Enhancement of Antioxidant Potential, Phytochemicals, Nutritional Properties, and Growth of Siphonochilus aethiopicus (Schweinf.) B.L.Burtt with Different Dosages of Compost Tea
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Extraction of the Compost Tea
2.3. Sample Measurement and Data Collection
2.4. Determination of Mineral Content
2.5. Preparation of Crude Extracts
2.6. Determination of Total Flavanol Content
2.7. Determination of Total Flavonol Content
2.8. Determination of Total Polyphenol Content
2.9. Determination of ABTS Antioxidant Capacity
2.10. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.11. Determination of Oxygen Radical Absorbance Capacity (ORAC)
2.12. Data Analysis
3. Results
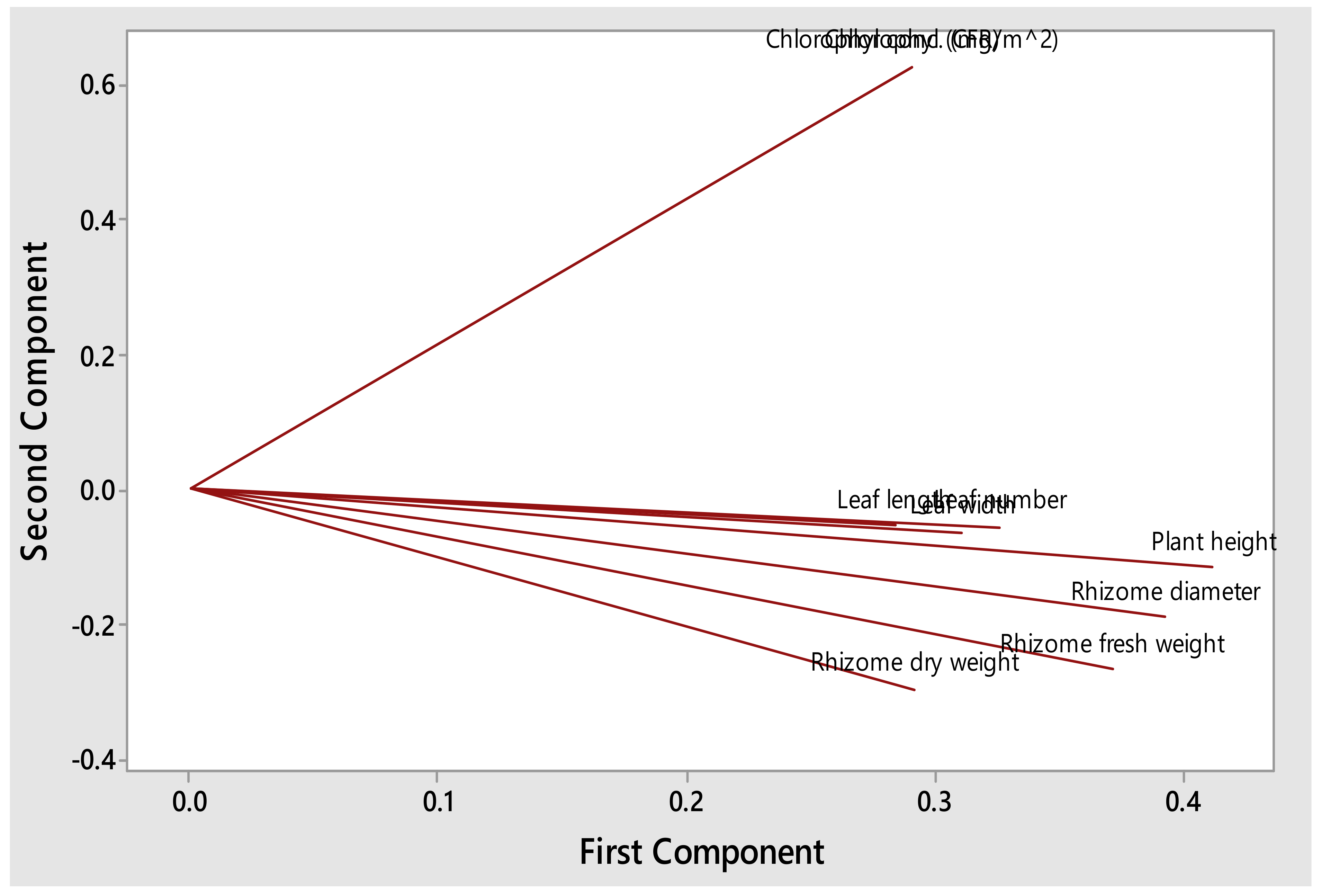
3.1. Impact of Compost Tea on Vegetative Growth of S. aethiopicus
Leaf Length, Leaf Width, Leaf Number, Plant Height, Chlorophyl and Rhizome Diameter
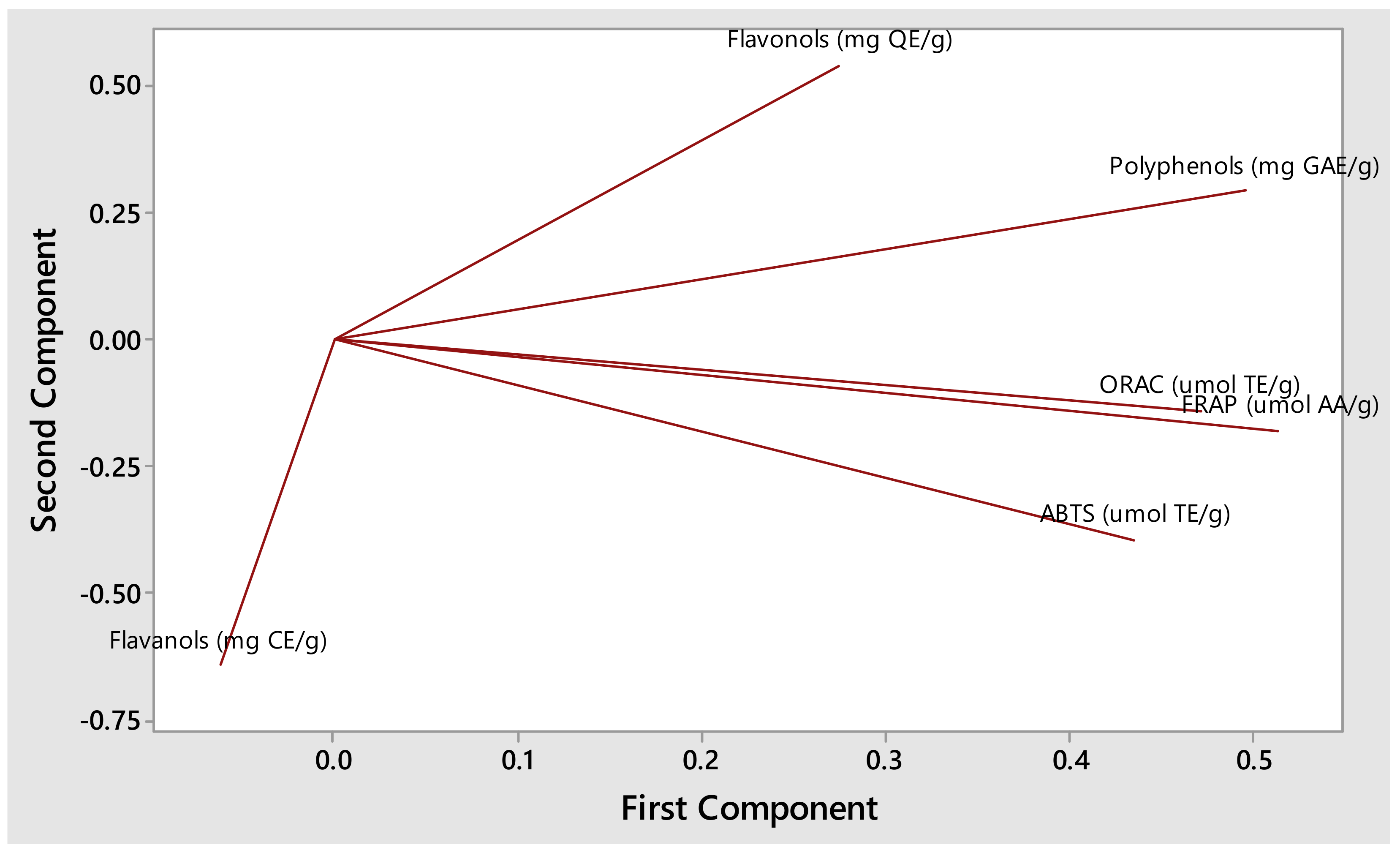
3.2. Impact of Compost Tea on Phytochemicals and Antioxidant Content of S. aethiopicus
3.2.1. Flavanols
3.2.2. Flavonols
3.2.3. Polyphenols
3.2.4. The ABTS, FRAP, and ORAC Antioxidant Contents
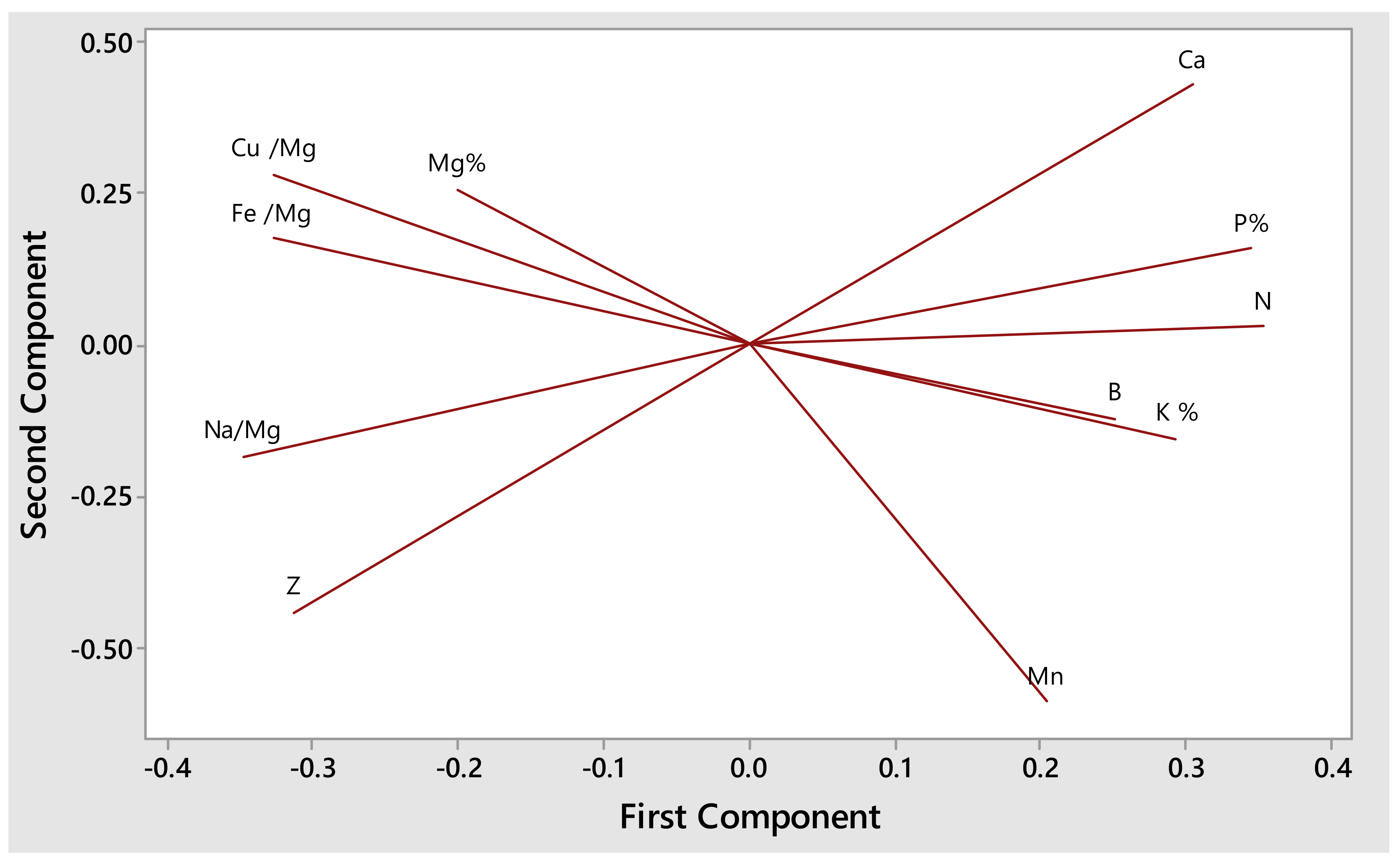
3.3. Effects of Varying Dosages of Compost Tea on Mineral Compositions of S. aethiopicus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Light, M.E.; McGaw, L.J.; Rabe, T.; Sparg, S.G.; Taylor, M.B.; Erasmus, D.G.; Jäger, A.K.; van Staden, J. Investigation of the Biological Activities of Siphonochilus aethiopicus and the Effect of Seasonal Senescence. South Afr. J. Bot. 2002, 68, 55–61. [Google Scholar] [CrossRef]
- Seile, B.P.; Bareetseng, S.; Koitsiwe, M.T.; Aremu, A.O. Indigenous Knowledge on the Uses, Sustainability and Conservation of African Ginger (Siphonochilus aethiopicus) among Two Communities in Mpumalanga Province, South Africa. Diversity 2022, 14, 192. [Google Scholar] [CrossRef]
- Hankey, A.; Reynolds, Y. Siphonochilus aethiopicus (Schweif.) B.L.Burtt. Available online: https://pza.sanbi.org/siphonochilus-aethiopicus (accessed on 29 November 2022).
- Mokgehle, S.N.; Tesfay, S.Z.; Makgato, M.J.; Araya, H.T. Phytochemical Profiling and Soluble Sugars of African Ginger (Siphonochilus aethiopicus) from Different Growing Regions in South Africa. South Afr. J. Plant Soil 2019, 36, 157–163. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Amoo, S.O.; Mokgehle, S.N.; Aremu, A.O. Ethnomedicinal Uses, Biological Activities, Phytochemistry and Conservation of African Ginger (Siphonochilus aethiopicus): A Commercially Important and Endangered Medicinal Plant. J. Ethnopharmacol. 2021, 266, 113459. [Google Scholar] [CrossRef]
- Noudogbessi, J.P.A.; Tchobo, P.F.; Alitonou, G.A.; Avlessi, F.; Soumanou, M.; Chalard, P.; Figueredo, G.; Chalchat, J.C.; Sohounhloue, D.C.K. Chemical Study of Extracts of Siphonochilus aethiopicus (Schweinf.) B.L.Burtt (Zingiberaceae) from Benin. Asian J. Chem. 2013, 25, 8489–8492. [Google Scholar] [CrossRef]
- Zongwe, F.K.; Muya, J.T.; Mutimana, R.; Virima, M.; Mayaliwa, M.; Chung, H.; Maharaj, V. Autoxidation of Siphonochilone in Processed Rhizomes and Stored Powders of Siphonochilus aethiopicus (Schweinf.) B.L.Burtt. ChemistrySelect 2018, 3, 8569–8574. [Google Scholar] [CrossRef]
- Igoli, N.P.; Al-Tannak, N.F.; Ezenyi, I.C.; Gray, A.I.; Igoli, J.O. Antiplasmodial Activity of a Novel Diarylheptanoid from Siphonochilus aethiopicus. Nat. Prod. Res. 2021, 35, 5588–5595. [Google Scholar] [CrossRef]
- Oladele, A.; Alade, G. Medicinal Plants Conservation and Cultivation by Traditional Medicine Practitioners (TMPs) in Aiyedaade Local Government Area of Osun State, Nigeria. Agric. Biol. J. N. Am. 2011, 2, 476–487. [Google Scholar] [CrossRef]
- Mbongwa, N.S.; Twine, W.C.; Williams, V.L. Medicinal Plant Cultivation: Beliefs and Perceptions of Traditional Healers and Muthi Traders in KwaZulu-Natal and Gauteng, South Africa. S. Afr. J. Bot. 2021, 143, 123–132. [Google Scholar] [CrossRef]
- Jimoh, M.A.; Jimoh, M.O.; Saheed, S.A.; Bamigboye, S.O.; Laubscher, C.P.; Kambizi, L. Commercialization of Medicinal Plants: Opportunities for Trade and Concerns for Biodiversity Conservation. In Sustainable Uses of Medicinal Plants; Kambizi, L., Bvenura, C., Eds.; Taylors & Francis, CRS Press: Boca Raton, FL, USA, 2023; p. 678. ISBN 9781032071732. [Google Scholar]
- Williams, V.L.; Balkwill, K.; Witkowski, E.T.F. Unraveling the Commercial Market for Medicinal Plants and Plant Parts on the Witwatersrand, South Africa. Econ. Bot. 2000, 54, 310–327. [Google Scholar] [CrossRef]
- Cunningham, A.B. Development of a Conservation Policy on Commercially Exploited Medicinal Plants: A Case Study from Southern Africa. In Conservation of Medicinal Plants; Cambridge University Press: Cambridge, UK, 1991; pp. 337–358. [Google Scholar]
- Van Wyk, A.S.; Prinsloo, G. Medicinal Plant Harvesting, Sustainability and Cultivation in South Africa. Biol. Conserv. 2018, 227, 335–342. [Google Scholar] [CrossRef]
- Zschocke, S.; Rabe, T.; Taylor, J.L.S.; Jäger, A.K.; van Staden, J. Plant Part Substitution—A Way to Conserve Endangered Medicinal Plants? J. Ethnopharmacol. 2000, 71, 281–292. [Google Scholar] [CrossRef]
- Jena, A.K.; Karan, M.; Vasisht, K. Plant Parts Substitution Based Approach as a Viable Conservation Strategy for Medicinal Plants: A Case Study of Premna Latifolia Roxb. J. Ayurveda Integr. Med. 2017, 8, 68–72. [Google Scholar] [CrossRef]
- Basak, B.B.; Saha, A.; Sarkar, B.; Kumar, B.P.; Gajbhiye, N.A.; Banerjee, A. Repurposing Distillation Waste Biomass and Low-Value Mineral Resources through Biochar-Mineral-Complex for Sustainable Production of High-Value Medicinal Plants and Soil Quality Improvement. Sci. Total Environ. 2021, 760, 143319. [Google Scholar] [CrossRef]
- Jasson, T.I. Effects of Compost Tea Extract on Growth, Nutritional Value, Soil Quality of Hypoxis Hemerocallidea and Siphonochilus aethiopicus. Master’s Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2017. [Google Scholar]
- Lu, S.; Pentico, D.; Castro, R.; Dinh, S.; Love, J.J.; Larom, D.L.; Pérez, R.L.; Liu, C. Effect of Ultraviolet Light Exposure and Compost Tea Supplementation on Growth, Antioxidant Activities, and Microbiome of Hydroponically Grown Mustard Greens. ACS Agric. Sci. Technol. 2022, 2, 521–533. [Google Scholar] [CrossRef]
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Paull, R.E. Biochemical Properties of Compost Tea Associated with Compost Quality and Effects on Pak Choi Growth. Sci. Hortic. 2012, 148, 138–146. [Google Scholar] [CrossRef]
- Nkcukankcuka, M.; Jimoh, M.O.; Griesel, G.; Laubscher, C.P. Growth Characteristics, Chlorophyll Content and Nutrients Uptake in Tetragonia Decumbens Mill. Cultivated under Different Fertigation Regimes in Hydroponics. Crop. Pasture Sci. 2022, 73, 67–76. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Germination Response of Amaranthus caudatus L. To Soil Types and Environmental Conditions. Thaiszia J. Bot. 2019, 29, 85–100. [Google Scholar] [CrossRef]
- Tshayingwe, A.; Jimoh, M.O.; Sogoni, A.; Wilmot, C.M.; Laubscher, C.P. Light Intensity and Growth Media Influence Growth, Nutrition, and Phytochemical Content in Trachyandra Divaricata Kunth. Agronomy 2023, 13, 247. [Google Scholar] [CrossRef]
- Salacha, M.I.; Kallithraka, S.; Tzourou, I. Browning of White Wines: Correlation with Antioxidant Characteristics, Total Polyphenolic Composition and Flavanol Content. Int. J. Food Sci. Technol. 2008, 43, 1073–1077. [Google Scholar] [CrossRef]
- Ngxabi, S.; Jimoh, M.O.; Kambizi, L.; Laubscher, C.P. Growth Characteristics, Phytochemical Contents, and Antioxidant Capacity of Trachyandra ciliata (L.f) Kunth Grown in Different Soilless Media in Hydroponics under Varying Degrees of Salinity. Horticulturae 2021, 7, 244. [Google Scholar] [CrossRef]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Phytochemical and Antioxidant Activities of Rumex Crispus L. in Treatment of Gastrointestinal Helminths in Eastern Cape Province, South Africa. Asian Pac. J. Trop. Biomed. 2017, 7, 1071–1078. [Google Scholar] [CrossRef]
- Jimoh, M.A.; Idris, O.A.; Jimoh, M.O. Cytotoxicity, Phytochemical, Antiparasitic Screening, and Antioxidant Activities of Mucuna pruriens (Fabaceae). Plants 2020, 9, 1249. [Google Scholar] [CrossRef] [PubMed]
- Bulawa, B.; Sogoni, A.; Jimoh, M.O.; Laubscher, C.P. Potassium Application Enhanced Plant Growth, Mineral Composition, Proximate and Phytochemical Content in Trachyandra divaricata Kunth (Sandkool). Plants 2022, 11, 3183. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC): New Horizons in Relating Dietary Antioxidants/Bioactives and Health Benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Naidu, Y.; Meon, S.; Kadir, J.; Siddiqui, Y. Microbial Starter for the Enhancement of Biological Activity of Compost Tea. Int. J. Agric. Biol. 2010, 12, 51–56. [Google Scholar]
- Gómez-Brandón, M.; Vela, M.; Martínez-Toledo, V.; Insam, H.; Domínguez, J. Effects of Compost and Vermicompost Teas as Organic Fertilizers. Adv. Fertil. Technol. Synth. 2015, 1, 300–318. [Google Scholar]
- Marín, F.; Santos, M.; Diánez, F.; Carretero, F.; Gea, F.J.; Yau, J.A.; Navarro, M.J. Characters of Compost Teas from Different Sources and Their Suppressive Effect on Fungal Phytopathogens. World J. Microbiol. Biotechnol. 2013, 29, 1371–1382. [Google Scholar] [CrossRef]
- Sanwal, S.K.; Yadav, R.K.; Singh, P.K. Effect of Organic Manure on Growth, Yield and Quality Parameters of Ginger (Zingiber Officinale). Indian J. Agric. Sci. 2007, 77, 67–72. [Google Scholar]
- St. Martin, C.C.G. Potential of Compost Tea for Suppressing Plant Diseases. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2014, 9, 1–38. [Google Scholar] [CrossRef]
- Xego, S. Hydroponic Propagation of Siphonochilus aethiopicus: An Endangered Medicinal Plant. Ph.D. Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2017. [Google Scholar]
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Talcott, S.T.; Krenek, K.A. Vermicompost Extracts Influence Growth, Mineral Nutrients, Phytonutrients and Antioxidant Activity in Pak Choi (Brassica rapa cv. Bonsai, Chinensis Group) Grown under Vermicompost and Chemical Fertiliser. J. Sci. Food Agric. 2009, 89, 2383–2392. [Google Scholar] [CrossRef]
- Holzapfel, C.W.; Marais, W.; Wessels, P.L.; van Wyk, B.E. Siphonochilone and Related Compounds and Uses Thereof. Phytochemistry 2013, 59, 405–407. [Google Scholar] [CrossRef]
- Al-Tannak, N.F.; Anyam, J.V.; Igoli, N.P.; Gray, A.I.; Alzharani, M.A.; Igoli, J.O. A New Sesquiterpene from South African Wild Ginger (Siphonochilus aethiopicus (Schweinf) B.L.Burtt). Nat. Prod. Res. 2022, 36, 4943–4948. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Egea-Gilabert, C. Application of Directly Brewed Compost Extract Improves Yield and Quality in Baby Leaf Lettuce Grown Hydroponically. Agronomy 2020, 10, 370. [Google Scholar] [CrossRef]
- Ros, M.; Hurtado-Navarro, M.; Giménez, A.; Fernández, J.A.; Egea-Gilabert, C.; Lozano-Pastor, P.; Pascual, J.A. Spraying Agro-Industrial Compost Tea on Baby Spinach Crops: Evaluation of Yield, Plant Quality and Soil Health in Field Experiments. Agronomy 2020, 10, 440. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Kambizi, L. Aquatic Phytotherapy: Prospects, Challenges and Bibliometric Analysis of Global Research Output on Medicinal Aquatic Plants from 2011 to 2020. In Medicinal Plants for Cosmetics, Health and Diseases; Lall, N., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 507–522. ISBN 9781003108375. [Google Scholar]
- Verrillo, M.; Salzano, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Bioactivity and Antimicrobial Properties of Chemically Characterized Compost Teas from Different Green Composts. Waste Manag. 2021, 120, 98–107. [Google Scholar] [CrossRef]
- Nofal, A.M.; El-Rahman, M.A.; Alharbi, A.A.; Abdelghany, T.M. Ecofriendly Method for Suppressing Damping-off Disease Caused by Rhizoctonia Solani Using Compost Tea. Bioresources 2021, 16, 6378–6391. [Google Scholar] [CrossRef]
- Apáti, P.; Szentmihályi, K.; Kristó, S.T.; Papp, I.; Vinkler, P.; Szoke, É.; Kéry, Á. Herbal Remedies of Solidago—Correlation of Phytochemical Characteristics and Antioxidative Properties. J. Pharm. Biomed. Anal. 2003, 32, 1045–1053. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Antioxidant and Phytochemical Activities of Amaranthus caudatus L. Harvested from Different Soils at Various Growth Stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef]
- Ng, Z.X.; Yong, P.H.; Lim, S.Y. Customized Drying Treatments Increased the Extraction of Phytochemicals and Antioxidant Activity from Economically Viable Medicinal Plants. Ind. Crop. Prod. 2020, 155, 112815. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Rosa, E.; Saavedra, M.J. Phytochemical characterization and antioxidant properties of baby-leaf watercress produced under organic production system. CyTA-J. Food 2013, 11, 343–351. [Google Scholar] [CrossRef]
- Katalinic, V.; Modun, D.; Music, I.; Boban, M. Gender Differences in Antioxidant Capacity of Rat Tissues Determined by 2,2′-Azinobis (3-Ethylbenzothiazoline 6-Sulfonate; ABTS) and Ferric Reducing Antioxidant Power (FRAP) Assays. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 47–52. [Google Scholar] [CrossRef]
- Ungur, R.A.; Borda, I.M.; Codea, R.A.; Ciortea, V.M.; Năsui, B.A.; Muste, S.; Sarpataky, O.; Filip, M.; Irsay, L.; Crăciun, E.C.; et al. A Flavonoid-Rich Extract of Sambucus nigra L. Reduced Lipid Peroxidation in a Rat Experimental Model of Gentamicin Nephrotoxicity. Materials 2022, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R.; Warman, P.R. The Effects of Municipal Solid Waste Compost And Compost Tea on Mineral Element Uptake And Fruit Quality of Strawberries. Compost. Sci. Util. 2013, 17, 85–94. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Heavy Metal Uptake and Growth Characteristics of Amaranthus caudatus L. under Five Different Soils in a Controlled Environment. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 417–425. [Google Scholar] [CrossRef]
- Alegría-Torán, A.; Barberá-Sáez, R.; Cilla-Tatay, A. Bioavailability of Minerals in Foods. In Handbook of Mineral Elements in Food; John Wiley & Sons: Chichester, UK, 2015; pp. 41–67. [Google Scholar] [CrossRef]
- Quintaes, K.D.; Diez-Garcia, R.W. The Importance of Minerals in the Human Diet. In Handbook of Mineral Elements in Food; John Wiley & Sons: Chichester, UK, 2015; pp. 1–21. [Google Scholar] [CrossRef]
- Singh, B.; Schulze, D.G. Soil Minerals and Plant Nutrition. Nat. Educ. Knowl. 2015, 6, 1–8. [Google Scholar]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Suitability of Amaranthus Species for Alleviating Human Dietary Deficiencies. South Afr. J. Bot. 2018, 115, 65–73. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Nutrients and Antinutrient Constituents of Amaranthus caudatus L. Cultivated on Different Soils. Saudi J. Biol. Sci. 2020, 27, 3570–3580. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The Importance of Minerals in Human Nutrition: Bioavailability, Food Fortification, Processing Effects and Nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]

| Treatments | Soil | pH | Resist. | H+ | P Bray II | K | Exchangeable Cations (cmol(+)/kg) | Cu | Zn | Mn | B | Fe | C% | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (KCl) | (Ohm) | (cmol/kg) | mg/kg | Na | K | Ca | Mg | mg/kg | mg/kg | |||||||
| 0.25 () | Sand | 4.85 ± 0.63 | 1095.00 ± 559.00 | 1.19 ± 0.78 | 123.50 ± 81.30 | 358 ± 23.50 | 0.91 ± 0.67 | 0.92 ± 0.60 | 12.56 ± 1.82 | 6.26 ± 2.60 | 4.12 ± 2.50 | 99.20 ± 5.97 | 527.00 ± 46.60 | 1.74 ± 0.06 bc | 331.50 ± 70.70 | 2.82 ± 0.92 |
| 0.50 () | Sand | 4.80 ± 0.57 | 1525.00 ± 290.00 | 1.34 ± 0.84 | 104.50 ± 16.30 | 282.5 ± 14.80 | 0.61 ± 0.08 | 0.73 ± 0.04 | 12.61 ± 1.69 | 5.52 ± 0.01 | 3.16 ± 1.99 | 56.1 ± 2.59 | 320.00 ± 22.40 | 1.57 ± 0.01 c | 240.00 ± 68.30 | 4.10 ± 1.82 |
| 0.75 () | Sand | 4.85 ± 0.59 | 1435.00 ± 163.00 | 1.17 ± 0.74 | 114.00 ± 49.50 | 326.0 ± 33.90 | 0.56 ± 0.02 | 0.84 ± 0.09 | 15.10 ± 0.43 | 5.40 ± 0.17 | 4.29 ± 1.87 | 91.60 ± 5.23 | 564.00 ± 42.50 | 2.15 ± 0.13 a | 355.00 ± 79.90 | 3.86 ± 2.39 |
| 1.00 () | Sand | 4.85 ± 0.50 | 975.00 ± 469.00 | 1.14 ± 0.42 | 117.50 ± 2.12 | 421.0 ± 77.80 | 0.86 ± 0.40 | 1.08 ± 0.19 | 11.56 ± 0.16 | 5.20 ± 0.22 | 3.22 ± 1.15 | 85.80 ± 4.86 | 483.00 ± 33.40 | 1.61 ± 0.23 c | 256.22 ± 12.81 | 2.25 ± 0.14 |
| Compost tea only | Sand | 4.95 ± 0.64 | 435.00 ± 247.00 | 1.05 ± 0.70 | 158.50 ± 51.60 | 558 ± 165.00 | 1.20 ± 0.42 | 1.43 ± 0.42 | 14.26 ± 1.70 | 6.34 ± 1.29 | 3.54 ± 1.44 | 83.50 ± 5.98 | 487.00 ± 35.20 | 2.16 ± 0.06 a | 314.70 ± 55.00 | 2.10 ± 0.01 |
| Soil + water | Sand | 4.80 ± 0.28 | 1920.00 ± 157.00 | 0.72 ± 0.11 | 105.5 ± 44.50 | 275.5 ± 23.30 | 0.57 ± 0.04 | 0.71 ± 0.06 | 12.06 ± 1.77 | 5.32 ± 0.04 | 4.35 ± 0.92 | 107.60 ± 9.73 | 547.00 ± 59.70 | 2.00 ± 0.15 ab | 294.10 ± 47.00 | 2.37 ± 0.17 |
| Analysis of Variance (ANOVA) at 95% confidence limit | ||||||||||||||||
| p-values | 1.000 ns | 0.579 ns | 0.947 ns | 0.871 ns | 0.317 ns | 0.51 ns | 0.316 ns | 0.248 ns | 0.865 ns | 0.954 ns | 0.966 ns | 0.991 ns | 0.010 * | 0.450 ns | 0.540 ns | |
| F-values | 0.02 | 0.82 | 0.21 | 0.34 | 1.50 | 0.95 | 1.50 | 1.80 | 0.35 | 0.19 | 0.17 | 0.09 | 8.71 | 1.09 | 0.90 | |
| Pooled SD | 0.53 | 314.17 | 0.65 | 48.27 | 56.38 | 0.36 | 0.31 | 1.44 | 1.19 | 1.73 | 61.07 | 41.62 | 0.13 | 59.80 | 1.29 | |
| Leaf Length (cm) | Leaf Width (cm) | Leaf Number | Plant Height (cm) | Rhizome Diameter (cm) | Fresh Rhizomes (g) | Dry Rhizomes (g) | Chlorophyl Concentration (mg/m2) | |
|---|---|---|---|---|---|---|---|---|
| Compost tea only | 13.00 ± 4.47 bc | 2.95 ± 0.67 b | 4.90 ± 1.73 | 44.25 ± 17.30 a | 3.22 ± 0.70 a | 20.38 ± 14.13 | 2.47 ± 1.78 | 277.70 ± 58.70 |
| Growth media + water | 21.05 ± 3.15 a | 3.69 ± 0.62 a | 5.20 ± 1.32 | 40.85 ± 10.28 ab | 3.22 ± 0.77 a | 16.57 ± 8.33 | 2.19 ± 1.22 | 268.00 ± 46.00 |
| 0.25 | 11.43 ± 3.53 bc | 2.73 ± 0.63 b | 4.20 ± 1.14 | 25.95 ± 8.28 c | 2.26 ± 0.73 b | 9.93 ± 4.21 | 2.00 ± 1.87 | 268.20 ± 58.70 |
| 0.50 | 10.81 ± 3.87 c | 2.54 ± 0.50 b | 4.50 ± 1.90 | 27.85 ± 15.33 c | 2.28 ± 0.88 b | 12.31 ± 17.67 | 1.44 ± 2.23 | 238.90 ± 33.50 |
| 0.75 | 12.21 ± 3.77 bc | 2.93 ± 0.58 b | 4.60 ± 1.65 | 29.10 ± 12.89 c | 2.26 ± 0.71 b | 12.86 ± 11.86 | 2.46 ± 3.20 | 236.60 ± 70.10 |
| 1.00 | 14.35 ± 2.87 b | 2.76 ± 0.45 b | 5.90 ± 2.81 | 30.55 ± 11.57 bc | 2.28 ± 0.67 b | 11.03 ± 8.03 | 1.36 ± 1.09 | 243.30 ± 60.90 |
| Analysis of Variance (ANOVA) at 95% confidence limit | ||||||||
| p-values | 0.000 * | 0.001 * | 0.380 ns | 0.010 * | 0.002 * | 0.348 ns | 0.714 ns | 0.39 ns |
| F-values | 10.62 | 4.74 | 1.09 | 3.40 | 4.32 | 1.14 | 0.58 | 1.07 |
| Pooled StDev | 3.65 | 0.58 | 1.84 | 12.96 | 0.75 | 11.58 | 2.03 | 54.67 |
| Flavanols (mg CE/g) | Flavonols (mg QE/g) | Polyphenols (mg GAE/g) | ABTS (umol TE/g) | FRAP (umol AA/g) | ORAC (umol TE/g) | |
|---|---|---|---|---|---|---|
| Compost tea only | 1.39 ± 0.63 bc | 4.83 ± 1.36 a | 7.34 ± 0.86 a | 31.66 ± 5.20 | 29.07 ± 1.85 ab | 208.16 ± 24.22 a |
| Growth media + water | 1.14 ± 0.05 bc | 3.08 ± 0.56 bc | 5.929 ± 0.39 c | 28.69 ± 1.81 | 24.56 ± 0.94 c | 145.93 ± 19.09 b |
| 0.25 | 1.05 ± 0.07 c | 2.68 ± 0.20 c | 7.27 ± 0.63 ab | 36.27 ± 10.43 | 29.48 ± 4.66 a | 212.50 ± 48.00 a |
| 0.50 | 1.87 ± 0.14 a | 3.17 ± 0.36 bc | 5.89 ± 1.04 c | 29.76 ± 4.16 | 27.39 ± 3.41 abc | 189.2 ± 34.10 a |
| 0.75 | 1.37 ± 0.38 bc | 3.58 ± 0.13 b | 6.45 ± 0.80 bc | 29.07 ± 4.59 | 28.89 ± 3.19 ab | 191.72 ± 15.34 a |
| 1.00 | 1.43 ± 0.11 b | 2.98 ± 0.56 bc | 5.70 ± 0.22 c | 32.37 ± 2.32 | 25.77 ± 2.03 bc | 193.20 ± 33.40 a |
| Analysis of Variance (ANOVA) at 95% confidence limit | ||||||
| p-values | 0.002 * | 0.000 * | 0.001 * | 0.196 ns | 0.036 * | 0.013 * |
| F-values | 5.12 | 8.02 | 6.13 | 1.58 | 2.77 | 3.48 |
| Pooled StDev | 0.31 | 0.66 | 0.714329 | 5.52 | 2.94 | 31.0129 |
| N% | P% | K % | Ca% | Mg% | Na (mg/kg) | Mn (mg/kg) | Fe (mg/kg) | Cu (mg/kg) | Zn (mg/kg) | B (mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compost tea only | 1.030 ± 0.34 bc | 0.29 ± 0.07 ab | 3.13 ± 0.55 bc | 0.49 ± 0.07 a | 0.34 ± 0.013 a | 1346.00 ± 98.50 a | 963.00 ± 28.10 cd | 311.00 ± 39.50 b | 3.17 ± 1.17 b | 41.33 ± 5.57 a | 9.50 ± 1.23 a |
| Growth media + water | 0.87 ± 0.45 c | 0.22 ± 0.09 b | 2.22 ± 0.99 c | 0.47 ± 0.16 a | 0.34 ± 0.07 a | 1331.00 ± 74.70 a | 617.00 ± 27.20 d | 1469.00 ± 13.93 a | 5.33 ± 3.01 a | 77.2 ± 7.58 a | 8.17 ± 2.14 a |
| 0.25 | 1.45 ± 0.20 a | 0.31 ± 0.01 a | 4.35 ± 0.74 a | 0.52 ± 0.17 a | 0.29 ± 0.01 b | 395.80 ± 82.60 b | 1932.00 ± 27.30 a | 83.33 ± 4.97 b | 2.00 ± 0.00 b | 41.33 ± 5.57 a | 13.17 ± 4.54 a |
| 0.50 | 1.11 ± 0.34 abc | 0.27 ± 0.06 ab | 3.72 ± 1.13 a | 0.50 ± 0.05 a | 0.32 ± 0.05 ab | 783.00 ± 76.20 ab | 1857.00 ± 48.90 a | 258.00 ± 41.70 b | 2.50 ± 0.84 b | 77.20 ± 7.58 a | 9.67 ± 1.21 a |
| 0.75 | 1.22 ± 0.27 abc | 0.29 ± 0.05 a | 3.65 ± 0.64 ab | 0.47 ± 0.21 a | 0.30 ± 0.04 ab | 898.00 ± 75.90 ab | 1534.00 ± 54.50 ab | 194.00 ± 26.80 b | 2.17 ± 0.41 b | 72.20 ± 7.73 a | 10.33 ± 1.97 a |
| 1.00 | 1.29 ± 0.073 ab | 0.31 ± 0.03 a | 4.59 ± 0.74 ab | 0.42 ± 0.16 a | 0.31 ± 0.03 ab | 589.20 ± 61.70 ab | 1358.00 ± 50.20 bc | 105.50 ± 12.83 b | 2.67 ± 0.52 b | 50.83 ± 3.92 a | 10.83 ± 0.75 a |
| Analysis of Variance (ANOVA) at 95% confidence limit | |||||||||||
| p-values | 0.038 * | 0.122 ns | 0.000 * | 0.884 ns | 0.210 ns | 0.107 ns | 0.000 * | 0.005 * | 0.003 * | 0.737 ns | 0.022 * |
| F-values | 2.74 | 1.91 | 6.55 | 0.34 | 1.53 | 2.00 | 9.30 | 4.31 | 4.68 | 0.55 | 3.12 |
| Pooled StDev | 0.30 | 0.06 | 0.82 | 0.15 | 0.04 | 78.27 | 39.37 | 23.29 | 1.39 | 2.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasson, T.I.; Jimoh, M.O.; Daniels, C.W.; Nchu, F.; Laubscher, C.P. Enhancement of Antioxidant Potential, Phytochemicals, Nutritional Properties, and Growth of Siphonochilus aethiopicus (Schweinf.) B.L.Burtt with Different Dosages of Compost Tea. Horticulturae 2023, 9, 274. https://doi.org/10.3390/horticulturae9020274
Jasson TI, Jimoh MO, Daniels CW, Nchu F, Laubscher CP. Enhancement of Antioxidant Potential, Phytochemicals, Nutritional Properties, and Growth of Siphonochilus aethiopicus (Schweinf.) B.L.Burtt with Different Dosages of Compost Tea. Horticulturae. 2023; 9(2):274. https://doi.org/10.3390/horticulturae9020274
Chicago/Turabian StyleJasson, Timothy Ivan, Muhali O. Jimoh, Christiaan W. Daniels, Felix Nchu, and Charles P. Laubscher. 2023. "Enhancement of Antioxidant Potential, Phytochemicals, Nutritional Properties, and Growth of Siphonochilus aethiopicus (Schweinf.) B.L.Burtt with Different Dosages of Compost Tea" Horticulturae 9, no. 2: 274. https://doi.org/10.3390/horticulturae9020274
APA StyleJasson, T. I., Jimoh, M. O., Daniels, C. W., Nchu, F., & Laubscher, C. P. (2023). Enhancement of Antioxidant Potential, Phytochemicals, Nutritional Properties, and Growth of Siphonochilus aethiopicus (Schweinf.) B.L.Burtt with Different Dosages of Compost Tea. Horticulturae, 9(2), 274. https://doi.org/10.3390/horticulturae9020274









