Seed Longevity Potential Predicted by Radicle Emergence (RE) Vigor Test in Watermelon Seed Cultivars
Abstract
1. Introduction
2. Materials and Methods
- n = number of seeds newly germinated at time t;
- t = days from setting to germination.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demir, I.; Ermis, S.; Mavi, K.; Matthews, S. Mean germination time of pepper seed lots (Capsicum annuum) predicts size and uniformity of seedlings in germination tests and transplant modules. Seed Sci. Technol. 2008, 36, 21–30. [Google Scholar] [CrossRef]
- Matthews, S.; Noli, E.; Demir, I.; Khajeh-Hosseini, M.; Wagner, M.H. Evaluation of seed quality: From physiology to international standardization. Seed Sci. Res. 2012, 22, 69–73. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2021. [Google Scholar]
- Powell, A.A.; Matthews, S. Seed ageing/repair hypothesis leads to new testing methods. Seed Technol. 2012, 34, 15–25. [Google Scholar]
- Powell, A.A. Seed vigour in the 21st century. Seed Sci. Technol. 2022, 50, 45–73. [Google Scholar] [CrossRef]
- Matthews, S.; Khajeh-Hosseini, M. Length of the lag period of germination and metabolic repair explain vigor differences in seed lots of maize (Zea mays). Seed Sci. Technol. 2007, 35, 200–212. [Google Scholar] [CrossRef]
- Matthews, S.; El-Khadem, R.; Casarini, E.; Khajeh Hosseini, M.; Nasehzadeh, M.; Wagner, M.-H. Rate of physiological germination compared with the cold test and accelerated ageing as a repeatable vigor test for maize (Zea mays). Seed Sci. Technol. 2010, 38, 379–389. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Wang, Y.R.; Powell, A.A. Frequent individual counts of radicle emergence and mean just germination time predict seed vigor of Avena sativa and Elymus nutans. Seed Sci. Technol. 2016, 44, 189–198. [Google Scholar] [CrossRef]
- Guan, Y.; Yin, M.; Jia, X.; An, J.; Wang, C.; Pan, R.; Song, W.; Hu, J. Single counts of radicle emergence can be used as a vigour test to predict seedling emergence potential of wheat. Seed Sci. Technol. 2018, 46, 349–357. [Google Scholar] [CrossRef]
- Munyaneza, V.; Chen, D.; Hu, X. Detection of seed vigour differences in Festuca sinensis seed lots. Seed Sci. Technol. 2022, 50, 61–75. [Google Scholar] [CrossRef]
- Mavi, K.; Demir, I.; Matthews, S. Mean germination time estimates the relative emergence of seed lots of three cucurbit crops under stress conditions. Seed Sci. Technol. 2010, 3, 14–25. [Google Scholar] [CrossRef]
- Ermis, S.; Karslıoglu, M.; Ozden, E.; Demir, I. Use of a single radicle emergence count as a vigor test in prediction of seedling emergence potential of leek seed lots. Seed Sci. Technol. 2015, 43, 308–312. [Google Scholar] [CrossRef]
- Shinohara, T.; Ducournau, S.; Matthews, S.; Wagner, M.-H.; Powell, A.A. Early counts of radicle emergence, counted manually and by image analysis, can reveal differences in the production of normal seedlings and the vigor of seed lots of cauliflower. Seed Sci. Technol. 2021, 49, 219–235. [Google Scholar] [CrossRef]
- Demir, I.; Kuzucu, C.O.; Ermis, S.; Oktem, G. Radicle emergence as seed vigour test estimates seedling quality of hybrid cucumber (Cucumis sativus L.) cultivars in low temperature and salt stress conditions. Horticulturae 2023, 9, 3. [Google Scholar] [CrossRef]
- Guloksuz, T.; Demir, I. Vigor tests in Geranium, Salvia, Gazania, and Impatiens seed lots to estimate seedling emergence potential in modules. Propag. Ornam. Plants 2012, 12, 133–138. [Google Scholar]
- Demir, I.; Celikkol, T.; Sarıkamıs, G.; Eksi, C. Vigor tests to estimate seedling emergence potential and longevity in viola seed lots. Hortscience 2011, 46, 402–405. [Google Scholar] [CrossRef]
- Ilbi, H.; Powell, A.A.; Alan, O. Single radicle emergence counts for predicting vigor of marigold (Tagetes spp.) seed lots. Seed Sci. Technol. 2020, 48, 381–389. [Google Scholar] [CrossRef]
- Demir, I.; Erturk, N.; Gokdas, Z. Seed vigor evaluation in petunia seed lots to predict seedling emergence and longevity. Seed Sci. Technol. 2020, 48, 391–400. [Google Scholar] [CrossRef]
- Mis, S.; Ermis, S.; Powell, A.A.; Demir, I. Radicle emergence (RE) test identifies differences in normal germination percentages (NG) of watermelon, lettuce and carrot seed lots. Seed Sci. Technol. 2022, 50, 257–267. [Google Scholar] [CrossRef]
- Ermis, S.; Oktem, G.; Mavi, K.; Hay, F.R.; Demir, I. The radicle emergence test and storage longevity of cucurbit rootstock seed lots. Seed Sci. Technol. 2022, 50, 1–10. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D. Quantitative response of the longevity of seed of twelve crops to temperature and moisture in hermetic storage. Seed Sci. Technol. 2007, 35, 432–444. [Google Scholar] [CrossRef]
- Yetisir, H. History and current status of grafted vegetables in Turkey. Cron. Hortic. 2017, 57, 13–17. [Google Scholar]
- Tao, Q.; Sun, J.; Zhang, Y.; Sun, X.; Li, Z.; Zhong, S.; Sun, J. Single count of radicle emergence and mean germination time estimate seed vigour of Chinese milk vetch (Astragalus sinicus). Seed Sci. Technol. 2022, 50, 47–59. [Google Scholar] [CrossRef]
- Ozden, E.; Mavi, K.; Sari, E.; Demir, I. Radicle emergence test predicts longevity (half viability period, P50) of leek seed lots. Seed Sci. Technol. 2017, 45, 243–247. [Google Scholar] [CrossRef]
- Mavi, K.; Demir, I. Controlled deterioration for vigour assessment and predicting seedling growth of winter squash (Cucurbita maxima) seed lots under salt stress. N. Z. J. Crop Hortic. Sci. 2005, 33, 193–197. [Google Scholar] [CrossRef]
- Matthews, S. Controlled deterioration: A new vigour test for crop seed. In Seed Production; Hebblethwaite, P.D., Ed.; Butterworth: London, UK, 1980; pp. 647–660. [Google Scholar]
- Demir, I.; Kuzucu, C.O.; Ermis, S.; Memis, N.; Kadıoglu, N. Estimation of seed longevity in onion seed lots by a vigor test of radicle emergence test in artificial ageing conditions. Horticulturae 2022, 8, 1063. [Google Scholar] [CrossRef]
- Demir, I.; Kenanoglu, B.B.; Ozden, E. Seed vigor tests to estimate seedling emergence in cress (Lepidium sativum L.) seed lots. Not. Bot. Horti Agrobot. 2019, 47, 881–886. [Google Scholar] [CrossRef]
- Matthews, S.; Powell, A.A. Towards automated single counts of radicle emergence to predict seed and seedling vigor. Seed Sci. 2011, 142, 44–48. [Google Scholar]
- Powell, A.A.; Harman, G.E. Absence of a consistent association of changes in the membranal lipids with the ageing of pea seeds. Seed Sci. Technol. 1985, 13, 659–667. [Google Scholar]
- McDonald, M.B. Seed deterioration: Physiology, repair, and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Joosen, R.V.L.; Kodde, J.; Willems, L.A.J.; Ligterink, W.; Hilhorst, H.W.M. The germinator automated germination scoring system. Seed Test. Int. 2010, 140, 4–8. [Google Scholar]
- Wagner, M.H.; Demilly, D.; Ducournau, S.; Durr, C.; Léchappé, J. Computer vision for monitoring seed germination from dry state to young seedlings. Seed Test. Int. 2011, 142, 49–51. [Google Scholar]
- Matthews, S.; Khajeh-Hosseini, M. Mean germination time as an indicator of emergence performance in soil of seed lots of maize (Zea mays). Seed Sci. Technol. 2006, 34, 339–347. [Google Scholar] [CrossRef]
- Khajeh-Hosseini, M.; Azimi, B.; Malekzadeh, S. Cell cycle in high and low vigour maize (Zea mays L.) seeds and its relation with RE. In Proceedings of the 30th ISTA Seed Symposium, Antalya, Turkey, 13–15 June 2013. [Google Scholar]
- Waterworth, W.M.; Masnavi, G.; Bhardwaj, R.M.; Jiang, Q.; Bray, C.M.; West, C.E. A plant DNA ligase is an important determinant of seed longevity. Plant J. 2010, 63, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Cheshmi, M.; Khajeh-Hosseini, M. Single count of radicle emergence DNA replication during seed germination and vigour in alfalfa seed lots. Seed Sci. Technol. 2020, 48, 367–380. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- Hay, F.R.; Davies, R.M.; Dickie, J.B.; Merritt, D.J.; Wolkis, D.M. More on seed longevity phenotyping. Seed Sci. Res. 2022, 32, 144–149. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological correlates of ex-situ seed longevity: A comparative study on 195 species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef]
- Merritt, D.J.; Martyn, A.J.; Ainsley, P.; Young, R.E.; Seed, L.U.; Thorpe, M.; Hay, F.R.; Commander, L.E.; Shackelford, N.; Offord, C.A.; et al. A continental-scale study of seed lifespan in experimental storage examining seed, plant, and environmental traits associated with longevity. Biodivers. Conserv. 2014, 23, 1081–1104. [Google Scholar] [CrossRef]

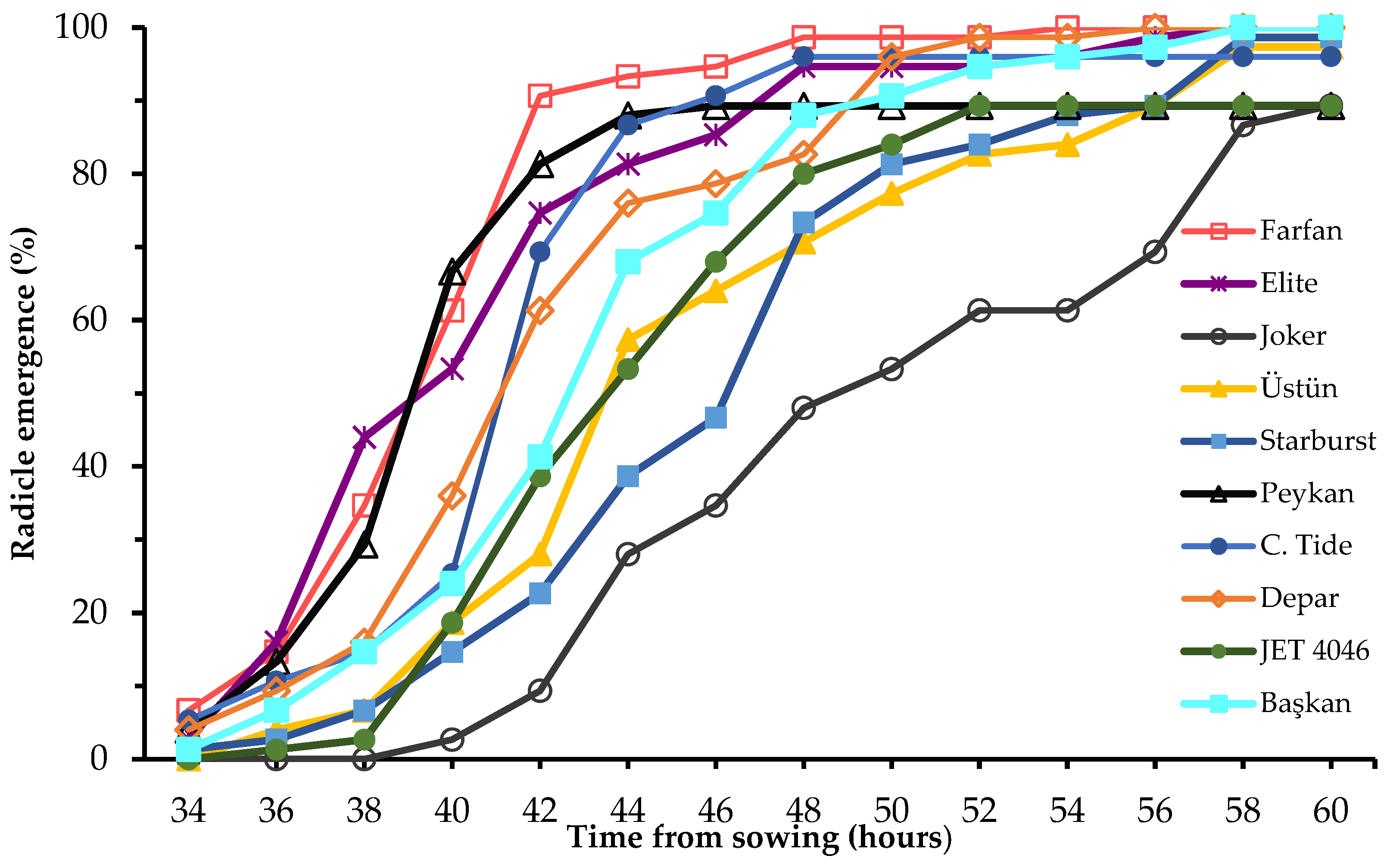
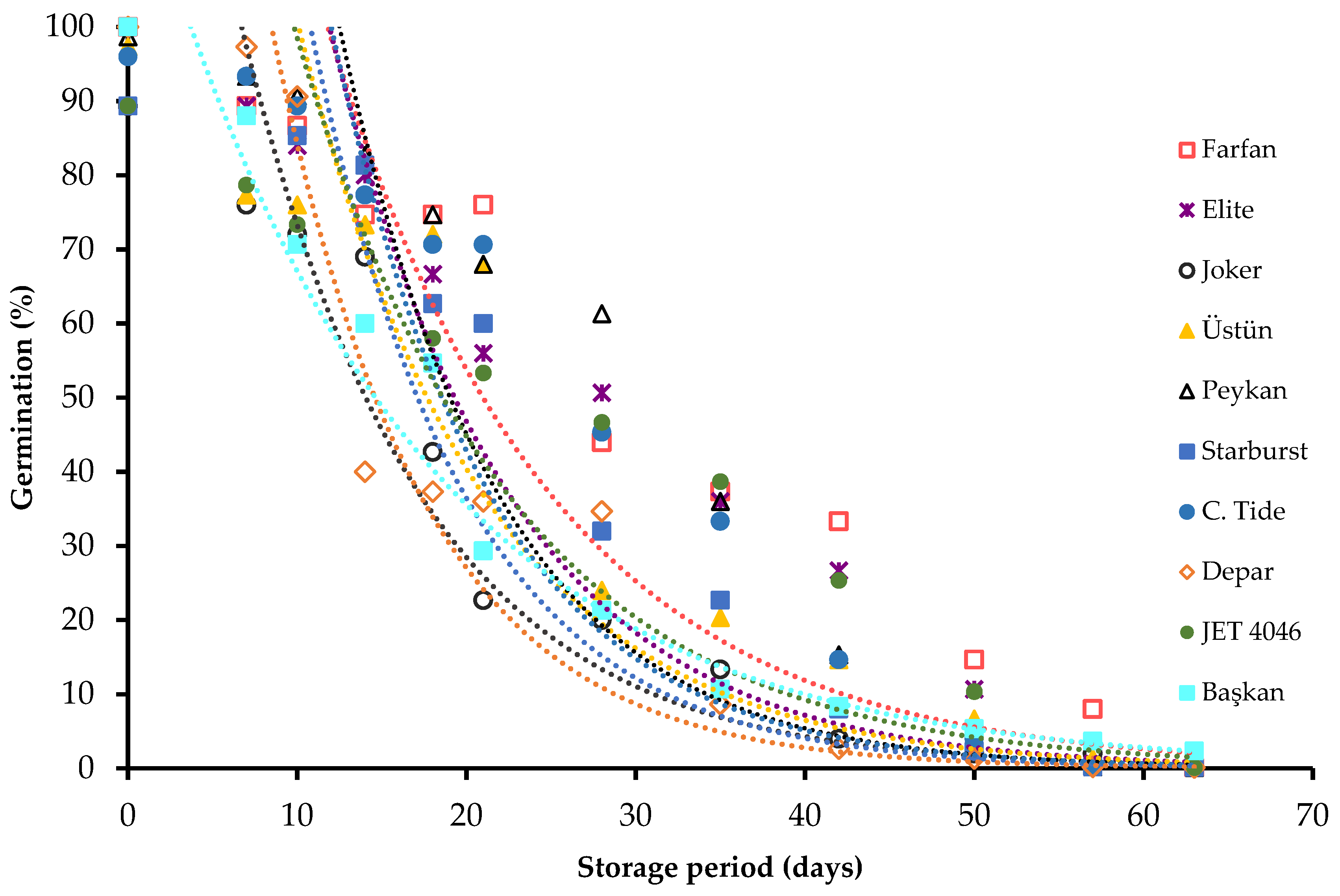
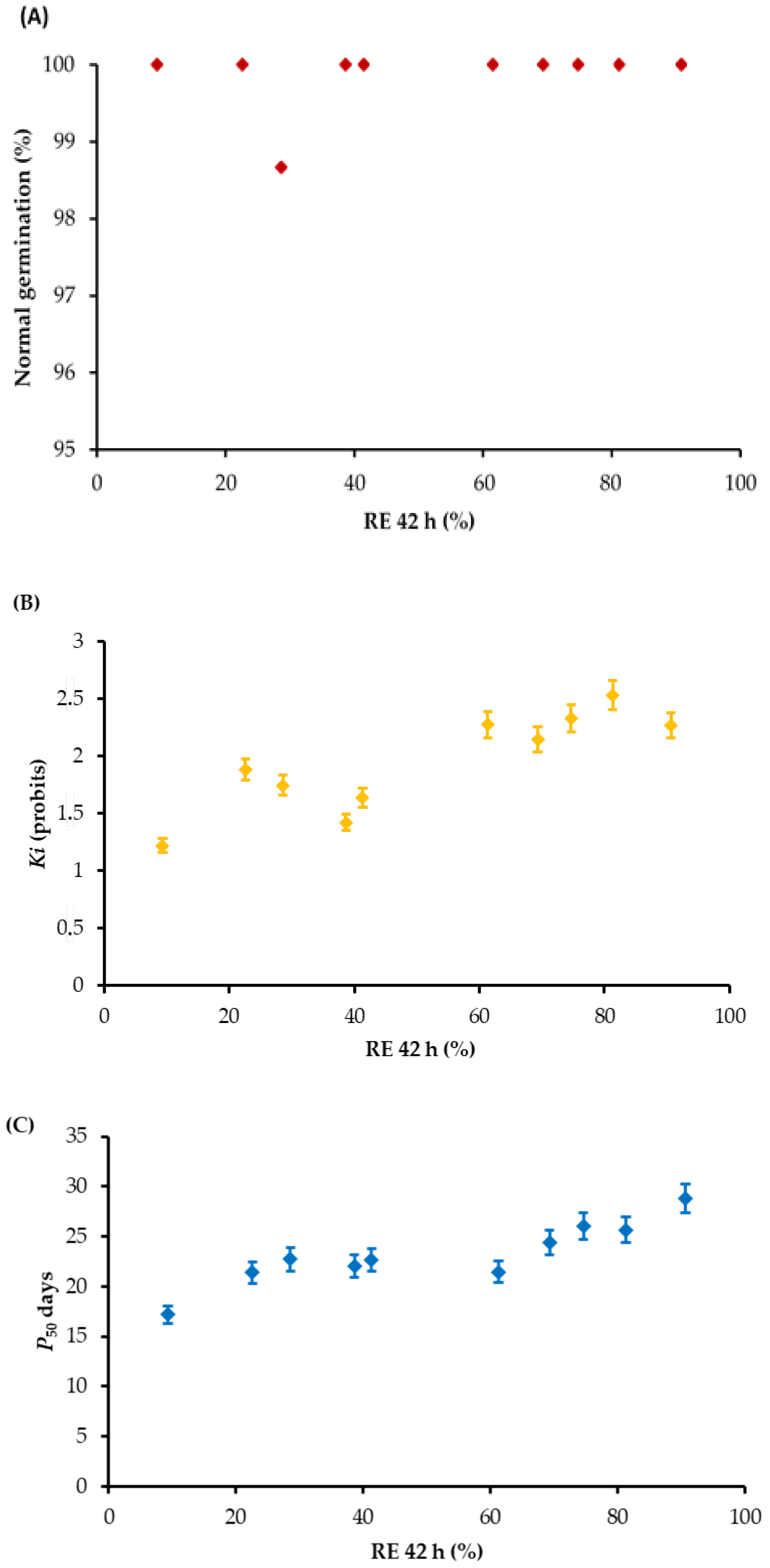
| Cultivars | GP (%) | MGT (h) | Seed m.c. (%) | 1000 Seed Weight (g) | Production Year |
|---|---|---|---|---|---|
| Farfan | 100 | 40.16 | 7.25 | 66.07 | 2021 |
| Elite | 100 | 42.13 | 7.39 | 80.73 | 2021 |
| Joker | 100 | 45.92 | 6.42 | 65.18 | 2022 |
| Üstün | 98.7 | 47.97 | 5.54 | 63.48 | 2022 |
| Starburst | 100 | 46.83 | 6.06 | 58.78 | 2021 |
| Peykan | 100 | 40.69 | 6.17 | 64.26 | 2022 |
| Crimson tide | 100 | 42.27 | 6.51 | 72.46 | 2021 |
| Depar | 100 | 42.88 | 7.04 | 64.83 | 2022 |
| Jet 4046 | 100 | 45.86 | 5.67 | 62.24 | 2021 |
| Başkan | 100 | 44.11 | 6.07 | 65.33 | 2022 |
| Cultivars | Ki (s.e.) (Probits) | Slope, −σ−1 (s.e.) (Probits Day−1) | P50 (s.e.) (Days) |
|---|---|---|---|
| Farfan | 2.27 (0.007) | −0.0788 (0.0064) | 28.79 (0.12) |
| Elite | 2.33 (0.010) | −0.0895 (0.0078) | 26.05 (0.15) |
| Joker | 1.22 (0.008) | −0.0710 (0.0062) | 17.17 (0.15) |
| Üstün | 1.74 (0.021) | −0.0768 (0.0089) | 22.71 (0.26) |
| Starburst | 1.88 (0.031) | −0.0888 (0.0059) | 21.38 (0.15) |
| Peykan | 2.53 (0.035) | −0.0917 (0.0075) | 25.67 (0.14) |
| Crimson tide | 2.15 (0.007) | −0.0879 (0.0077) | 24.42 (0.14) |
| Depar | 2.27 (0.013) | −0.106 (0.0092) | 21.45 (0.17) |
| Jet 4046 | 1.42 (0.029) | −0.0644 (0.0059) | 22.05 (0.25) |
| Başkan | 1.64 (0.031) | −0.0725 (0.0050) | 22.61 (0.18) |
| Time of RE Count (h) | Ki | P50 |
|---|---|---|
| 34 | 0.6342 * | 0.5367 |
| 36 | 0.8063 ** | 0.7389 ** |
| 38 | 0.6527 * | 0.6963 * |
| 40 | 0.7513 ** | 0.7383 ** |
| 42 | 0.7538 ** | 0.7936 ** |
| 44 | 0.7008 * | 0.7616 ** |
| 46 | 0.6356 * | 0.7669 ** |
| 48 | 0.6100 * | 0.8030 ** |
| 50 | 0.6668 * | 0.6665 * |
| 52 | 0.6072 * | 0.6055 * |
| 54 | 0.6050 * | 0.6183 * |
| 56 | 0.5612 | 0.6378 * |
| 58 | 0.6263 * | 0.6775 * |
| 60 | 0.7160 * | 0.7339 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eren, E.; Ermis, S.; Oktem, G.; Demir, I. Seed Longevity Potential Predicted by Radicle Emergence (RE) Vigor Test in Watermelon Seed Cultivars. Horticulturae 2023, 9, 280. https://doi.org/10.3390/horticulturae9020280
Eren E, Ermis S, Oktem G, Demir I. Seed Longevity Potential Predicted by Radicle Emergence (RE) Vigor Test in Watermelon Seed Cultivars. Horticulturae. 2023; 9(2):280. https://doi.org/10.3390/horticulturae9020280
Chicago/Turabian StyleEren, Erkan, Sıtkı Ermis, Guleda Oktem, and Ibrahim Demir. 2023. "Seed Longevity Potential Predicted by Radicle Emergence (RE) Vigor Test in Watermelon Seed Cultivars" Horticulturae 9, no. 2: 280. https://doi.org/10.3390/horticulturae9020280
APA StyleEren, E., Ermis, S., Oktem, G., & Demir, I. (2023). Seed Longevity Potential Predicted by Radicle Emergence (RE) Vigor Test in Watermelon Seed Cultivars. Horticulturae, 9(2), 280. https://doi.org/10.3390/horticulturae9020280







