alseekh, S.; Fernie, A.R.; Fraser, P.D.; et al. Analysis of tomato post-harvest properties: Fruit color, shelf life, and fungal susceptibility. Curr. Protoc. Plant Bio. 2020, 5, e20108. [Google Scholar] [CrossRef]
- Hu, Y.; Han, Z.; Sun, Y.; Wang, S.; Wang, T.; Wang, Y.; Xu, K.; Zhang, X.; Xu, X.; Han, Z.; et al. ERF4 affects fruit firmness through TPL4 by reducing ethylene production. Plant J. 2020, 103, 937–950. [Google Scholar] [CrossRef]
- Meng, F.; Li, Y.; Li, S.; Chen, H.; Shao, Z.; Jian, Y.; Mao, Y.; Liu, L.; Wang, Q. Carotenoid biofortification in tomato products along whole agro-food chain from field to fork. Trends Food Sci. Tech. 2022, 124, 296–308. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. The genetics of fruit flavour preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.; Resende, M.F., Jr.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Cara, B.; Giovannoni, J.J. Molecular biology of ethylene during tomato fruit development and maturation. Plant Sci. 2008, 175, 106–113. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Mukhtar Ahmed, K.B.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production- a review. Carbohyd. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Khan, M.M.; Jaleel, H.; Sadiq, Y.; Shabbir, A.; Uddin, M. Exogenously sourced γ-irradiated chitosan-mediated regulation of growth, physiology, quality attributes, and yield in Mentha piperita L. Turk. J. Biol. 2017, 41, 388–401. [Google Scholar] [CrossRef]
- Dzung, P.D.; Phu, D.V.; Du, B.D.; Ngoc, L.S.; Duy, N.N.; Hiet, H.D.; Nghia, D.H.; Thang, N.T.; Le, B.V.; Hien, N.Q. Effect of foliar application of oligochitosan with different molecular weight on growth promotion and fruit yield enhancement of chili plant. Plant Prod. Sci. 2017, 20, 389–395. [Google Scholar] [CrossRef]
- El-Sawy, N.M.; Abd El-Rehim, H.A.; Elbarbary, A.M.; Hegazy, E.-S.A. Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohyd. Polym. 2010, 79, 555–562. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Liu, S.; Zou, P.; Xing, R.; Yu, H.; Chen, X.; Qin, Y.; Li, P. Relationship between the degree of polymerization of chitooligomers and their activity affecting the growth of wheat seedlings under salt stress. J. Agr. Food Chem. 2017, 65, 501–509. [Google Scholar] [CrossRef]
- Gu, L. Effects of chitosan oligosaccharide on seed germination of tomato. Southwest China J. Agric. Sci. 2014, 27, 1233–1236. [Google Scholar]
- Zhang, X.; Li, K.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, P. Size effects of chitooligomers on the growth and photosynthetic characteristics of wheat seedlings. Carbohyd. Polym. 2016, 138, 27–33. [Google Scholar] [CrossRef]
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Ali, A.; Mir, S.R.; Varshney, L. Effect of Co-60 gamma irradiated chitosan and phosphorus fertilizer on growth, yield and trigonelline content of Trigonella foenum-graecum L. J. Radiat. Res. Appl. Sc. 2015, 8, 446–458. [Google Scholar] [CrossRef]
- Jaleel, H.; Khan, M.M.A.; Ahmad, B.; Shabbir, A.; Sadiq, Y.; Uddin, M.; Varshney, L. Essential oil and citral production in field-grown lemongrass in response to gamma-irradiated chitosan. J. Herbs Spices Med. Plants 2017, 23, 378–392. [Google Scholar] [CrossRef]
- Dzung, N.A.; Khanh, V.T.P.; Dzung, T.T. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohyd. Polym. 2011, 84, 751–755. [Google Scholar] [CrossRef]
- Hemantaranjan, A.; Katiyar, D.; Singh, B.; Bhanu, A.N. A future perspective in crop protection chitosan and its oligosaccharides. Adv. Plants Agric. Res. 2014, 1, 23–30. [Google Scholar] [CrossRef]
- Zou, P.; Li, K.; Liu, S.; Xing, R.; Qin, Y.; Yu, H.; Zhou, M.; Li, P. Effect of chitooligosaccharides with different degrees of acetylation on wheat seedlings under salt stress. Carbohyd. Polym. 2015, 126, 62–69. [Google Scholar] [CrossRef]
- He, J.; Han, W.; Wang, J.; Qian, Y.; Saito, M.; Bai, W.; Song, J.; Lv, G. Functions of oligosaccharides in improving tomato seeding growth and chilling resistance. J. Plant Growth Regul. 2022, 41, 535–545. [Google Scholar] [CrossRef]
- Islam, M.M.; Kabir, M.H.; Mamun, A.N.K.; Islam, M.; Islam, M.M.; Das, P. Studies on yield and yield attributes in tomato and chilli using foliar application of oligo-chitosan. GSC Biol. Pharm. Sci. 2018, 3, 20–28. [Google Scholar] [CrossRef]
- Lei, F.; Zhang, D.; Tan, H.; Pan, X.; Wu, Y.; Liu, G.; Xiao, T. Effect of oligochitosan on yield, quality, and Phytophthora infestans control of cherry tomato. Guizhou Nong Ye Ke Xue 2019, 47, 74–77. [Google Scholar]
- Sultana, S.; Islam, M.; Khatun, M.; Hassain, M.; Huque, R. Effect of foliar application of oligo-chitosan on growth, yield and quality of tomato and eggplant. Asian J. Agric. Res. 2017, 11, 36–42. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Liang, D.; Zhang, M.; Jia, C.; Qi, M.; Liu, Y.; Shao, Z.; Meng, F.; Hu, S.; et al. SlBES1 promotes tomato fruit softening through transcriptional inhibition of PMEU1. iScience 2021, 24, 102926. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Zhang, M.; Zhang, Y.; Wang, Q. Effect of carbon dioxide enrichment on health-promoting compounds and organoleptic properties of tomato fruits grown in greenhouse. Food Chem. 2014, 153, 157–163. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, W.; Chen, K.; Zhang, B. Transcriptome and methylome analysis reveals effects of ripening on and off the vine on flavor quality of tomato fruit. Postharvest Biol. Technol. 2020, 162, 111096. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y. Evaluation of the effects of irrigation and fertilization on tomato fruit yield and quality: A principal component analysis. Sci. Rep. 2017, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Chen, H.; Hu, S.; Liu, H.; Meng, F.; Li, S.; Zhang, B.; Zhu, C.; Wang, G.; Liu, L.; et al. Chitosan oligosaccharide treatment improves quality attributes of tomato fruit stored under room temperature. Postharvest Biol. Technol. 2022, 189, 111914. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.A.; Bai, J. Recent advance in aromatic volatile research in tomato fruit: The metabolisms and regulations. Food Bioproc. Tech. 2016, 9, 203–216. [Google Scholar] [CrossRef]
- Rambla, J.L.; Tikunov, Y.M.; Monforte, A.J.; Bovy, A.G.; Granell, A. The expanded tomato fruit volatile landscape. J. Exp. Bot. 2014, 65, 4613–4623. [Google Scholar] [CrossRef]
- Llop-Tous, I.; Barry, C.S.; Grierson, D. Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol. 2000, 123, 971–978. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Murachi, S.; Okunishi, H.; Shiomi, S.; Nakano, R.; Kubo, Y.; Inaba, A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998, 118, 1295–1305. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef]
- Meng, X.; Yang, D.; Li, X.; Zhao, S.; Sui, N.; Meng, Q. Physiological changes in fruit ripening caused by overexpression of tomato SlAN2, an R2R3-MYB factor. Plant Physiol. Bioch. 2015, 89, 24–30. [Google Scholar] [CrossRef]
- Fray, R.G.; Grierson, D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol. Biol. 1993, 22, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Diretto, G.; Purgatto, E.; Danoun, S.; Zouine, M.; Li, Z.; Roustan, J.-P.; Bouzayen, M.; Giuliano, G.; Chervin, C. Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Kerch, G.; Sabovics, M.; Kruma, Z.; Kampuse, S.; Straumite, E. Effect of chitosan and chitooligosaccharide on vitamin C and polyphenols contents in cherries and strawberries during refrigerated storage. Eur. Food Res. Technol. 2011, 233, 351–358. [Google Scholar] [CrossRef]
- Ma, L.; Cao, J.; Xu, L.; Zhang, X.; Wang, Z.; Jiang, W. Effects of 1-methylcyclopropene in combination with chitosan oligosaccharides on post-harvest quality of aprium fruits. Sci. Hortic. 2014, 179, 301–305. [Google Scholar] [CrossRef]
- Deng, L.; Yin, B.; Yao, S.; Wang, W.; Zeng, K. Postharvest application of oligochitosan and chitosan reduces calyx alterations of citrus fruit induced by ethephon degreening treatment. J. Agr. Food Chem. 2016, 64, 7394–7403. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Zhao, H.; Chen, N.; Cao, J.; Jiang, W. Effects of oligochitosan on postharvest Alternaria rot, storage quality, and defense responses in Chinese jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. J. Food Protect. 2011, 74, 783–788. [Google Scholar] [CrossRef]
- Winter, H.; Huber, S.C. Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit. Rev. Biochem. Mol. 2000, 35, 253–289. [Google Scholar] [CrossRef]
- Khan, W.M.; Prithiviraj, B.; Smith, D.L. Effect of foliar application of chitin and chitosan oligosaccharides on photosynthesis of maize and soybean. Photosynthetica 2002, 40, 621–624. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, M.; Liu, M.; Su, D.; Chen, J.; Gao, Y.; Bouzayen, M.; Li, Z. The molecular regulation of ethylene in fruit ripening. Small Methods 2020, 4, 1900485. [Google Scholar] [CrossRef]

| Volatiles | Precursors | Content (μg·kg−1 FW) | Overall Liking | Overall Flavor Intensity | |
|---|---|---|---|---|---|
| Control | COS Treatment | ||||
| 1-penten-3-one | Lipid | 53.1 ± 1.1 | 59.4 ± 1.4 * | + | + |
| (E)-2-pentenal | Lipid | 11.7 ± 0.6 | 30.3 ± 0.5 * | + | + |
| Hexanal | Lipid | 382.6 ± 11.2 | 787.6 ± 16.2 * | ||
| (Z)-3-hexenal | Lipid | 136.9 ± 4.1 | 110.3 ± 3.7 * | ||
| (E)-2-hexenal | Lipid | 1069.8 ± 59.8 | 1734.8 ± 28.2 * | + | |
| (E)-3-hexen-1-ol | Lipid | 3.6 ± 0.1 | 4.3 ± 0.2 * | + | + |
| Hexanol | Lipid | 37.6 ± 5.7 | 77.1 ± 3.9 * | ||
| Heptanal | Lipid | 15.2 ± 0.4 | 19.1 ± 0.5 * | ||
| (E)-2-heptenal | Lipid | 60.0 ± 1.5 | 99.6 ± 3.3 * | + | + |
| 2-octanone | Lipid | 323.4 ± 2.5 | 301.9 ± 6.2 * | ||
| 1-octen-3-one | Lipid | 26.5 ± 1.8 | 34.5 ± 0.8 * | + | |
| 1-nonanal | Lipid | 26.3 ± 2.0 | 26.1 ± 1.6 | ||
| (E, E)-2,4-decadienal | Lipid | U.d | 7.3 ± 0.4 * | + | |
| 2-isobutylthiazole | Branched-chain amino acid | 288.1 ± 7.5 | 350.4 ± 6.1 * | + | + |
| Isovaleric acid | Branched-chain amino acid | 15.9 ± 0.3 | 15.4 ± 0.4 | + | + |
| Phenylacetaldehyde | Phenylalanine | 2.4 ± 0.1 | 7.0 ± 0.1 * | + | + |
| Benzyl alcohol | Phenylalanine | 2.7 ± 0.1 | 5.0 ± 0.2 * | + | |
| 2-phenylethanol | Phenylalanine | 5.7 ± 0.2 | 23.1 ± 0.6 * | + | + |
| Geranylacetone | Carotenoid | 142.1 ± 3.4 | 253.9 ± 8.6 * | ||
| 6-methyl-5-hepten-2-one | Carotenoid | 307.1 ± 12.8 | 496.8 ± 6.1 * | + | |
| 6-methyl-5-hepten-2-ol | Carotenoid | 42.8 ± 0.1 | 46.7 ± 0.9 * | + | + |
| β-ionone | Carotenoid | 11.3 ± 0.5 | 17.1 ± 0.7 * | + | + |
| Total volatiles | 2964.9 ± 116.7 | 4507.5 ± 90.4 * | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Chen, H.; Wang, T.; Mustafa, G.; Liu, L.; Wang, Q.; Shao, Z. Quality Improvement of Tomato Fruits by Preharvest Application of Chitosan Oligosaccharide. Horticulturae 2023, 9, 300. https://doi.org/10.3390/horticulturae9030300
Zheng J, Chen H, Wang T, Mustafa G, Liu L, Wang Q, Shao Z. Quality Improvement of Tomato Fruits by Preharvest Application of Chitosan Oligosaccharide. Horticulturae. 2023; 9(3):300. https://doi.org/10.3390/horticulturae9030300
Chicago/Turabian StyleZheng, Jirong, Hao Chen, Tonglin Wang, Ghazala Mustafa, Lihong Liu, Qiaomei Wang, and Zhiyong Shao. 2023. "Quality Improvement of Tomato Fruits by Preharvest Application of Chitosan Oligosaccharide" Horticulturae 9, no. 3: 300. https://doi.org/10.3390/horticulturae9030300
APA StyleZheng, J., Chen, H., Wang, T., Mustafa, G., Liu, L., Wang, Q., & Shao, Z. (2023). Quality Improvement of Tomato Fruits by Preharvest Application of Chitosan Oligosaccharide. Horticulturae, 9(3), 300. https://doi.org/10.3390/horticulturae9030300








