Loss Assessment during Postharvest and Handling of Thai Garlic Used for Processing
Abstract
1. Introduction
2. Materials and Methods
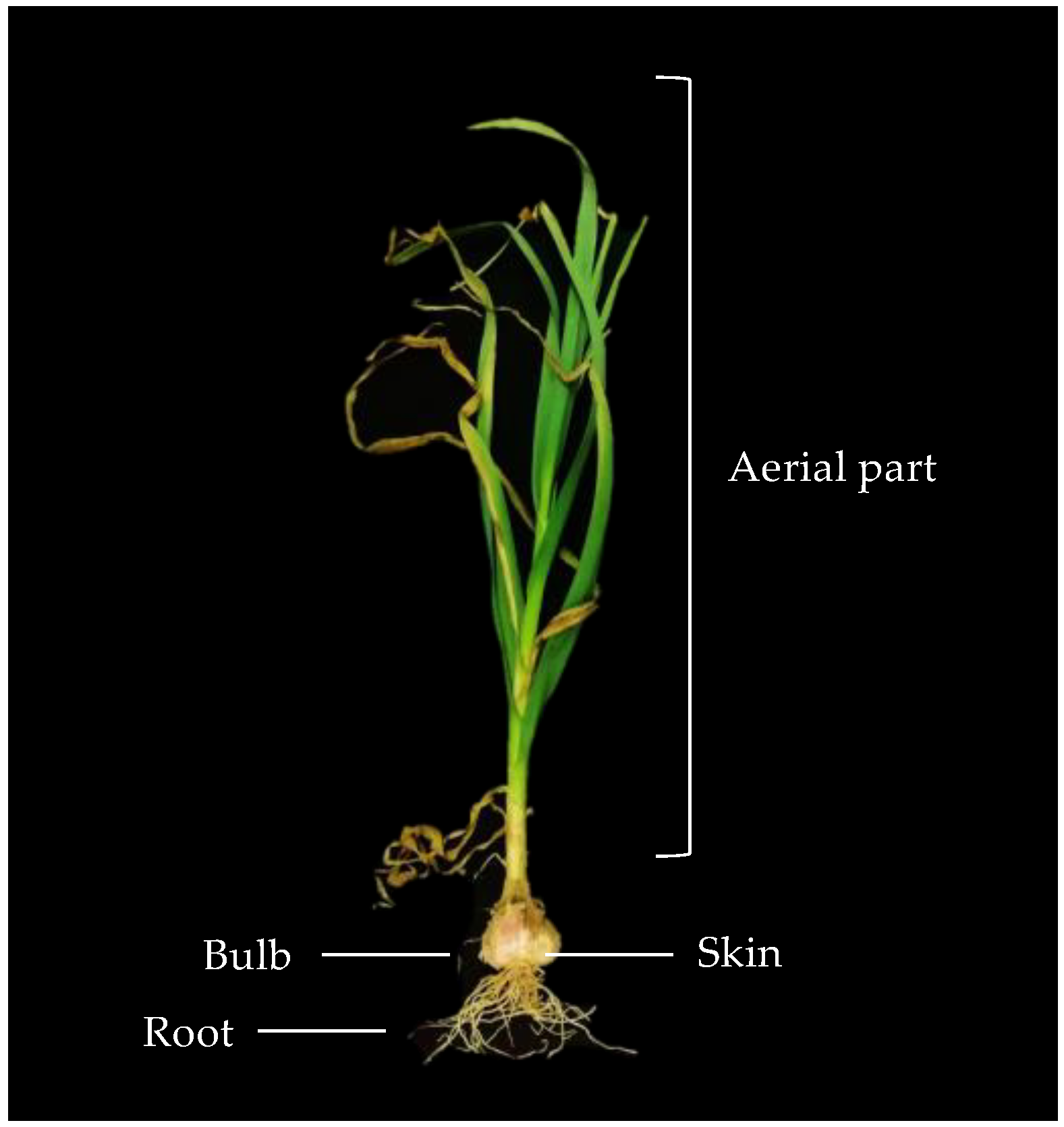
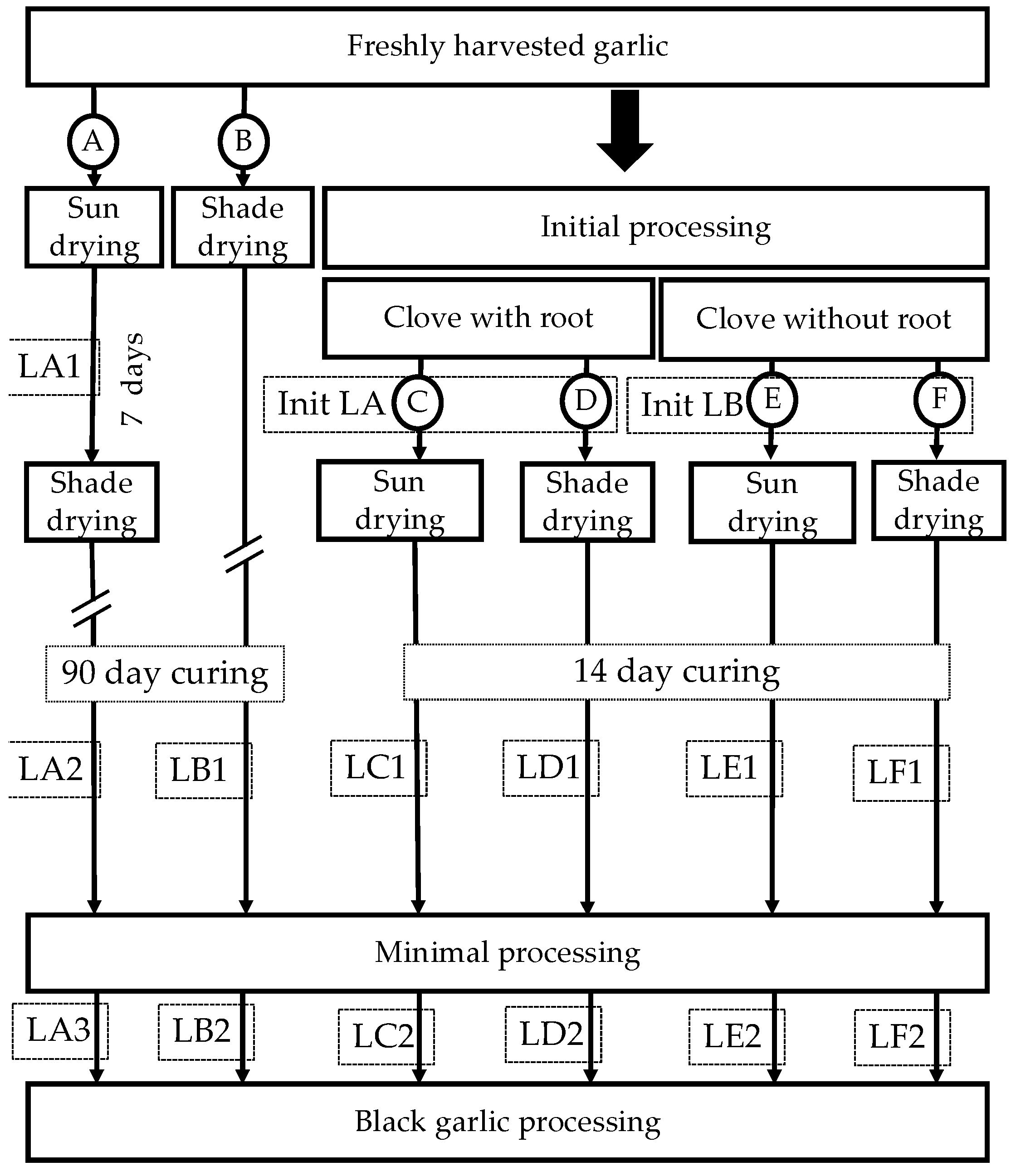
2.1. Process Flow Diagram and Mass Flow Acquisition
- Whole semi-sun drying: garlic plants were sun-dried in the field for seven days before being combined and hung in the shade for 90 days.
- Whole shade drying: garlic plants were bundled and hung in the shade for 90 days.
- Root bulb sun drying: bulbs without an aerial part, but with root, remained sun-dried on a grate for 14 days.
- Root bulb shade drying: bulbs without an aerial part, but with root remaining, were dried on a grate in the shade for 14 days.
- Bulb sun drying: bulbs without an aerial part and root were sun-dried on a grate for 14 days.
- Bulb shade drying: bulbs without aerial parts and roots were dried on a grate in the shade for 14 days.
2.2. Loss Assessment and Calculation
2.3. Quality Assessments
2.3.1. Total Phenolic Content
2.3.2. Total Flavonoid Content
2.3.3. DPPH Scavenging Activity
2.3.4. ABTS Scavenging Activity
2.3.5. Total Reducing Sugar
2.4. Statistical Analysis
3. Results
3.1. Process Flow Diagram and Mass Flow Acquisition
3.2. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. World Food and Agriculture 2021. In Statistical Year Book; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2021. [Google Scholar]
- Sunanta, P.; Chung, H.H.; Kunasakdakul, K.; Ruksiriwanich, W.; Jantrawut, P.; Hongsibsong, S.; Sommano, S.R. Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing. Food Sci. Nutr. 2020, 8, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Singh, D.; Bhonde, S.; Banerjee, S. Post Harvest Losses and Quality in Garlic as Influenced by Curing and Top Cut. Natl. Hortic. Res. Dev. Found. News Lett. 1999, 19, 12–18. [Google Scholar]
- Madhu, B.; Mudgal, V.D.; Champawat, P.S. Storage of garlic bulbs (Allium sativum L.): A review. J. Food Process Eng. 2019, 42, e13177. [Google Scholar] [CrossRef]
- Rotta, N.M.; Curry, S.; Han, J.; Reconco, R.; Spang, E.; Ristenpart, W.; Donis-González, I.R. A comprehensive analysis of operations and mass flows in postharvest processing of washed coffee. Resour. Conserv. Recycl. 2021, 170, 105554. [Google Scholar] [CrossRef]
- Kallel, F.; Ellouz Chaabouni, S. Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: A review. Environ. Prog. Sustain. Energy 2017, 36, 1765–1777. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Cantwell, M.; Voss, R.; Hanson, B.; May, D.; Rice, B. Water and Fertilizer Management for Garlic: Productivity, Nutrient and Water Use Efficiency, and Post Harvest Quality; Report of a FREP Contact; CDFA: Sacramento, CA, USA, 2006.
- Atanda, S. The concepts and problems of post-harvest food losses in perishable crops. Afr. J. Food Sci. 2011, 5, 603–613. [Google Scholar]
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre-and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef]
- Sunanta, P.; Pankasemsuk, T.; Jantanasakulwong, K.; Chaiyaso, T.; Leksawasdi, N.; Phimolsiripol, Y.; Rachtanapun, P.; Seesuriyachan, P.; Sommano, S.R. Does Curing Moisture Content Affect Black Garlic Physiochemical Quality? Horticulturae 2021, 7, 535. [Google Scholar] [CrossRef]
- Vinson, J.A.; Hao, Y.; Su, X.; Zubik, L. Phenol antioxidant quantity and quality in foods: Vegetables. J. Agric. Food Chem. 1998, 46, 3630–3634. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.-W. Isolation and characterization of cellulose nanocrystals from garlic skin. Mater. Lett. 2014, 129, 20–23. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.; Oh, Y.; Ahmadi, F.; Kwak, W. Yield survey and nutritional evaluation of garlic stalk for ruminant feed. J. Anim. Sci. Technol. 2017, 59, 22. [Google Scholar] [CrossRef]
- Del Mar Contreras, M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef]
- Arzanlou, M.; Bohlooli, S. Introducing of green garlic plant as a new source of allicin. Food Chem. 2010, 120, 179–183. [Google Scholar] [CrossRef]
- Lebersorger, S.; Schneider, F. Discussion on the methodology for determining food waste in household waste composition studies. Waste Manag. 2011, 31, 1924–1933. [Google Scholar] [CrossRef]
- Amicarelli, V.; Bux, C.; Lagioia, G. How to measure food loss and waste? A material flow analysis application. Br. Food J. 2021, 123, 67–85. [Google Scholar] [CrossRef]
- Nath, B.; Hossen, M.A.; Paul, S.; Rahman, M. Postharvest Loss Assessment of Rice at Selected Areas of Gazipur District. Bangladesh Rice J. 2016, 20, 23–32. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Jimoh, F.O.; Afolayan, A.J.; Masika, P.J. Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera. BMC Complement. Altern. Med. 2008, 8, 54. [Google Scholar] [CrossRef]
- Marsden, W.L.; Gray, P.P.; Nippard, G.J.; Quinlan, M.R. Evaluation of the DNS method for analysing lignocellulosic hydrolysates. J. Chem. Technol. Biotechnol. 1982, 32, 1016–1022. [Google Scholar] [CrossRef]
- Dhingra, D.; Paul, S. Post Harvest Technology of Garlic—A Review. J. Agric. Eng. 2005, 42, 1–18. [Google Scholar]
- Opara, L.U. Onions: Post-Harvest Operation. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2003; pp. 1–16. [Google Scholar]
- Ahmad, M.S.; Siddiqui, M.W.; Ahmad, M.S.; Siddiqui, M.W. Factors affecting postharvest quality of fresh fruits. In Postharvest Quality Assurance of Fruits: Practical Approaches for Developing Countries; Springer: Cham, Switzerland, 2015; pp. 7–32. [Google Scholar]
- Vázquez-Barrios, M.E.; López-Echevarría, G.; Mercado-Silva, E.; Castaño-Tostado, E.; León-González, F. Study and prediction of quality changes in garlic cv. Perla (Allium sativum L.) stored at different temperatures. Sci. Hortic. 2006, 108, 127–132. [Google Scholar] [CrossRef]
- Brewster, J.L. Onions and Other Vegetable Alliums; CABI: Wallingford, UK, 2008; Volume 15.
- Zhu, Z.; Ma, R.; Draganic, A.; Orovic, I.; Zhang, X.; Wang, X.; Wang, J. Postharvest quality monitoring and cold chain management of fresh garlic scapes based on a wireless multi-sensors system. J. Food Process Eng. 2022, 45, e13918. [Google Scholar] [CrossRef]
- Atashi, S.; Akbarpour, V.; Mashayekhi, K.; Mousavizadeh, S.J. Garlic physiological characteristics from harvest to sprouting in response to low temperature. J. Stored Prod. Postharvest Res. 2011, 2, 285–291. [Google Scholar]
- Hong, G.; Kang, J.; Nie, X.; Cantwell, M. Controlled atmospheres retard sprout growth, affect compositional changes, and maintain visual quality attributes of garlic. In Proceedings of the VIII International Controlled Atmosphere Research Conference 600, Rotterdam, The Netherlands, 8–13 July 2001; pp. 791–794. [Google Scholar]
- Naheed, Z.; Cheng, Z.; Wu, C.; Wen, Y.; Ding, H. Total polyphenols, total flavonoids, allicin and antioxidant capacities in garlic scape cultivars during controlled atmosphere storage. Postharvest Biol. Technol. 2017, 131, 39–45. [Google Scholar] [CrossRef]
- Petropoulos, S.; Ntatsi, G.; Fernandes, Â.; Barros, L.; Barreira, J.; Ferreira, I.C.; Antoniadis, V. Long-term storage effect on chemical composition, nutritional value and quality of Greek onion landrace “Vatikiotiko”. Food Chem. 2016, 201, 168–176. [Google Scholar] [CrossRef]
- Wongsa, P.; Bhuyar, P.; Tongkoom, K.; Spreer, W.; Müller, J. Influence of hot-air drying methods on the phenolic compounds/allicin content, antioxidant activity and α-amylase/α-glucosidase inhibition of garlic (Allium sativum L.). Eur. Food Res. Technol. 2023, 249, 523–535. [Google Scholar] [CrossRef]
- Kayacan, S.; Karasu, S.; Akman, P.K.; Goktas, H.; Doymaz, I.; Sagdic, O. Effect of different drying methods on total bioactive compounds, phenolic profile, in vitro bioaccessibility of phenolic and HMF formation of persimmon. LWT 2020, 118, 108830. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Dharmasiri, T.S.K.; Song, X.; Liu, F.; Wang, X. Novel ultrasonic-assisted vacuum drying technique for dehydrating garlic slices and predicting the quality properties by low field nuclear magnetic resonance. Food Chem. 2020, 306, 125625. [Google Scholar] [CrossRef]
- Mohd Zainol, M.; Abdul-Hamid, A.; Abu Bakar, F.; Pak Dek, S. Effect of different drying methods on the degradation of selected flavonoids in Centella asiatica. Int. Food Res. J. 2009, 16, 531–537. [Google Scholar]
- Davey, M.W.; Montagu, M.V.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, B.; ElGasim, A.; Yagoub, A.; Ma, H.; Sun, Y.; Xu, X.; Yu, X.; Zhou, C. Role of drying techniques on physical, rehydration, flavor, bioactive compounds and antioxidant characteristics of garlic. Food Chem. 2021, 343, 128404. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Lech, K. The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. LWT Food Sci. Technol. 2016, 66, 484–489. [Google Scholar] [CrossRef]
- Fei, M.L.I.; Tong, L.I.; Wei, L.I.; De Yang, L. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crops Prod. 2015, 69, 137–142. [Google Scholar] [CrossRef]
- Salama, A.; Hicks, J.; Nock, J. Sugar and organic acid changes in stored onion bulbs treated with maleic hydrazide. HortScience 1990, 25, 1625–1628. [Google Scholar] [CrossRef]

| Step/Procedure | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Initial process (%) | ||||||
| aerial part | ND | ND | (Init LA) 48.60 ± 0.57 | (Init LA) 48.60 ± 0.57 | (Init LB) 49.00 ± 0.05 | (Init LB) 49.00 ± 0.05 |
| Root | ND | ND | ND | ND | 6.00 ± 0.07 | 6.00 ± 0.07 |
| Pre-drying process (%) | ||||||
| Water | (LA1) 30.47 ± 0.72 | ND | ND | ND | ND | ND |
| Curing process (%) | ||||||
| Water | (LA2) 34.16 ± 0.38 | (LB1) 66.06 ± 0.73 | (LC1) 21.29 ± 0.23 | (LD1) 20.35 ± 0.22 | (LE1) 18.54 ± 0.05 | (LF1) 17.90 ± 0.05 |
| Minimal process (%) | ||||||
| aerial part | (LA3) 7.54 ± 0.11 | (LB2) 7.23 ± 0.16 | ND | ND | ND | ND |
| Skin | (LA3) 0.98 ± 0.01 | (LB2) 0.94 ± 0.02 | (LC2) 0.04 ± 0.00 | (LD2) 0.04 ± 0.00 | (LE2) 0.03 ± 0.00 | (LF2) 0.04 ± 0.00 |
| Root | (LA3) 1.77 ± 0.03 | (LB2) 1.70 ± 0.04 | (LC2) 1.05 ± 0.01 | (LD2) 1.08 ± 0.01 | ND | ND |
| Total losses | 74.92 ± 0.37 A | 75.27 ± 0.47 A | 70.98 ± 0.32 D | 70.07 ± 0.33 E | 73.58 ± 0.07 B | 72.94 ± 0.07 C |
| Chemical Properties | Harvested | A | B | C | D | E | F |
|---|---|---|---|---|---|---|---|
| Moisture contents (%) | 79.93 ± 0.10 A | 49.33 ± 0.36 D | 49.55 ± 0.41 D | 58.37 ± 0.33 B | 58.40 ± 0.42 B | 56.45 ± 0.14 C | 56.36 ± 0.21 C |
| Total phenolic content (mg/g GAE) | 0.74 ± 0.02 A | 0.69 ± 0.03 AB | 0.61 ± 0.02 BC | 0.66 ± 0.02 AB | 0.55 ± 0.03 C | 0.73 ± 0.02 A | 0.73 ± 0.03 A |
| Total flavonoid content (mg/g CE) | 0.07 ± 0.02 A | 0.04 ± 0.01 BC | 0.04 ± 0.01 BC | 0.06 ± 0.01 AB | 0.05 ± 0.00 ABC | 0.04 ± 0.00 BC | 0.03 ± 0.00 C |
| DPPH scavenging activity (mg/g TE) | 0.75 ± 0.01 A | 0.67 ± 0.02 B | 0.57 ± 0.02 C | 0.52 ± 0.03 CD | 0.57 ± 0.04 C | 0.41 ± 0.01 E | 0.49 ± 0.01 D |
| ABTS scavenging activity (mg/g TE) | 1.94 ± 0.03 A | 1.87 ± 0.04 ABC | 1.79 ± 0.01 BCD | 1.66 ± 0.02 D | 1.72 ± 0.05 CD | 1.72 ± 0.10 D | 1.93 ± 0.03 AB |
| Total reducing sugar (mg/g) | 7.60 ± 0.82 A | 3.61 ± 0.49 B | 3.81 ± 0.71 B | 3.41 ± 0.87 B | 3.36 ± 0.21 B | 3.92 ± 0.70 B | 5.41 ± 0.80 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunanta, P.; Kontogiorgos, V.; Leksawasdi, N.; Phimolsiripol, Y.; Wangtueai, S.; Wongkaew, M.; Sommano, S.R. Loss Assessment during Postharvest and Handling of Thai Garlic Used for Processing. Horticulturae 2023, 9, 482. https://doi.org/10.3390/horticulturae9040482
Sunanta P, Kontogiorgos V, Leksawasdi N, Phimolsiripol Y, Wangtueai S, Wongkaew M, Sommano SR. Loss Assessment during Postharvest and Handling of Thai Garlic Used for Processing. Horticulturae. 2023; 9(4):482. https://doi.org/10.3390/horticulturae9040482
Chicago/Turabian StyleSunanta, Piyachat, Vassilis Kontogiorgos, Noppol Leksawasdi, Yuthana Phimolsiripol, Sutee Wangtueai, Malaiporn Wongkaew, and Sarana Rose Sommano. 2023. "Loss Assessment during Postharvest and Handling of Thai Garlic Used for Processing" Horticulturae 9, no. 4: 482. https://doi.org/10.3390/horticulturae9040482
APA StyleSunanta, P., Kontogiorgos, V., Leksawasdi, N., Phimolsiripol, Y., Wangtueai, S., Wongkaew, M., & Sommano, S. R. (2023). Loss Assessment during Postharvest and Handling of Thai Garlic Used for Processing. Horticulturae, 9(4), 482. https://doi.org/10.3390/horticulturae9040482









