Large-Scale In Vitro Propagation and Ex Vitro Adaptation of the Endangered Medicinal Plant Eryngium maritimum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Germination
2.3. Micropropagation
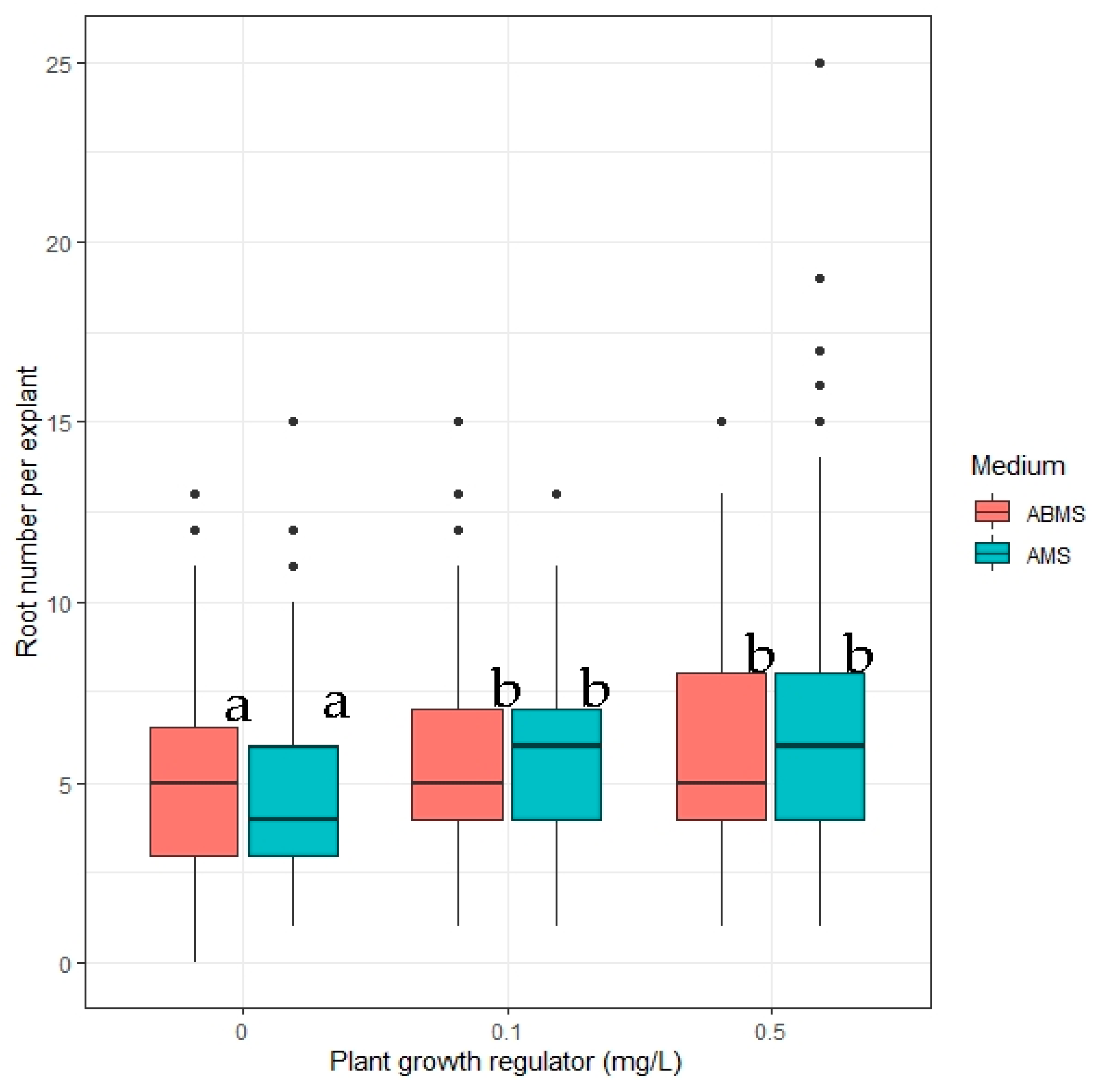
2.4. In Vitro Rooting
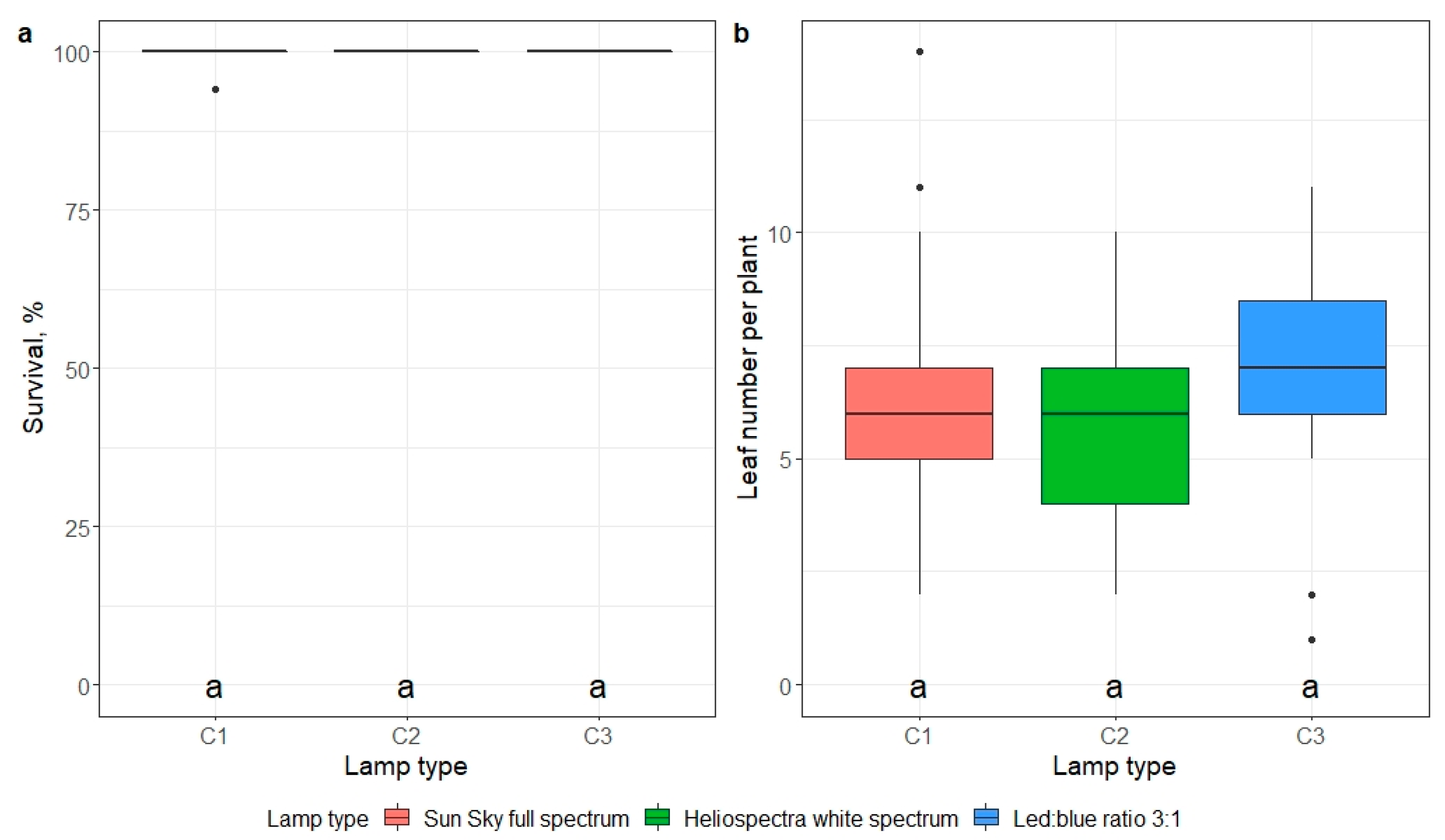
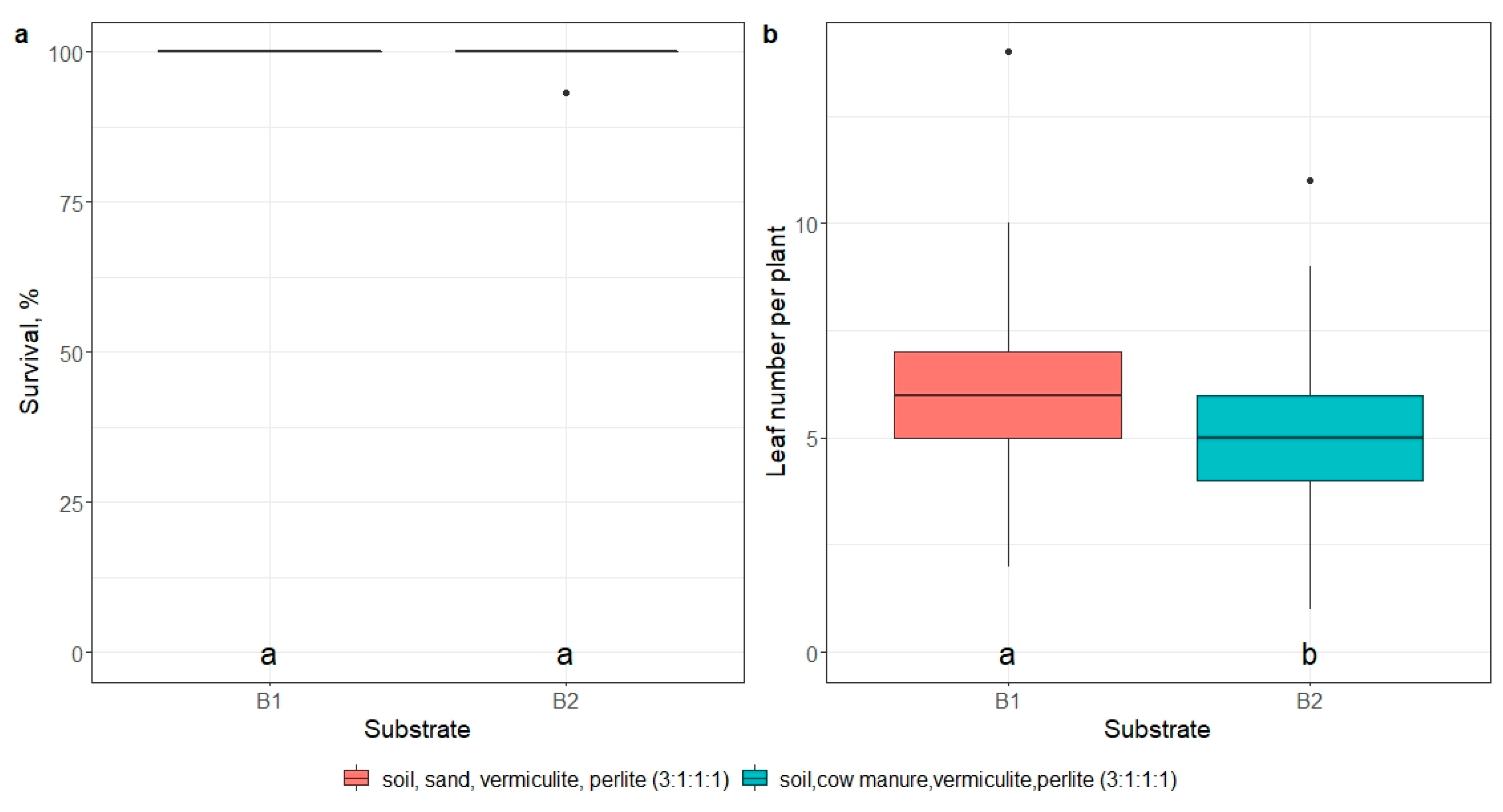
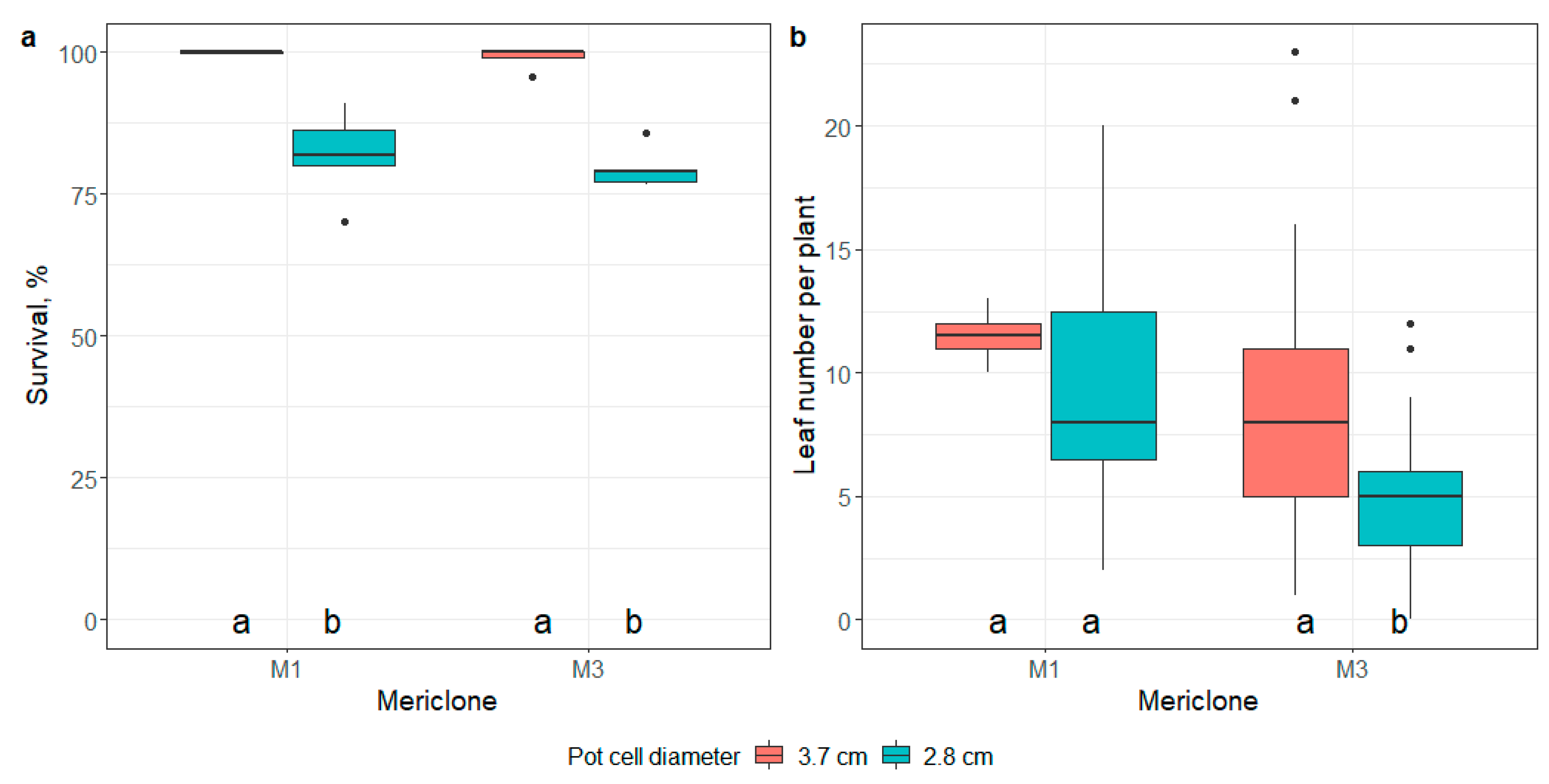
2.5. Ex Vitro Adaptation
2.6. Morphological Characterisation
2.7. Statistical Analysis
- -
- the impact of the disinfection on the seed infection rate;
- -
- the impact of the seed origin on the seed germination rate;
- -
- the impact of mericlone on the root development of in vitro plantlets;
- -
- impact of mericlone, substrate, or artificial light quality on the survival and number of leaves of E. maritimum ex vitro plantlets 21 days after ex vitro transplantation.
- -
- The impact of in vitro media macrosalt content and concentration of indole-3-acetic on the number of roots formed;
- -
- the impact of pot size on the survival and number of leaves of E. maritimum ex vitro plantlets 21 days after transplantation;
- -
- the impact of mericlone and cultivation length on the field survival rate;
- -
- the impact of planting date and cultivation length on the field survival rate.
- -
- The impact of basal media composition and supplementation of media with various cytokinins and the concentration of the cytokinins on the number of regenerated axillary shoots, the number of optimal sizes (>16 mm) and small (10–15 mm) axillary shoots, and shoot basal callus size;
3. Results
3.1. Micropropagation
3.2. Rooting
3.3. Ex Vitro Adaptation and Transfer to Field Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clausing, G.; Vickers, K.; Kadereit, J.W. Historical Biogeography in a Linear System: Genetic Variation of Sea Rocket (Cakile Maritima) and Sea Holly (Eryngium Maritimum) along European Coasts. Mol. Ecol. 2000, 9, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Ben Lajnef, H.; Ferioli, F.; Pasini, F.; Politowicz, J.; Khaldi, A.; Filippo D’Antuono, L.; Caboni, M.F.; Nasri, N. Chemical Composition and Antioxidant Activity of the Volatile Fraction Extracted from Air-Dried Fruits of Tunisian Eryngium Maritimum L. Ecotypes. J. Sci. Food Agric. 2018, 98, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Darriet, F.; Andreani, S.; De Cian, M.C.; Costa, J.; Muselli, A. Chemical Variability and Antioxidant Activity of Eryngium Maritimum L. Essential Oils from Corsica and Sardinia. Flavour Fragr. J. 2014, 29, 3–13. [Google Scholar] [CrossRef]
- Darriet, F.; Znini, M.; Majidi, L.; Muselli, A.; Hammouti, B.; Bouyanzer, A.; Costa, J. Evaluation of Eryngium Maritimum Essential Oil as Environmentally Friendly Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Int. J. Electrochem. Sci 2013, 8, 4328–4345. [Google Scholar]
- Kikowska, M.; Kalemba, D.; Dlugaszewska, J.; Thiem, B. Chemical Composition of Essential Oils from Rare and Endangered Species—Eryngium maritimum L. and E. alpinum L. Plants 2020, 9, 417. [Google Scholar] [CrossRef]
- Maggio, A.; Bruno, M.; Formisano, C.; Rigano, D.; Senatore, F. Chemical Composition of the Essential Oils of Three Species of Apiaceae Growing Wild in Sicily: Bonannia Graeca, Eryngium Maritimum and Opopanax Chironium. Nat. Prod. Commun. 2013, 8, 1934578X1300800640. [Google Scholar] [CrossRef]
- Kikowska, M.; Thiem, B.; Sliwinska, E.; Rewers, M.; Kowalczyk, M.; Stochmal, A.; Oleszek, W. The Effect of Nutritional Factors and Plant Growth Regulators on Micropropagation and Production of Phenolic Acids and Saponins from Plantlets and Adventitious Root Cultures of Eryngium maritimum L. J. Plant Growth Regul. 2014, 33, 809–819. [Google Scholar] [CrossRef]
- Kikowska, M.; Chanaj-Kaczmarek, J.; Derda, M.; Budzianowska, A.; Thiem, B.; Ekiert, H.; Szopa, A. The Evaluation of Phenolic Acids and Flavonoids Content and Antiprotozoal Activity of Eryngium Species Biomass Produced by Biotechnological Methods. Molecules 2022, 27, 363. [Google Scholar] [CrossRef]
- Sultana, K.W.; Das, S.; Chandra, I.; Roy, A. Efficient Micropropagation of Thunbergia Coccinea Wall. and Genetic Homogeneity Assessment through RAPD and ISSR Markers. Sci. Rep. 2022, 12, 1683. [Google Scholar] [CrossRef]
- Ayuso, M.; García-Pérez, P.; Ramil-Rego, P.; Gallego, P.P.; Barreal, M.E. In Vitro Culture of the Endangered Plant Eryngium Viviparum as Dual Strategy for Its Ex Situ Conservation and Source of Bioactive Compounds. Plant Cell Tissue Organ Cult. 2019, 138, 427–435. [Google Scholar] [CrossRef]
- Pence, V.C.; Meyer, A.; Linsky, J.; Gratzfeld, J.; Pritchard, H.W.; Westwood, M.; Bruns, E.B. Defining Exceptional Species—A Conceptual Framework to Expand and Advance Ex Situ Conservation of Plant Diversity beyond Conventional Seed Banking. Biol. Conserv. 2022, 266, 109440. [Google Scholar] [CrossRef]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.-C. Cryobiotechnologies: Tools for Expanding Long-Term Ex Situ Conservation to All Plant Species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Necajeva, J.; Ievinsh, G. Seed Dormancy and Germination of an Endangered Coastal Plant Eryngium Maritimum (Apiaceae). Est. J. Ecol. 2013, 62, 150–161. [Google Scholar] [CrossRef]
- Bączek, K.; Pawełczak, A.; Przybył, J.L.; Kosakowska, O.; Węglarz, Z. Secondary Metabolites of Various Eleuthero (Eleutherococcus Senticosus/Rupr. et Maxim./Maxim) Organs Derived from Plants Obtained by Somatic Embryogenesis. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 433–466. ISBN 978-3-030-30185-9. [Google Scholar]
- Kikowska, M.; Thiem, B. In Vitro Systems of Selected Eryngium Species (E. Planum, E. Campestre, E. Maritimum, and E. Alpinum) for Studying Production of Desired Secondary Metabolites (Phenolic Acids, Flavonoids, Triterpenoid Saponins, and Essential Oil). In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 869–901. ISBN 978-3-030-30185-9. [Google Scholar]
- Kikowska, M.; Kruszka, D.; Derda, M.; Hadaś, E.; Thiem, B. Phytochemical Screening and Acanthamoebic Activity of Shoots from in Vitro Cultures and in Vivo Plants of Eryngium Alpinum L.—The Endangered and Protected Species. Molecules 2020, 25, 1416. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Thiem, B.; Sliwinska, E.; Rewers, M.; Kowalczyk, M.; Stochmal, A.; Długaszewska, J. Micropropagation of Eryngium Campestre L. Via Shoot Culture Provides Valuable Uniform Plant Material with Enhanced Content of Phenolic Acids and Antimicrobial Activity. Acta Biol. Cracoviensia Ser. Bot. 2016, 58, 43–56. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Krawczyk, A.; Więckowska, B.; Sliwinska, E. Phenolic Acid and DNA Contents of Micropropagated Eryngium planum L. Plant Cell, Tissue Organ Cult. 2013, 114, 197–206. [Google Scholar] [CrossRef]
- Kļaviņa, D.; Gailīte, A.; Ievinsh, G. Initial Responses of Explants from Rare and Endangered Coastal Plant Species during Initiation of Tissue Culture. Acta Univ. Latv. 2006, 710, 81–91. [Google Scholar]
- Nagananda, G.S.; Rajath, S.; Mathew, R.K.; Rajan, S.S. Effect of Adjuvants and Nitrogen Sources on in Vitro Shoot Regeneration and Clonal Propagation of Medicinally Important Plant Eryngium foetidum L. Res. Biotechnol. 2012, 3, 21–25. [Google Scholar]
- Kikowska, M.; Thiem, B.; Szopa, A.; Klimek-Szczykutowicz, M.; Rewers, M.; Sliwinska, E.; Ekiert, H. Comparative Analysis of Phenolic Acids and Flavonoids in Shoot Cultures of Eryngium Alpinum L.: An Endangered and Protected Species with Medicinal Value. Plant Cell, Tissue Organ Cult. 2019, 139, 167–175. [Google Scholar] [CrossRef]
- Fay, M.F. Conservation of Rare and Endangered Plants Using in Vitro Methods. Vitr. Cell. Dev. Biol. Plant 1992, 28, 1–4. [Google Scholar] [CrossRef]
- Benson, E.E.; Danaher, J.E.; Pimbley, I.M.; Anderson, C.T.; Wake, J.E.; Daley, S.; Adams, L.K. In Vitro Micropropagation of Primula Scotica: A Rare Scottish Plant. Biodivers. Conserv. 2000, 9, 711–726. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Theodorou, P.; Aretaki, P.-E. In Vitro Propagation of the Mount Parnitha Endangered Species Sideritis Raeseri Subsp. Attica. Horticulturae 2022, 8, 1114. [Google Scholar] [CrossRef]
- Ezz, A.L.; Dura, S.; Daradkeh, N. Propagation Physiology of Juniperus Phoenicea L. from Jordan Using Seeds and in Vitro Culture Techniques: Baseline Information for a Conservation Perspective. African J. Biotechnol. 2012, 11, 7684–7692. [Google Scholar]
- Kim, M.; Shim, C.; Lee, J.; Wangchuk, C. Hot Water Treatment as Seed Disinfection Techniques for Organic and Eco-Friendly Environmental Agricultural Crop Cultivation. Agriculture 2022, 12, 1081. [Google Scholar] [CrossRef]
- Sen, M.K.; Jamal, M.; Nasrin, S. Sterilization Factors Affect Seed Germination and Proliferation of Achyranthes Aspera Cultured in Vitro. Environ. Exp. Biol. 2013, 11, 119–123. [Google Scholar]
- Gammoudi, N.; Nagaz, K.; Ferchichi, A. Establishment of Optimized in Vitro Disinfection Protocol of Pistacia Vera L. Explants Mediated a Computational Approach: Multilayer Perceptron–Multi−objective Genetic Algorithm. BMC Plant Biol. 2022, 22, 324. [Google Scholar] [CrossRef]
- Yildiz, M.; Fatih Ozcan, S.; T Kahramanogullari, C.; Tuna, E. The Effect of Sodium Hypochlorite Solutions on the Viability and in Vitro Regeneration Capacity of the Tissue. Nat. Prod. J. 2012, 2, 328–331. [Google Scholar] [CrossRef]
- Sahu, P.K.; Tilgam, J.; Mishra, S.; Hamid, S.; Gupta, A.; Verma, S.K.; Kharwar, R.N. Surface Sterilization for Isolation of Endophytes: Ensuring What (Not) to Grow. J. Basic Microbiol. 2022, 62, 647–668. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent Development in Micropropagation Techniques for Rare Plant Species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Tripathi, S.; Lal, M.; Mishra, S. Screening of Some Chemical Disinfectants for Media Sterilization During In Vitro Micropropagation of Sugarcane. Sugar Tech 2012, 14, 364–369. [Google Scholar] [CrossRef]
- Subramanya, S.H.; Pai, V.; Bairy, I.; Nayak, N.; Gokhale, S.; Sathian, B. Potassium Permanganate Cleansing Is an Effective Sanitary Method for the Reduction of Bacterial Bioload on Raw Coriandrum Sativum. BMC Res. Notes 2018, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Grauda, D.; Lapiņa, L.; Jansone, B.; Jansons, A.; Rashal, I. Recovering Genetic Resources of Some Legume Species of Latvian Origin by Plant Tissue Culture. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2013, 67, 224–228. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Bakulin, S.D.; Ermolaeva, O.Y.; Kozlovsky, B.L.; Dmitriev, P.A.; Stepanenko, V.V.; Kornienko, I.V.; Bushkova, A.A.; Rajput, V.D.; Varduny, T.V. Investigation of Growth Factors and Mathematical Modeling of Nutrient Media for the Shoots Multiplication In Vitro of Rare Plants of the Rostov Region. Horticulturae 2023, 9, 60. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant Tissue Culture Media and Practices: An Overview. Vitr. Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kadapatti, S.S.; Murthy, H.N. In Vitro Micropropagation of Andrographis Macrobotrys. J. Herbs. Spices Med. Plants 2022, 28, 89–98. [Google Scholar] [CrossRef]
- Asensio, E.; de Medinacelli Juan-Méndez, R.; Juan-Vicedo, J. In Vitro Propagation and Phytochemistry of Thymol-Producing Plants from a Horticultural Form of Thymus × Josephi-Angeli Mansanet & Aguil. (Lamiaceae). Horticulturae 2022, 8, 1188. [Google Scholar]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the Cell Cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, Cytokinin and the Control of Shoot Branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An Academic and Technical Overview on Plant Micropropagation Challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Kikowska, M.; Sliwinska, E.; Thiem, B. Micropropagation and Production of Somatic Seeds for Short-Term Storage of the Endangered Species Eryngium alpinum L. Plants 2020, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Beatrice, P.; Chiatante, D.; Scippa, G.S.; Montagnoli, A. Photoreceptors’ Gene Expression of Arabidopsis Thaliana Grown with Biophilic LED-Sourced Lighting Systems. PLoS ONE 2022, 17, e0269868. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Hwang, H.; An, S.; Lee, B.; Chun, C. Improvement of Growth and Morphology of Vegetable Seedlings with Supplemental Far-Red Enriched LED Lights in a Plant Factory. Horticulturae 2020, 6, 109. [Google Scholar] [CrossRef]
- Akimova, S.; Radzhabov, A.; Esaulko, A.; Samoshenkov, E.; Nechiporenko, I.; Kazakov, P.; Voskoboinikov, Y.; Matsneva, A.; Zubkov, A.; Aisanov, T. Improvement of Ex Vitro Growing Completion of Highbush Blueberry (Vaccinium Corymbosum L.) in Containers. Forests 2022, 13, 1550. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Chen, F.; Zhao, J.; Wang, X.; Khan, A.T.; Yuegao, X. Effects of Red and Blue LEDs on in Vitro Growth and Microtuberization of Potato Single-Node Cuttings. Front. Agric. Sci. Eng. 2018, 5, 197–205. [Google Scholar]
- Dutta Gupta, S.; Jatothu, B. Fundamentals and Applications of Light-Emitting Diodes (LEDs) in in Vitro Plant Growth and Morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Randall, W.C.; Lopez, R.G. Comparison of Supplemental Lighting from High-Pressure Sodium Lamps and Light-Emitting Diodes during Bedding Plant Seedling Production. HortScience 2014, 49, 589–595. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.-H.; Kim, S.-K.; Lee, K.-H.; Park, J.-Y.; Nam, M.-W.; Choi, D.-H.; Lee, H.-I. LED Light for in Vitro and Ex Vitro Efficient Growth of Economically Important Highbush Blueberry (Vaccinium Corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Kobori, M.M.R.G.; da Costa Mello, S.; de Freitas, I.S.; Silveira, F.F.; Alves, M.C.; Azevedo, R.A. Supplemental Light with Different Blue and Red Ratios in the Physiology, Yield and Quality of Impatiens. Sci. Hortic. 2022, 306, 111424. [Google Scholar] [CrossRef]
- Tarakanov, I.G.; Kosobryukhov, A.A.; Tovstyko, D.A.; Anisimov, A.A.; Shulgina, A.A.; Sleptsov, N.N.; Kalashnikova, E.A.; Vassilev, A.V.; Kirakosyan, R.N. Effects of Light Spectral Quality on the Micropropagated Raspberry Plants during Ex Vitro Adaptation. Plants 2021, 10, 2071. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shawon, M.; Ahmed, R.; An, J.H.; Yun, Y.J.; Park, S.J.; Na, J.K.; Choi, K.Y. Influence of Substrate Composition and Container Size on the Growth of Tissue Culture Propagated Apple Rootstock Plants. Agronomy 2021, 11, 2450. [Google Scholar] [CrossRef]
- Mohammed, M.; Munir, M.; Ghazzawy, H.S. Design and Evaluation of a Smart Ex Vitro Acclimatization System for Tissue Culture Plantlets. Agronomy 2023, 13, 78. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic Substrate for Transplant Production in Organic Nurseries. A Review. Agron. Sustain. Dev. 2018, 38, 35. [Google Scholar] [CrossRef]
- Neri, J.C.; Meléndez-Mori, J.B.; Tejada-Alvarado, J.J.; Vilca-Valqui, N.C.; Huaman-Huaman, E.; Oliva, M.; Goñas, M. An Optimized Protocol for Micropropagation and Acclimatization of Strawberry (Fragaria× Ananassa Duch.) Variety ‘Aroma.’. Agronomy 2022, 12, 968. [Google Scholar] [CrossRef]
- Bouzo, C.A.; Favaro, J.C. Container Size Effect on the Plant Production and Precocity in Tomato (Solanum Lycopersicum L.). Bulg. J. Agric. Sci. 2015, 21, 325–332. [Google Scholar]
- Oh, H.J.; Park, Y.G.; Park, J.E.; Jeong, B.R. Effect of Cell Size on Growth and Development of Plug Seedlings of Three Indigenous Medicinal Plants. J. Bio-Environ. Control 2014, 23, 71–76. [Google Scholar] [CrossRef]
- Kumar, R.R.; Purohit, V.K.; Prasad, P.; Nautiyal, A.R. Efficient In Vitro Propagation Protocol of Swertia Chirayita (Roxb. Ex Fleming) Karsten: A Critically Endangered Medicinal Plant. Natl. Acad. Sci. Lett. 2018, 41, 123–127. [Google Scholar] [CrossRef]
- Modgil, M.; Sharma, T.; Thakur, M. Commercially Feasible Protocol for Rooting and Accimatization of Micropropagated Apple Rootstocks. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; pp. 209–214. [Google Scholar]
- Smith, R.H. Chapter 5-Contamination, 3rd ed.; Academic Press: San Diego, CA, USA, 2013; pp. 53–62. ISBN 978-0-12-415920-4. [Google Scholar]
- Ngezahayo, F.; Liu, B. Axillary Bud Proliferation Approach for Plant Biodiversity Conservation and Restoration. Int. J. Biodivers. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Mežaka, I.; Kronberga, A.; Nakurte, I.; Taškova, I.; Jakovels, D.; Primavera, A. Genetic, Chemical and Morphological Variability of Chamomile (Chamomilla Recutita L.) Populations of Latvia. Ind. Crops Prod. 2020, 154, 112614. [Google Scholar] [CrossRef]
- Olaitan, M.M.; Mangse, G.; Ogbaga, C.C.; Uthman, T.O. Gibberellic Acid Influences Growth Indices and Biochemical Parameters in Micropropagated Ocimum Gratissimum L. Explants. J. Med. Plants Econ. Dev. 2022, 6, 7. [Google Scholar] [CrossRef]
- Pepe, M.; Hesami, M.; Jones, A.M. Machine Learning-Mediated Development and Optimization of Disinfection Protocol and Scarification Method for Improved In Vitro Germination of Cannabis Seeds. Plants 2021, 10, 2397. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Fernández, I.; Cerrato, M.D.; Ribas-Serra, A.; Cardona, C.; González, C.; Gil, L. Evidence of Interpopulation Variation in the Germination of Eryngium Maritimum L.(Apiaceae). Plant Ecol. 2021, 222, 1101–1112. [Google Scholar] [CrossRef]
- Cortés-Fernández, I.; Cerrato, M.D.; Ribas-Serra, A.; Gil Vives, L. Floral Traits and Reproductive Success Variation among Inflorescence Orders in Eryngium Maritimum. Plant Biol. 2022, 24, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Aviziene, D.; Pakalnis, R.; Sendzikaite, J. Status of Red-Listed Species Eryngium Maritimum L. on the Lithuanian Coastal Dunes. In Proceedings of the 7th International Conference Environmental Engineering, Vilnius, Lithuania, 22–23 May 2008; pp. 22–28. [Google Scholar]
- Raturi, M.K.; Thakur, A. Silver Nitrate and Silver-Thiosulphate Mitigates Callus and Leaf Abscission during Shisham Clonal Micro-Propagation. J. Plant Biotechnol. 2021, 48, 173–178. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A.; Eftekhari, M.; Ahmadi, H.; Akbari, M.; Khorami, S.S. Modeling and Optimizing a New Culture Medium for In Vitro Rooting of G×N15 Prunus Rootstock Using Artificial Neural Network-Genetic Algorithm. Sci. Rep. 2018, 8, 9977. [Google Scholar] [CrossRef] [PubMed]
- Cabral, N.N.; Pescador, R.; Pinheiro, M.V.M.; Ornellas, T.S.; Rizzolo, R.G.; Bordallo, S.U.; Guterres, S.M.; Gris, T.; Schvambach, M.I.; de Souza, P.F. Different Spectral Qualities Do Not Influence the in Vitro and Ex Vitro Survival of Epidendrum Denticulatum Barb. Rod.: A Brazilian Orchid. Vegetos 2022, 1–15. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot Size Matters: A Meta-Analysis of the Effects of Rooting Volume on Plant Growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Łabuz, T.A. Evaluation of Past and Present Sea Holly (Eryngium Maritimum) Habitats on Polish Coastal Dunes. Acta Univ. Latv. 2007, 723, 114. [Google Scholar]
- Rizwan, H.M.; Irshad, M.; He, B.; Liu, S.; Lu, X.; Sun, Y.; Qiu, D. Role of Reduced Nitrogen for Induction of Embryogenic Callus Induction and Regeneration of Plantlets in Abelmoschus esculentus L. South African J. Bot. 2020, 130, 300–307. [Google Scholar] [CrossRef]
- Shekhawat, M.S.; Mehta, S.R.; Manokari, M.; Priyadharshini, S.; Badhepuri, M.K.; Jogam, P.; Dey, A.; Rajput, B.S. Morpho-Anatomical and Physiological Changes of Indian Sandalwood (Santalum Album L.) Plantlets in Ex Vitro Conditions to Support Successful Acclimatization for Plant Mass Production. Plant Cell, Tissue Organ Cult. 2021, 147, 423–435. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, M.; Singh, D. V Growth, Yield and Quality of Peppermint (Mentha x Piperita L.) as Influenced by Planting Time. J. Herbs. Spices Med. Plants 1998, 5, 33–39. [Google Scholar] [CrossRef]
- Olesen, J.E.; Trnka, M.; Kersebaum, K.C.; Skjelvåg, A.O.; Seguin, B.; Peltonen-Sainio, P.; Rossi, F.; Kozyra, J.; Micale, F. Impacts and Adaptation of European Crop Production Systems to Climate Change. Eur. J. Agron. 2011, 34, 96–112. [Google Scholar] [CrossRef]
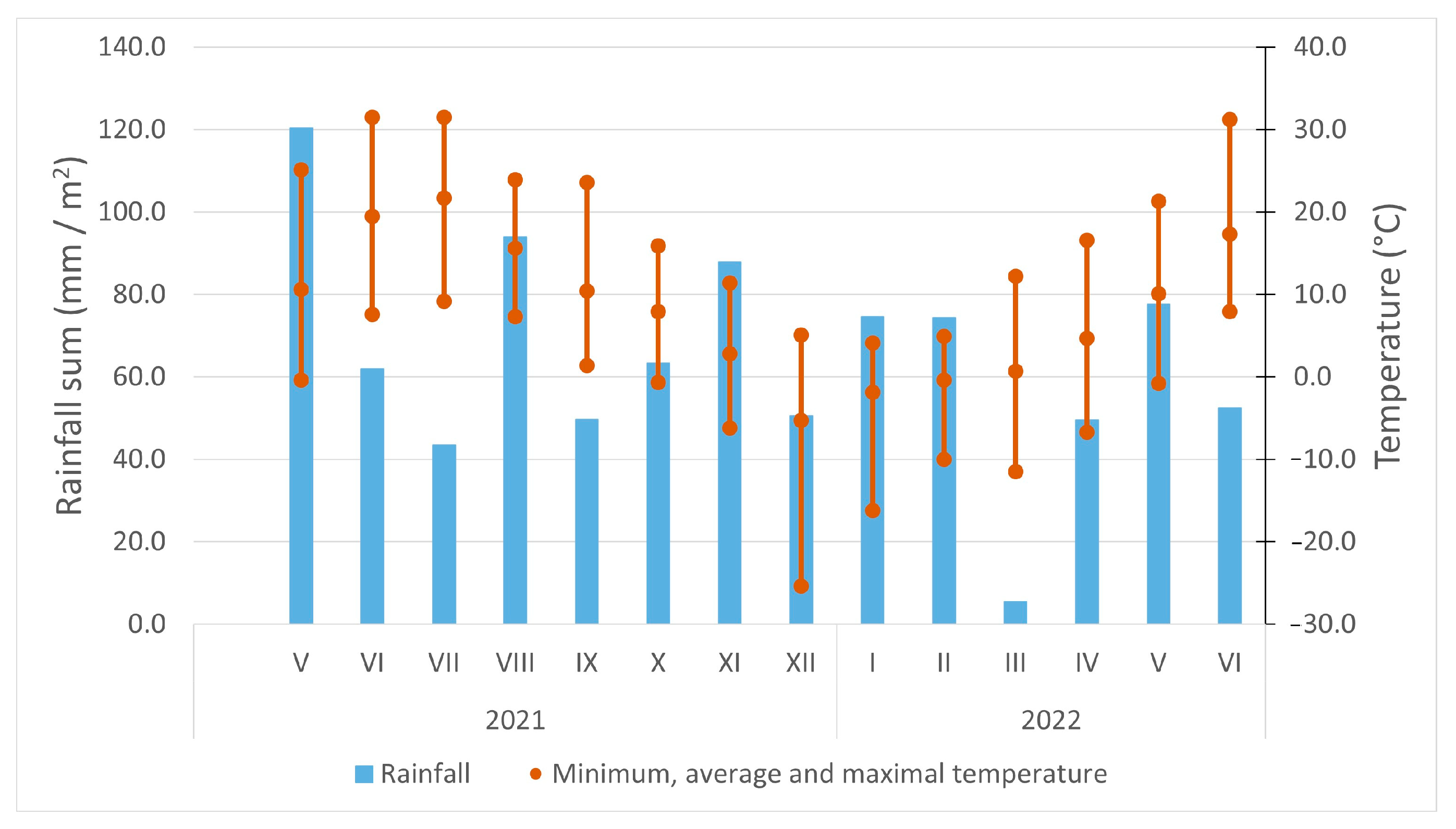
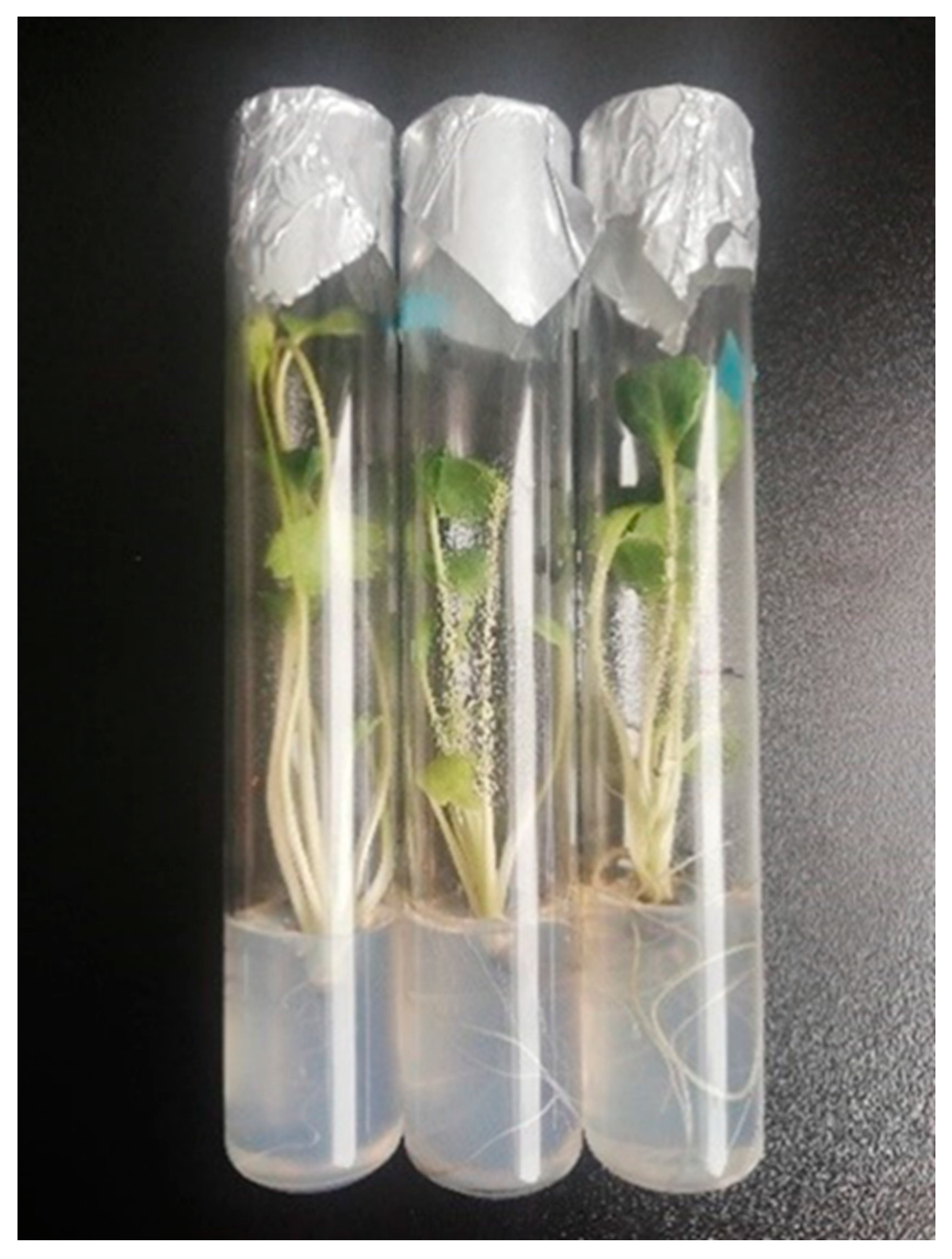
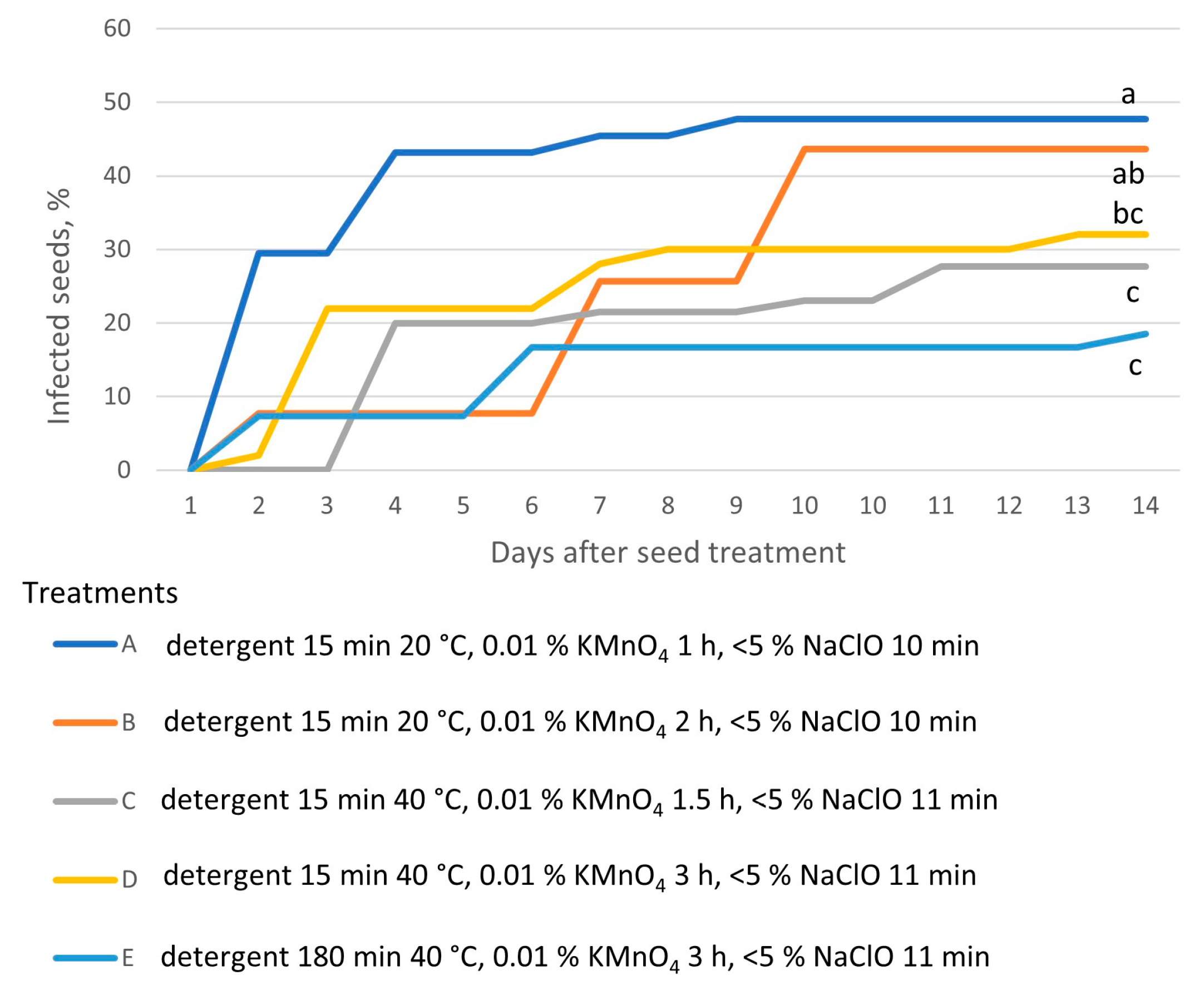
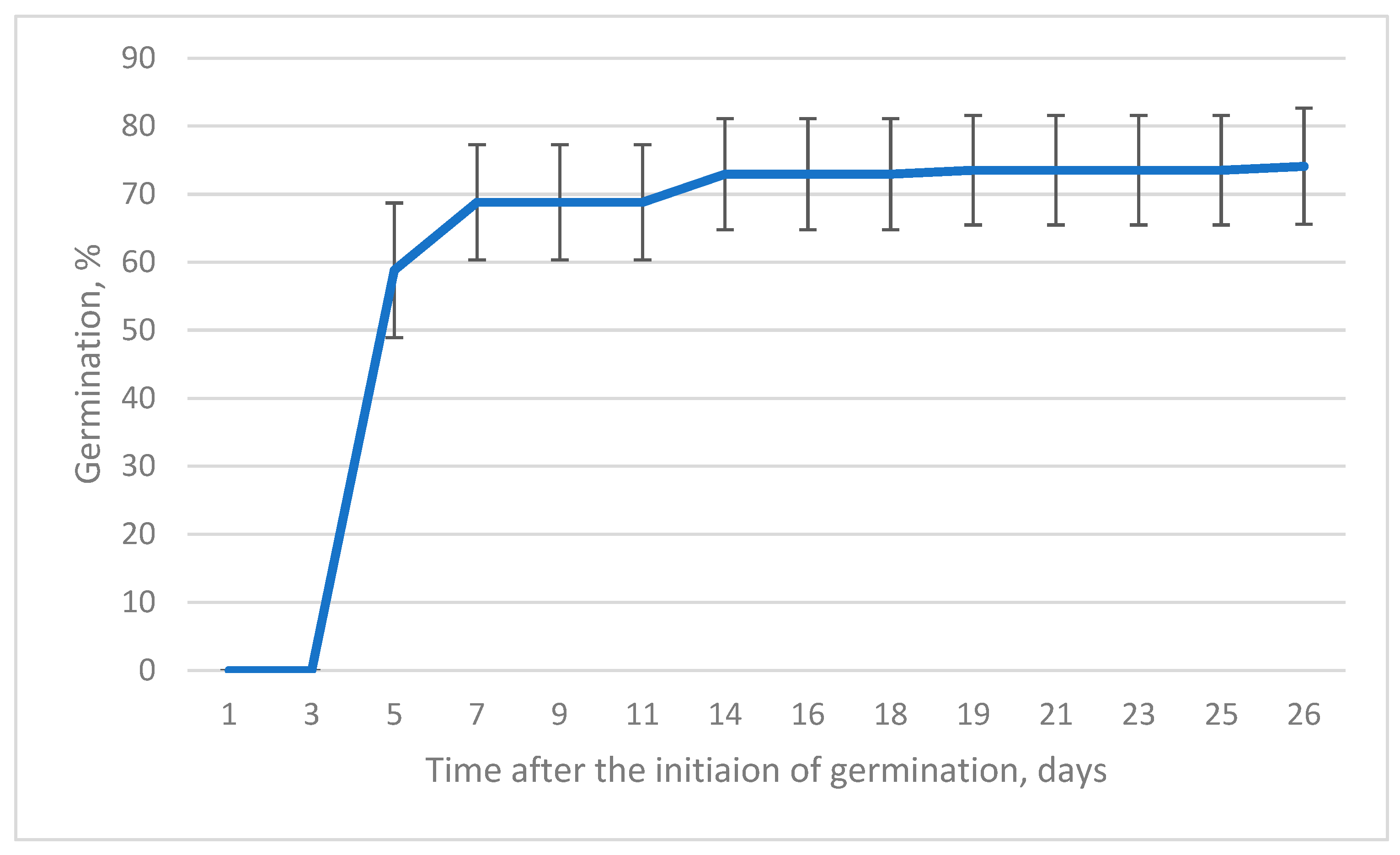
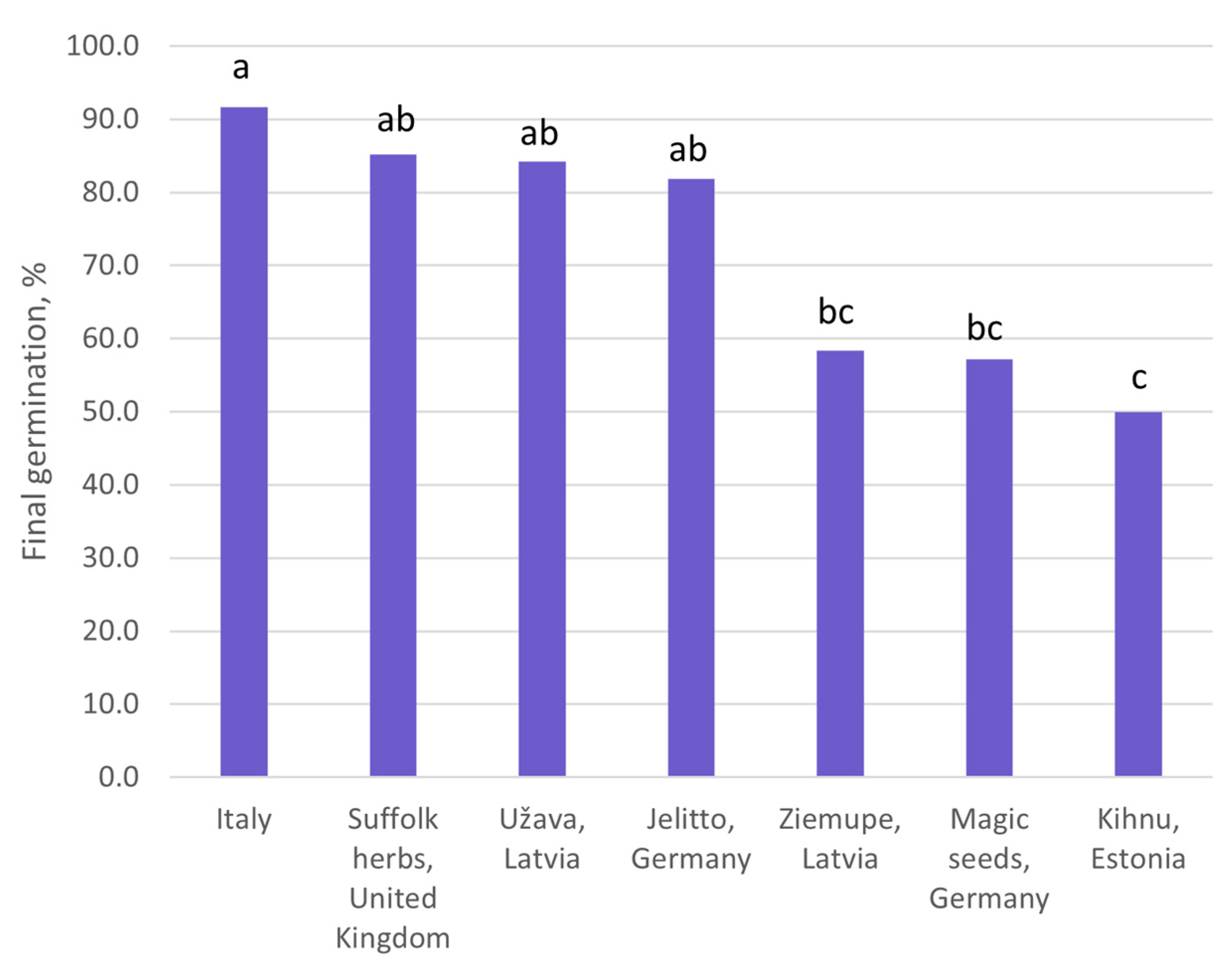
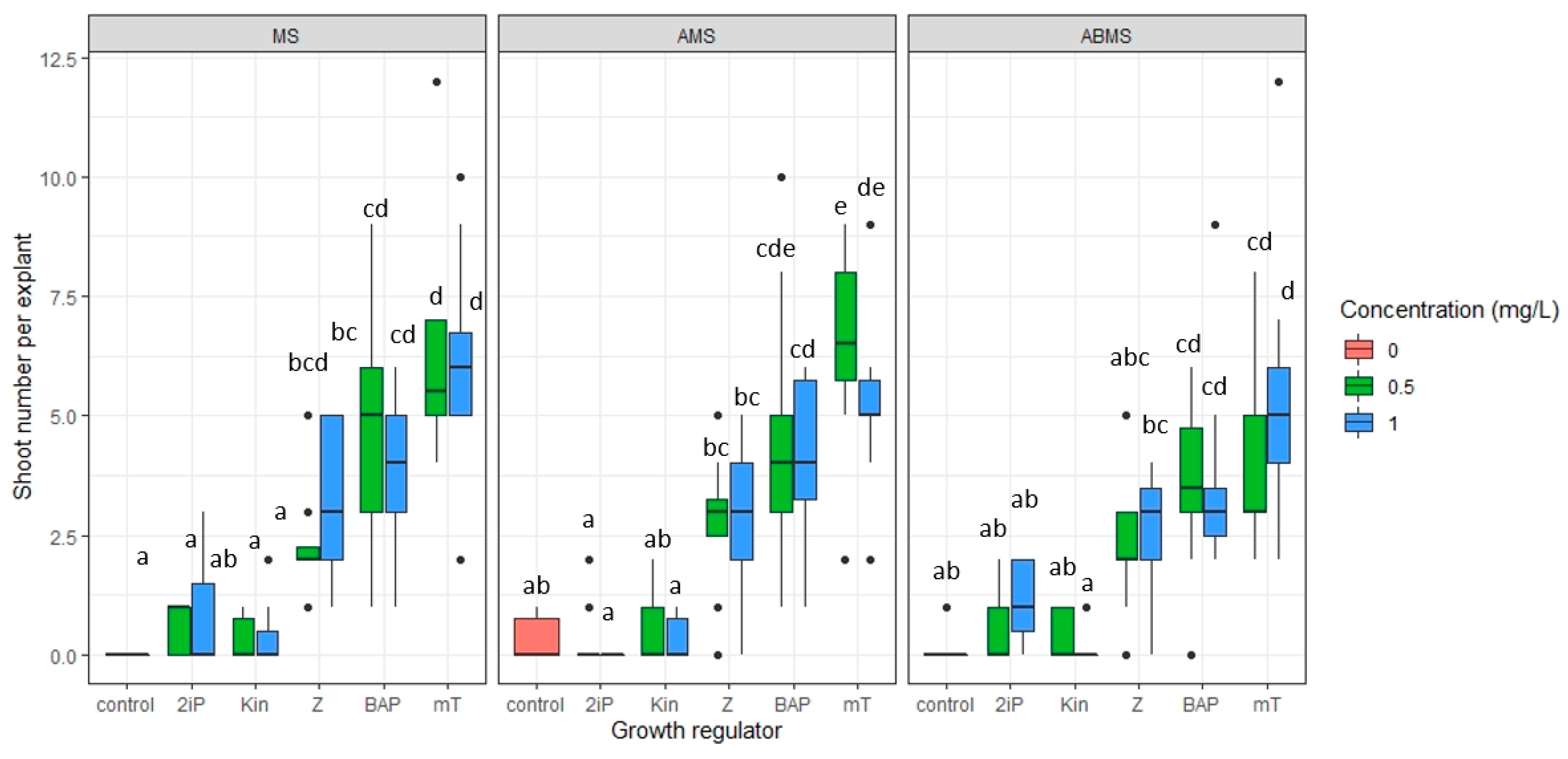
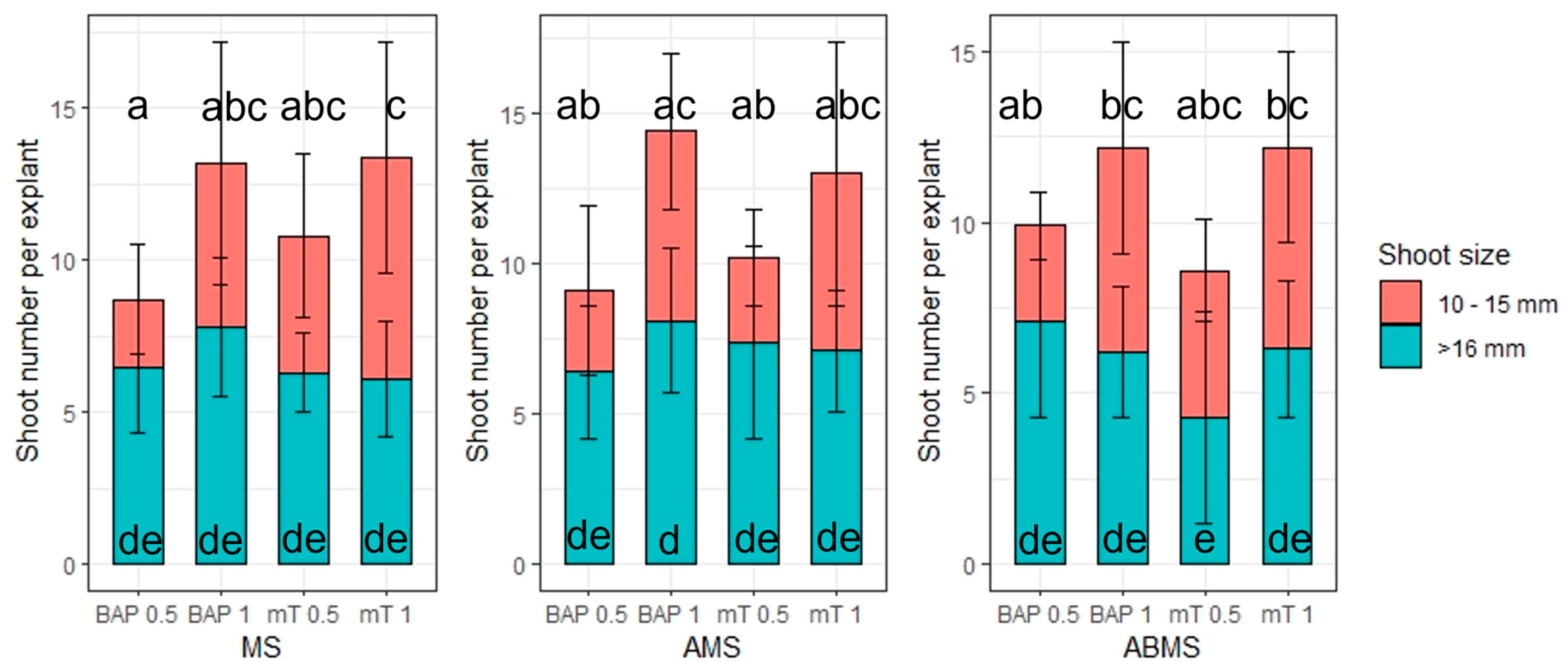
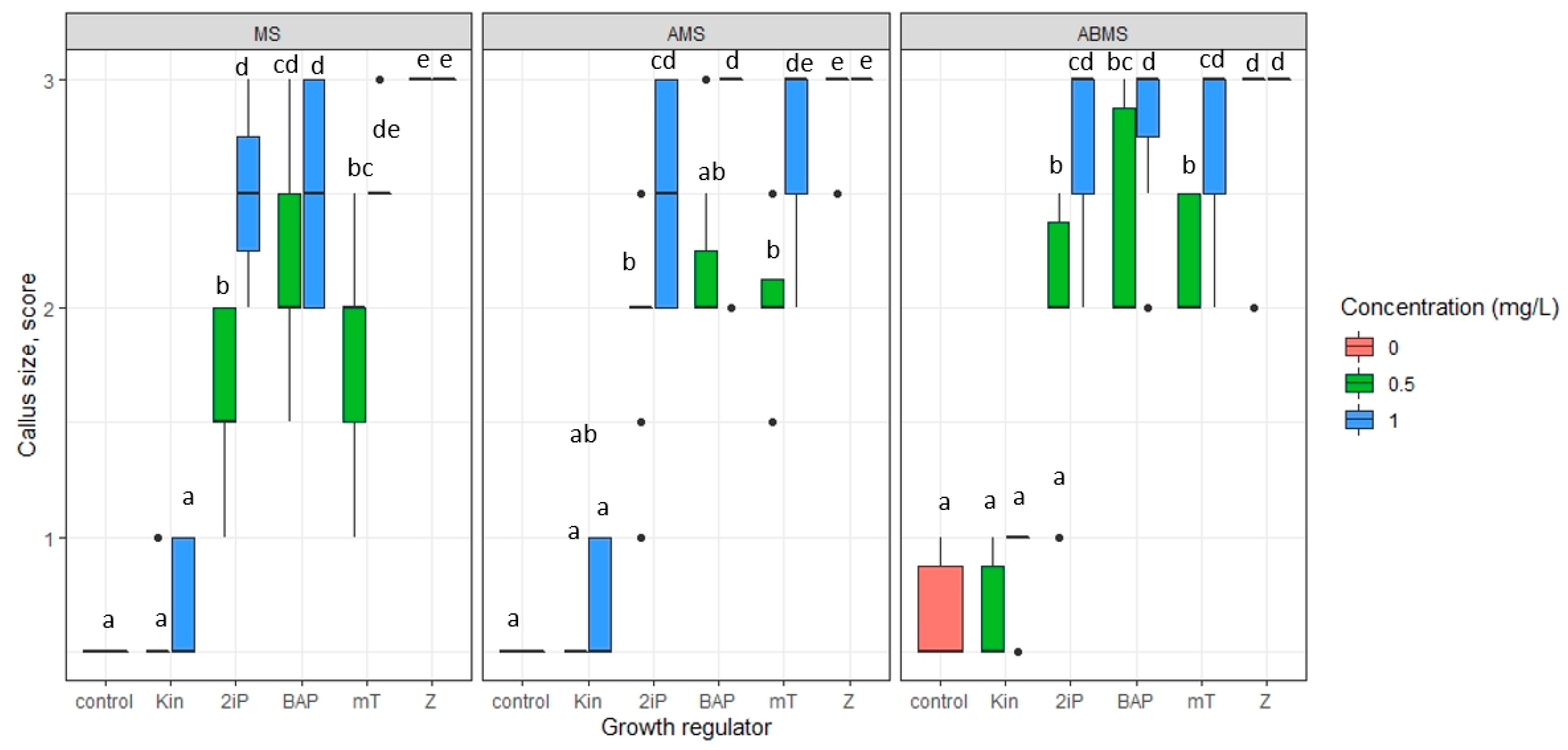
| Origin | Source | Coordinates |
|---|---|---|
| Jellito, Germany | Commercial | |
| Magic seeds, Germany | Commercial | |
| Suffolk herbs, United Kingdom | Commercial | |
| Kihnu, Estonia | Wild | N58°7′49″ E23°57′32″ |
| Saaremaa, Estonia | Wild | N58°28′36″ E21°54′59″ |
| Ziemupe, Latvia | Wild | N56°48′4″ E21°4′4″ |
| Užava, Latvia | Wild | N57°14′49″ E21°25′52″ |
| Italy | University of Pisa botanical garden collection |
| Experimental Variant | Diluted Commercial Detergent “Fairy” | KMnO4 (0.01%) | Commercial Bleach “ACE” |
|---|---|---|---|
| A | 15 min 20 °C | 1 h | 10 min |
| B | 15 min 20 °C | 2 h | 10 min |
| C | 15 min 40 °C | 1.5 h | 11 min |
| D | 15 min 40 °C | 3 h | 11 min |
| E | 180 min 40 °C | 3 h | 11 min |
| Survival, % | Root Development (Score, 1–3) | Survival, % | Number of Developed Leaves per Plant |
|---|---|---|---|
| M1 | 1.3a 1 | 99.0a | 5.7a |
| M2 | 1.3a | 99.4a | 6.2a |
| M3 | 1.8a | 99.0a | 6.5a |
| Planting Date | June 2021 | July 2021 | ||
|---|---|---|---|---|
| Survival, % | Autumn 2021 | Spring 2022 | Autumn 2021 | Spring 2022 |
| M1 | 90.8ab 1 | 72.5b | - | - |
| M2 | 99.2a | 77.5b | 55.8c | 22.5d |
| M3 | 86.7ab | 72.5b | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mežaka, I.; Kļaviņa, D.; Kaļāne, L.; Kronberga, A. Large-Scale In Vitro Propagation and Ex Vitro Adaptation of the Endangered Medicinal Plant Eryngium maritimum L. Horticulturae 2023, 9, 271. https://doi.org/10.3390/horticulturae9020271
Mežaka I, Kļaviņa D, Kaļāne L, Kronberga A. Large-Scale In Vitro Propagation and Ex Vitro Adaptation of the Endangered Medicinal Plant Eryngium maritimum L. Horticulturae. 2023; 9(2):271. https://doi.org/10.3390/horticulturae9020271
Chicago/Turabian StyleMežaka, Ieva, Dace Kļaviņa, Laura Kaļāne, and Arta Kronberga. 2023. "Large-Scale In Vitro Propagation and Ex Vitro Adaptation of the Endangered Medicinal Plant Eryngium maritimum L." Horticulturae 9, no. 2: 271. https://doi.org/10.3390/horticulturae9020271
APA StyleMežaka, I., Kļaviņa, D., Kaļāne, L., & Kronberga, A. (2023). Large-Scale In Vitro Propagation and Ex Vitro Adaptation of the Endangered Medicinal Plant Eryngium maritimum L. Horticulturae, 9(2), 271. https://doi.org/10.3390/horticulturae9020271








