Analysis of the Postharvest Storage Characteristics of Two New Pear Cultivars ‘Shannongsu’ and ‘Xincixiang’
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of Fruit Firmness
2.2.2. Determination of Ethylene Release Rate
2.2.3. Extraction and Detection of Fruit Aroma Components
2.2.4. Determination of Enzyme Activities
2.2.5. RNA Extraction and qRT-PCR Analysis
3. Results
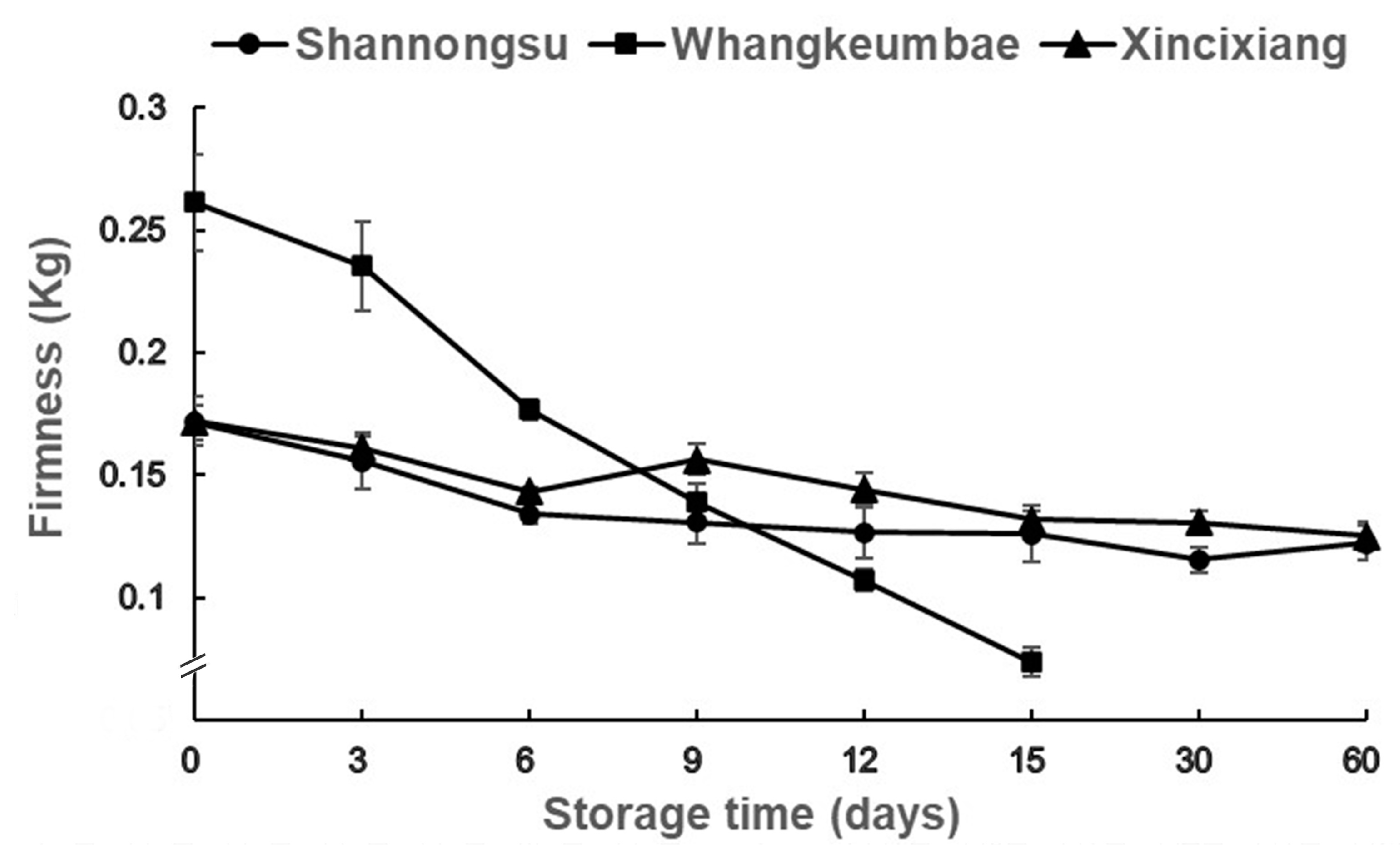
3.1. Changes in Fruit Firmness
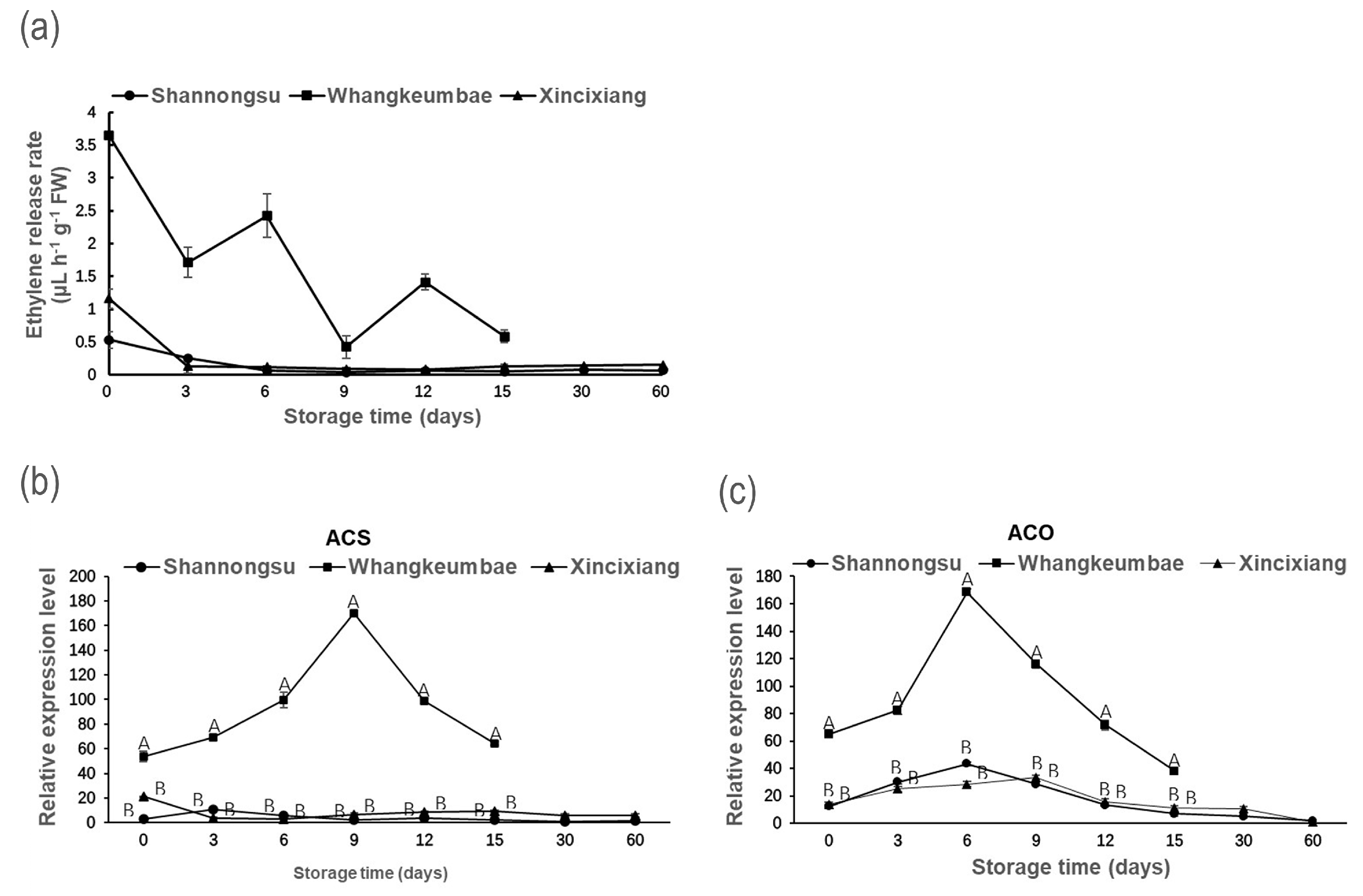
3.2. The Production of Ethylene
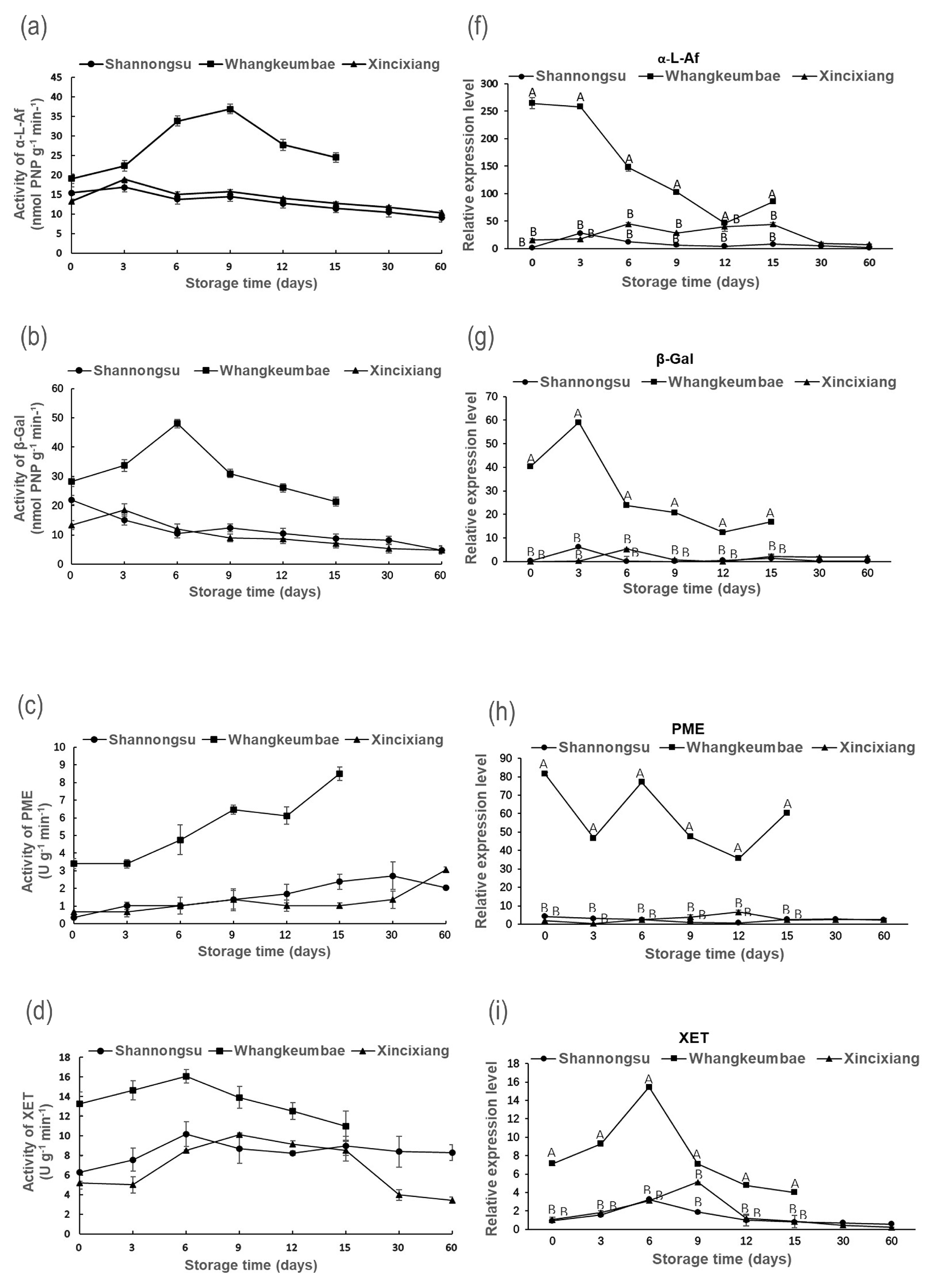
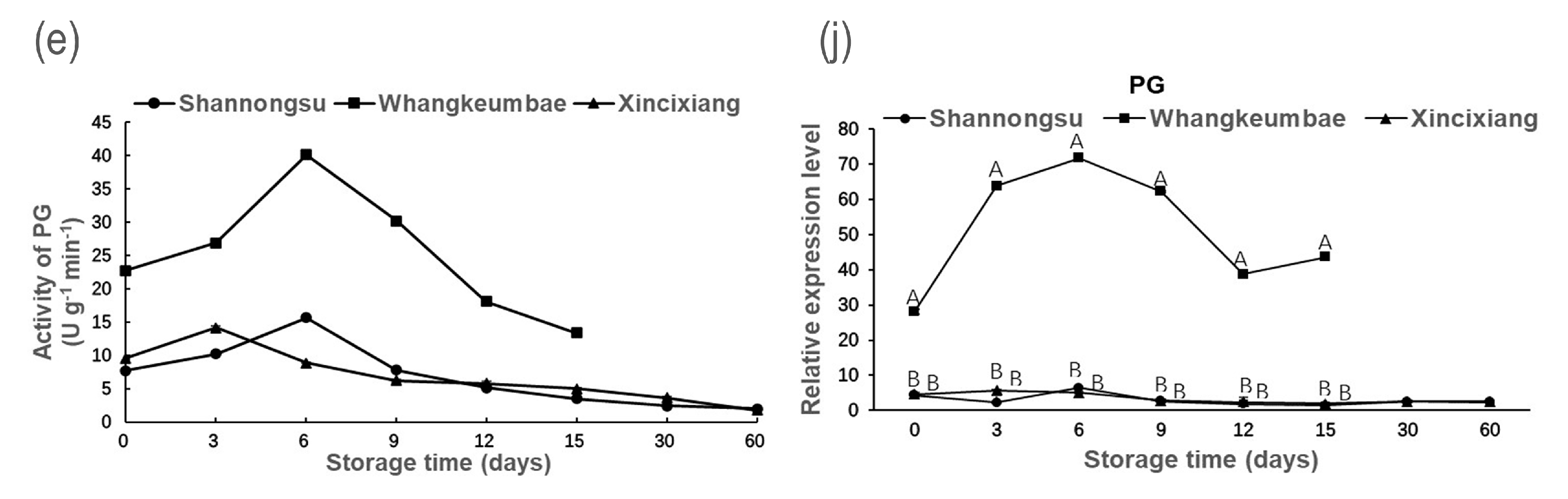
3.3. Fruit Softening-Related Enzyme Activity and Gene Expression
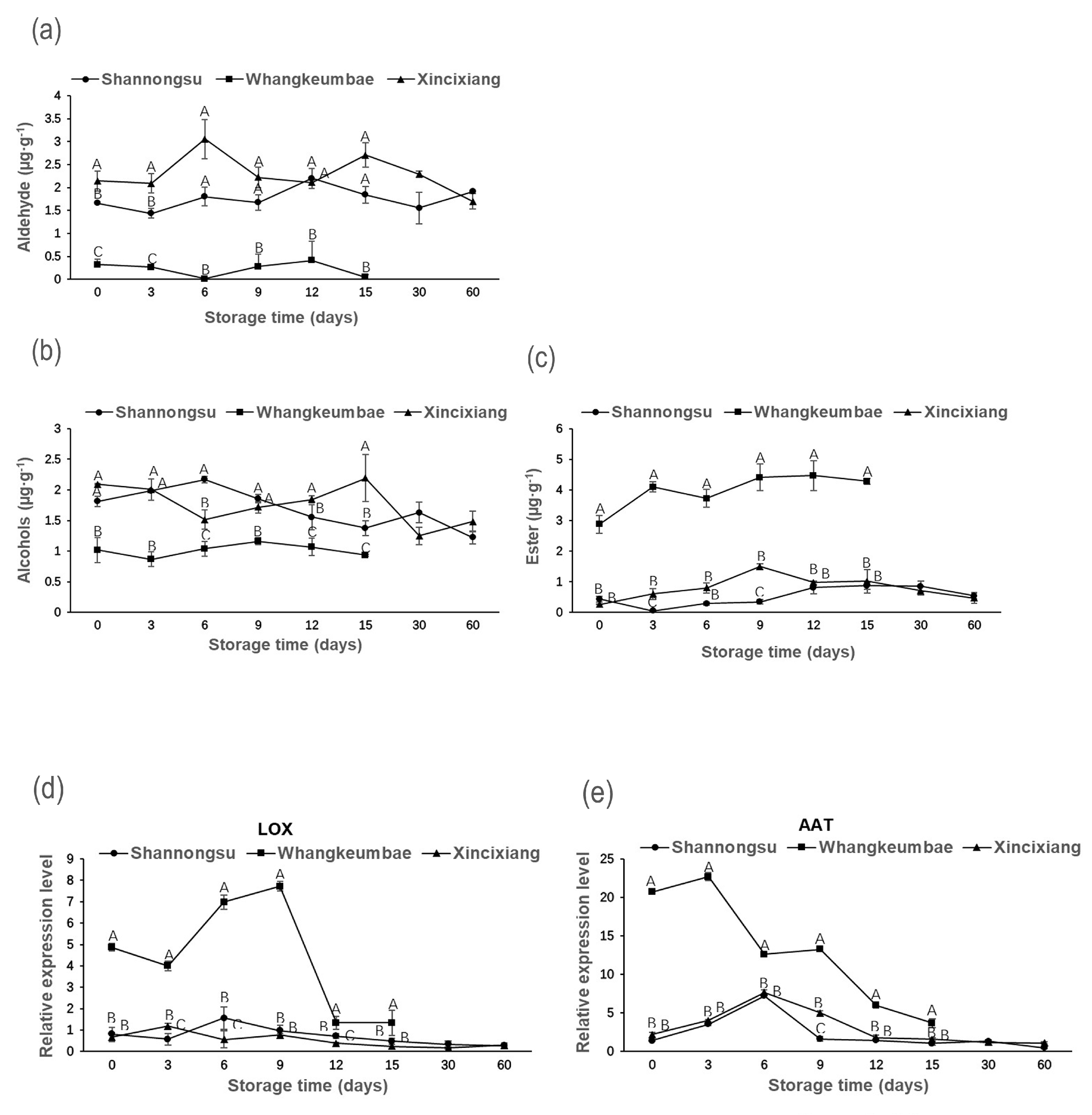
3.4. The Contents of Aroma Volatile and Expression of Key Gene Related to Aroma
4. Discussion
4.1. The Pivotal Role of Ethylene in Regulating Postharvest Storage Quality
4.2. The Relationship between Aroma Volatile and Storage Quality
4.3. The Exploitation and Utilization of Excellent Germplasm Resources
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, G.M.; Gu, C.; Qiao, X.; Zhao, B.Y.; Ke, Y.Q.; Guo, B.B.; Hao, P.P.; Qi, K.J.; Zhang, S.L. Characteristic of pollen tube that grew into self style in pear cultivar and parent assignment for cross-pollination. Sci. Hortic. 2017, 216, 226–233. [Google Scholar] [CrossRef]
- Ma, Y.R.; Yang, M.N.; Wang, J.J.; Jiang, C.Z.; Wang, Q.G. Application of Exogenous Ethylene Inhibits Postharvest Peel Browning of ‘Huangguan’ Pear. Front. Plant Sci. 2017, 7, 2029. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.N.; Gao, Y.; Wan, C.P.; Chen, M.; Zhao, X.Y.; Liu, S.J.; Chen, J.Y. Influence of different cold storage times on quality of ‘Cuiguan’ pear fruits during shelf life. J. Food Process. Preserv. 2019, 43, e14245. [Google Scholar] [CrossRef]
- Chen, X.S.; Wang, N.; Zhang, Z.Y.; Feng, S.Q.; Chen, X.L.; Mao, Z.Q. Progress on the resource and breeding of kernel fruits I: Progress on the germplasm resources, quality development and genetics and breeding of pear in china. J. Plant Genet. Resour. 2019, 20, 791–800. [Google Scholar]
- Tian, L.M.; Cui, Y.F.; Dong, X.G.; Zhang, Y.; Qi, D.; Xu, J.Y.; Huo, H.L. Research Progress of pear variety improvement in our country. China Fruits 2019, 2, 14–19. [Google Scholar]
- Chen, X.S.; Wang, N.; Zhang, Z.Y.; Mao, Z.Q.; Wang, Z.G.; Jiang, Z.T.; Shan, Y.Z. Variety structure with high-quality, late-maturing and storage-tolerant varieties as the main driving force for the efficient development of apple and pear industry in China. China Fruits 2019, 3, 1–4. [Google Scholar]
- Feng, S.Q.; Wang, D.Y.; Wang, N.; Jiang, S.H.; Xu, H.F.; Liu, J.X.; Chen, X.L.; Wu, S.J.; Mao, Z.Q.; Chen, X.S. A new late ripening pear cultivar ‘Shannongsu’. Acta Hortic. Sin. 2016, 43, 2685–2686. [Google Scholar]
- Feng, S.Q.; Wang, N.; Jiang, S.H.; Xu, H.F.; Wang, D.Y.; Liu, J.X.; Chen, X.L.; Wu, S.J.; Mao, Z.Q.; Chen, X.S. A new late ripening pear cultivar ‘Xincixiang’. Acta Hortic. Sin. 2016, 43, 2683–2684. [Google Scholar]
- Barry, C.; Giovannoni, J. Ethylene and Fruit Ripening. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Fu, J.R. Ethylene regulation of fruit ripening: Molecular aspects. Plant Growth Regul. 2000, 30, 193–200. [Google Scholar] [CrossRef]
- Tan, D.M.; Li, T.Z.; Wang, A.D. Apple 1-aminocyclopropane-1-carboxylic acid synthase genes, mdacs1 and mdacs3a, are expressed in different systems of ethylene biosynthesis. Plant Mol. Biol. Report. 2013, 31, 204–209. [Google Scholar] [CrossRef]
- Wang, A.D.; Tan, D.M.; Tatsuki, M.; Kasai, A.; Li, T.Z.; Saito, H.; Harada, T. Molecular mechanism of distinct ripening profiles in ‘Fuji’ apple fruit and its early maturing sports. Postharvest Biol. Technol. 2009, 52, 38–43. [Google Scholar] [CrossRef]
- Dawson, D.; Melton, L.; Watkins, C. Cell wall changes in nectarines (Prunus persica): Solubilization and depolymerization of pectic and neutral polymers during ripening and in mealy fruit. Plant Physiol. 1992, 100, 1203–1210. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Wang, N.; Jiang, S.H.; Xu, H.H.; Wang, Y.C.; Wang, C.Z.; Li, M.; Liu, J.X.; Qu, C.Z.; Liu, W.; et al. Analysis of the xyloglucan endotransglucosylase/hydrolase gene family during apple fruit ripening and softening. J. Agric. Food Chem. 2017, 65, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Zhang, Y.M.; Xu, Y.T.; Dong, F.; Song, Y.; Liu, M.Y.; Liu, J.; Chen, X.S. Analysis of Aroma Components and Related Enzymes of Fatty Acid Metabolism of Red Bud Sports. Acta Hortic. Sin. 2012, 39, 2447–2456. [Google Scholar]
- Zhou, H.W.; Sonego, L.; Khalchitski, A.; Ben-Arie, R.; Lers, A.; Lurie, S. Cell wall enzymes and cell wall changes in ‘Flavortop’ nectarines: mRNA abundance, enzyme activity, and changes in pectic and neutral polymers during ripening and in woolly fruit. J. Am. Soc. Hortic. Sci. 2000, 125, 630–637. [Google Scholar] [CrossRef]
- Percy, A.E.; Obrien, I.E.W.; Jameson, P.E.; Melton, L.D.; MacRae, E.A.; Redgwell, R.J. Xyloglucan endotransglycosylase activity during fruit development and ripening of apple and kiwifruit. Physiol. Plant. 1996, 96, 43–50. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef]
- Liu, J.; Xu, H.; Wang, D.; Zhang, Z.; Wang, Y.; Zuo, W.; Wang, N.; Jiang, S.; Mao, Z.; Chen, X. Changes of firmness, aroma, cell wall-modifying enzyme activities and analysis of related-gene expression in 2 red flesh apple strains during fruit storage. YuanYi XueBao 2017, 44, 330–342. [Google Scholar]
- Zhang, Z.Y.; Jiang, S.H.; Wang, N.; Li, M.; Ji, X.H.; Sun, S.S.; Liu, J.X.; Wang, D.Y.; Xu, H.F.; Qi, S.M. Identification of Differentially Expressed Genes Associated with Apple Fruit Ripening and Softening by Suppression Subtractive Hybridization. PLoS ONE 2015, 10, e0146061. [Google Scholar] [CrossRef]
- Costa, F.; Stella, S.; Van, W.; Guerra, W.; Cecchinel, M.; Dallavia, J.; Koller, B.; Sansavini, S. Role of the genes Md-ACO1 and Md-ACS1 in ethylene production and shelf life of apple (Malus domestica Borkh). Euphytica 2005, 141, 181–190. [Google Scholar] [CrossRef]
- Tian, M.S.; Prakash, S.; Elgar, H.J.; Young, H.; Burmeister, D.M.; Ross, G.S. Responses of strawberry fruit to 1-Methylcyclopropene (1-MCP) and ethylene. Plant Growth Regul. 2000, 32, 83–90. [Google Scholar] [CrossRef]
- Yang, X.T.; Song, J.; Campbell-Palmer, L.; Fillmore, S.; Zhang, Z.Q. Effect of ethylene and 1-MCP on expression of genes involved in ethylene biosynthesis and perception during ripening of apple fruit. Postharvest Biol. Technol. 2013, 78, 55–66. [Google Scholar] [CrossRef]
- Ampa, K.; Saito, T.; Okawa, K.; Ohara, H.; Kondo, S. Effects of ethephon and abscisic acid application on ripening-related genes in ‘Kohi’ kiwifruit (Actinidia chinensis) on the Vine. Hortic. Plant J. 2017, 3, 29–33. [Google Scholar] [CrossRef]
- Qi, X.D.; Wei, J.M.; Gao, H.S.; Jia, Y.R.; Zhang, H.E. Relationship between fruit development and softening and degradation of Pectin polysaccharide in pear. Sci. Agric. Sin. 2015, 48, 3027–3037. [Google Scholar]
- Schaffer, R.J.; Friel, E.N.; Souleyre, E.J.F.; Bolitho, K.; Thodey, K.; Ledger, S.; Bowen, J.H.; Ma, J.H.; Nain, B.; Cohen, D.; et al. A Genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway(w). Plant Physiol. 2007, 144, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.P.; Wang, Y.L.; Liu, Z.C.; Wang, C.Z.; Sun, J.Z.; Wang, N.; Chen, X.S. Effect of 1-methylcyclopropene and nitric oxide on main fruit quality of ‘Whangkeumbae’ pear and related enzymes of fatty acid metabolism during storage. Sci. Agric. Sin. 2010, 43, 2962–2972. [Google Scholar]
- Chen, X.S.; Song, J.; Gao, L.P.; Ji, X.H.; Zhang, Z.Y.; Li, M.; Liu, D.L.; Zhang, R. Developing mechanism of fruits texture in ‘Jonagold’ apple and its crisp flesh sport. Sci. Agric. Sin. 2014, 47, 727–735. [Google Scholar]
- Bellincontro, A.; Morganti, F.; De Santis, D.; Botondi, R.; Mencarelli, F. Inhibition of ethylene via different ways affects LOX and ADH activities, and related volatiles compounds in peach (cv.‘Royal Gem’). Acta Hortic. 2005, 682, 445–452. [Google Scholar] [CrossRef]
- Mattheis, J.; Fan, X.; Argenta, L. Interactive responses of gala apple fruit volatile production to controlled atmosphere storage and chemical inhibition of ethylene action. J. Agric. Food Chem. 2005, 53, 4510–4516. [Google Scholar] [CrossRef]
- Chen, X.S.; Guo, W.W.; Xu, J.; Cong, P.H.; Wang, L.R.; Liu, C.H.; Li, X.G.; Wu, S.J.; Yao, Y.X.; Chen, X.L. Genetic improvement and promotion of fruit quality of main fruit trees. Sci. Agric. Sin. 2015, 48, 3524–3540. [Google Scholar]
- Chen, X.S.; Li, X.G.; Mao, Z.Q.; Wang, N.; Zhang, Z.Y. Breeding new late season varities with good quality and storability increase pear industry profits in China. Deciduous Fruits 2020, 52, 5–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Qi, S.; Li, B.; Zhang, J.; Cui, C.; Zhang, R.; Mao, Z.; Wang, N.; Chen, X.; Zhang, Z. Analysis of the Postharvest Storage Characteristics of Two New Pear Cultivars ‘Shannongsu’ and ‘Xincixiang’. Horticulturae 2023, 9, 281. https://doi.org/10.3390/horticulturae9020281
Zhang S, Qi S, Li B, Zhang J, Cui C, Zhang R, Mao Z, Wang N, Chen X, Zhang Z. Analysis of the Postharvest Storage Characteristics of Two New Pear Cultivars ‘Shannongsu’ and ‘Xincixiang’. Horticulturae. 2023; 9(2):281. https://doi.org/10.3390/horticulturae9020281
Chicago/Turabian StyleZhang, Susu, Sumin Qi, Bin Li, Jing Zhang, Can Cui, Rui Zhang, Zhiquan Mao, Nan Wang, Xuesen Chen, and Zongying Zhang. 2023. "Analysis of the Postharvest Storage Characteristics of Two New Pear Cultivars ‘Shannongsu’ and ‘Xincixiang’" Horticulturae 9, no. 2: 281. https://doi.org/10.3390/horticulturae9020281
APA StyleZhang, S., Qi, S., Li, B., Zhang, J., Cui, C., Zhang, R., Mao, Z., Wang, N., Chen, X., & Zhang, Z. (2023). Analysis of the Postharvest Storage Characteristics of Two New Pear Cultivars ‘Shannongsu’ and ‘Xincixiang’. Horticulturae, 9(2), 281. https://doi.org/10.3390/horticulturae9020281







