Abstract
Essential oils (EOs) and Plant extracts (PEs) are gaining attention as eco-friendly alternatives to synthetic fungicides for the management of soil-borne fungi related to root rot and the wilt of marigolds. Here, EOs of Cinnamomum zeylanicum (cinnamon), Mentha piperita (peppermint), Syzygium aromaticum (clove), Thymus vulgaris (thyme), PEs of Cymbopogon citratus (lemongrass), Lantana camara (lantana), Ocimum basilicum (basil), and Zingiber officinales (ginger) were first evaluated in vitro for their inhibitory activity against the mycelium growth of the root rot and wilt fungi of marigold: Rhizoctonia solani, Sclerotinia sclerotiorum, Fusarium oxysporum, and F. solani, and in vivo for their activity in reducing disease progression. The results show that all EOs had a strong inhibitory activity on the mycelium growth of pathogens. Maximum inhibition of mycelium growth was achieved at a concentration of 1 mL/Lof S. aromaticum, C. zeylanicum, and M. piperita. The inhibition values were 100, 80.9, and 72.4% for F. solani, 100, 81.1, and 70% for S. sclerotiorum, 90.2, 79.4, and 69.1% for F. oxysporum, and 85.4, 78.2, and 68.7% for R. solani, respectively. Regarding plant extracts, the highest inhibition of mycelium growth was attained at a concentration of 20% of Z. officinales, C. citratus, and L. camara. The inhibition values were 77.4, 69.1, and 60.6% for F. solani, 76.5, 67.2, and 58% for S. sclerotiorum, 73.5, 68.2, and 56.3% for F. oxysporum, and 72, 64.8, and 55.2% for R. solani, respectively. In pot experiments, the application of EOs and PEs showed high efficiency in suppressing root rot and wilt of marigold at all concentrations used, especially at 3 mL/L for seed soaking (along with 1.5 mL/L for foliar spraying) for S. aromaticum, C. zeylanicum, and M. piperita EOs, and (40% for seed soaking along with 20% for foliar spraying) for Z. officinales, C. citratus, and L. camara PEs. All the treatments applied in the field greatly reduced the diseases in both seasons, especially S. aromaticum EO, C. zeylanicum EO, Z. officinales PE, and carbendazim treatments. This was accompanied by a significant improvement in morphological, yield, and phytochemical parameters of marigold as well as a significant increase in the activity of defense-related antioxidant enzymes. Overall, essential oils and plant extracts can be used effectively to control root rot and wilt in marigold as sustainable and eco-friendly botanical biofungicides.
1. Introduction
Marigold (Calendula officinalis L.) is an annual herb belonging to the Asteraceae family, native to the Mediterranean, and widespread in central Europe and Asia [1]. It is also known by other names such as pot marigold, garden marigold, and English marigold [2]. The plant is mainly cultivated for its yellow and orange flowers for the purpose of decoration or the food and cosmetic industry [3]. Medicinally, it is used for wound healing, jaundice, blood purification, antispasmodic, and anti-inflammatory [4]. The plant contains volatile oils, flavonoids, carotenoids, terpenoids, coumarins, quinones, and carbohydrates to which all their biological properties are attributed [5]. Marigold is susceptible to many fungal diseases such as bloom blight, stem blight, gray mold, powdery mildew, and leaf spot [6,7,8,9]. However, root rot and wilt caused by soil-borne fungi are the most devastating to the crop, especially in the last decade [10].
Synthetic fungicides have long been used to effectively control fungal diseases of marigold [11]. However, their extensive use and residual effects in soil, plant, and water have led to many serious side effects, including environmental disorders, human health risks, damage to aquatic ecosystems, reduction of beneficial soil microorganisms, development of fungicide-resistant fungi, and depletion of ozone layer [12]. Because of these problems, there is an urgent need to develop new safe and effective alternatives for the management of fungal diseases [13]. Among these alternatives are plant extracts, essential oils, and isolated bioactive compounds [14]. Medicinal and aromatic plants are renewable natural sources full of bioactive substances belonging to different chemical classes [15]. Previous studies of antimicrobial properties show the potential of plant extracts and essential oils of these plants as antimicrobial, antibacterial, antifungal, and antiviral [16].
Plant extracts have drawn attention in the fungicides industry as potential agents for controlling plant pathogens in the field and post-harvest. This is primarily based on their antimicrobial properties due to the broad spectrum of their substance secondary metabolites such as phenols, polyphenols, alkaloids, flavonoids, glycosides, tannins, and other compounds [17]. Numerous studies have demonstrated the potential of plant extracts against a wide range of pathogenic fungi, including Aspergillus spp., Fusarium spp., Penicillium spp., Rhizoctomia saloni, Macrophomina phaseolina, Sclerotinia minör, S. sclerotiorum, Alternaria alternaria, Verticillium dahlia, and Mucor racemosus. Among these plant extracts, lemongrass (Cymbopogon citratus) [18,19,20], lantana (Lantana camara) [21,22], basil (Ocimum basilicum) [23,24,25], and ginger (Zingiber officinales) [23,25,26]. Essential oils contain mixtures of diverse compounds, e.g., monoterpenes, diterpenes, sesquiterpenes, aliphatic, and other aromatic compounds that are volatile in nature [27]. It is usually obtained from medicinal and aromatic plants, herbs, and spices [28]. Several studies have demonstrated the antimicrobial, antiparasitic, antioxidant, and insecticidal activities of essential oils [29]. Moreover, essential oils have been investigated as controlling agents against molds growth and production of aflatoxins [24]. These metabolites can lead to new classes of plant fungicides that can be used to control crop diseases including those caused by soil-borne fungi. Many studies have shown the antifungal activity of essential oils such as cinnamon (Cinnamomum zeylanicum) [30,31,32], peppermint (Mentha piperita) [19,23,33], clove (Syzygium aromaticum) [25,34,35], and thyme (Thymus vulgaris) [24,35] through their inhibitory effects on mycelium growth of phytopathogenic fungi belonging to Fusarium spp., Aspergillus spp., Penicillium spp., Colletotrichum spp., Botrytis cinerea, Phytophthora palmivora, Sclerotinia sclerotiorum, Macrophomina phaseolina, Alternaria citrii, and Rhizoctonia solani.
Plant extracts and essential oils were found to inhibit pathogenic fungi through three modes of action: increasing fungal mortality (fungicidal effect), inhibiting fungal growth and development (fungistatic effect), and/or improving plant growth by inducing defense responses of infected plants [36]. According to Lagrouh et al. [37], plant extracts and essential oils can affect pathogenic fungi through six mechanisms of action: inhibition of electron transport in mitochondria, inhibition of cell division, interference with nucleic acids synthesis and/or inhibition of protein synthesis, and inhibition of efflux pumps. The host plant activates defense processes involving the production of enzymatic and non-enzymatic antioxidants, including the production of soluble sugars, phenols, flavonoids, and hormones [38]. Phenols and flavonoids protect diverse components of the cell from damage and play an important role in plant growth and development by altering cellular processes [39]. Another mechanism to prevent pathogen infection is the production of enzymatic antioxidants and scavenging of reactive oxygen species (ROS) [40]. The accumulation of cell-damaging ROS is abrogated by activation of enzymatic antioxidants as well as non-enzymatic antioxidants [41].
Therefore, the present research aims to investigate the potential antifungal activity of essential oils of cinnamon, peppermint, clove, and thyme and plant extracts of lemongrass, lantana, basil, and ginger against soil-borne fungi related to root rot and wilt of marigold in vitro and in vivo, as well as recording possible alterations in morphological and phytochemical parameters and the activity of defense-related enzymes.
2. Results
2.1. Isolation and Frequency of Fungi Associated with Root Rot and Wilt of Marigold
Data given in Table 1 show that seven soil-borne fungi were isolated from rotted and wilted marigold. Purified isolates were identified as Fusarium nygamai Burgess and Trimboli, F. oxysporum (Schlecht.) Snyder and Hansen, F. solani (Mart.) Sacc., F. verticillioides (Sacc.) Nirenberg, Pythium splendens Hans Braun, Rhizoctonia solani Kühn, and Sclerotinia sclerotiorum (Lib.) de Bary. Fungi were shown at different frequencies: F. oxysporum (22.9%), R. solani (20.2%), F. solani (18.3%), S. sclerotiorum (15.6%), P. splendens (10.1%), and F. nygamai (4.6%). The results also confirm that the plants artificially infected with these fungi exhibited the same symptoms as the original ones, and the fungi isolated from them have the same characteristics as the original isolates.

Table 1.
Occurrence and frequency of seven soil-borne fungi isolated from rotted and wilted marigold samples, collected from the three districts at Beni-Suef Governorate, Egypt during the period from November 2017 to April 2018.
2.2. Pathogenicity Test of Root Rot and Wilt Fungi towards Potted Marigold Seedlings
Data provided in Table 2 reveal that all fungi were pathogenic to marigold seedlings causing damping off, root rot, and wilt with varying capabilities. The highest incidence of damping-off at pre- and post-emergence was recorded with S. sclerotiorum and R. solani, respectively. Additionally, the highest incidence of root rot/wilt was recorded with R. solani (33.3%), followed equally by S. sclerotiorum with F. oxysporum (26.7%). The fungi R. solani, S. sclerotiorum, F. oxysporum, and F. solani were the most destructive, recording 26.7, 33.3, 40, and 46.7% surviving plants, respectively. Therefore, these fungi were used in the following in vitro and/or in vivo trials.

Table 2.
Pathogenicity test of five soil-borne fungi for damping off, root rot, and wilt in marigold seedlings (cv. Orange Flower) growing in greenhouse conditions.
2.3. Antifungal Activity of Essential Oils against the Growth of Pathogenic Fungi in an In Vitro Assay
All essential oils had a strong inhibitory activity on the mycelium growth of pathogenic fungi (Table 3). Maximum inhibition of mycelium growth was achieved at a concentration of 1 mL/L of S. aromaticum, C. zeylanicum, and M. piperita, with significant differences. The inhibition values were 100, 80.9, and 72.4% for F. solani, 100, 81.1, and 70% for S. sclerotiorum, 90.2, 79.4, and 69.1% for F. oxysporum, and 85.4, 78.2, and 68.7% for R. solani, respectively, while minimum inhibition values of 30.6, 31.1, 32.4, and 34.6% were recorded at a concentration of 0.25 mL/L of T. vulgaris against F. oxysporum, R. solani, F. solani, and S. sclerotiorum, respectively.

Table 3.
In vitro, the inhibitory activity of essential oils at concentrations of 0.25, 0.5, and 1.0 mL/L against mycelium growth of R. solani, S. sclerotiorum, F. solani, and F. oxysporum.
2.4. Antifungal Activity of Plant Extracts against the Growth of Pathogenic Fungi in an In Vitro Assay
Data presented in Table 4 reveal that all plant extracts (PEs) showed antifungal activity against the mycelium growth of pathogenic fungi except for a 5% concentration of O. basilicum on S. sclerotiorum and F. oxysporum. The highest inhibition of mycelium growth was attained at a concentration of 20% of Z. officinales, followed by C. citratus and L. camara, with significant differences. The inhibition values were 77.4, 69.1, and 60.6% for F. solani, 76.5, 67.2, and 58% for S. sclerotiorum, 73.5, 68.2, and 56.3% for F. oxysporum, and 72, 64.8, and 55.2% for R. solani, respectively.

Table 4.
In vitro, the inhibitory activity of plant extracts at concentrations of 5, 10, and 20% against mycelium growth of R. solani, S. sclerotiorum, F. solani, and F. oxysporum.
2.5. Efficiency of Essential Oils, Plant Extracts, and Fungicides in Reducing Root Rot and Wilt Diseases of Potted Marigold in Artificially Infested Soil
The application of essential oils showed high efficiency in suppressing root rot and wilt in potted marigold (Table 5). Diseases were significantly reduced, especially at a concentration of 3 mL/L for seed soaking along with 1.5 mL/L for foliar spraying. In this regard, S. aromaticum EO was the best, followed by C. zeylanicum and M. piperita, with significant differences. They reduced damping-off caused by F. solani to 84.3, 79.5, and 65%, respectively, S. sclerotiorum to 79.6, 74.8, and 60.4%, respectively, F. oxysporum to 79.5, 69.9, and 55.4%, respectively, and R. solani to 75, 65.4, and 51.1%, respectively. Additionally, they reduced root rot/wilt caused by F. solani to 88.5, 82.9, and 66.2%, respectively, F. oxysporum to 83.5, 73, and 57.2%, respectively, S. sclerotiorum to 83.2, 67.8, and 61.6%, respectively, and R. solani to 78.2, 67.7, and 51.95%, respectively. Thymus vulgaris was the least disease-reducing essential oil. Data given in Table 5 also show that treatments of Z. officinales, C. citratus, and L. camara plant extracts at a concentration of 40% for seed soaking along with 20% for foliar spraying, led to a significant reduction in diseases. The reduction values of damping-off were 70.5, 60, and 51.3% for F. solani, 65.8, 55.4, and 46.8% for S. sclerotiorum, 61.2, 55.7, and 42.4% for R. solani, and 60.9, 55.2, and 46.5% for F. oxysporum, respectively. The reduction values of root rot/wilt were 73.9, 61.2, and 51.1% for F. solani, 68.8, 56.6, and 46.8% for S. sclerotiorum, 64, 57.4, and 47.9% for F. oxysporum, and 64, 57.4, and 42.6% for R. solani, respectively. The lowest values were recorded with O. basilicum PE. As shown in Table 5, both fungicides showed superior antifungal activity against all diseases without significant differences. Carbendazim outperformed topsin M, recording the highest reduction of damping-off, root rot, and wilt.

Table 5.
Effect of essential oils, methanol plant extracts, and fungicides applied as seed soaking and foliar spraying in reducing root rot and wilt of marigold grown in soil infested with R. solani, S. sclerotiorum, F. solani, and F. oxysporum under greenhouse conditions.
2.6. Management of Marigold Root Rot and Wilt Diseases under Open Field Conditions
All treatments significantly reduced the diseases in both seasons (Table 6). The lowest damping-off at pre-emergence was achieved with S. aromaticum EO, C. zeylanicum EO, and Z. officinales PE, with significant differences. The incidence values were 14.9, 18.6, and 22.1%, respectively, while the highest value was recorded with L. camara PE (35.1%) compared to 60.2% in control. The lowest post-emergence damping-off was achieved with S. aromaticum EO, Z. officinales PE, and C. zeylanicum EO, with significant differences, as they recorded 15.8, 20.4, and 21.4%, respectively, while the highest value was noted with L. camara PE (30.2%) compared to 55.1% in control. Similarly, the lowest incidence of root rot and wilt was recorded with S. aromaticum EO (8.3 and 16.4%, respectively), followed by C. zeylanicum EO (11.6 and 19.7%, respectively), and Z. officinales PE (12.9 and 22.9%, respectively), with significant differences. The highest values were recorded with L. camara PE (20.3 and 34.1%, respectively) compared to (43.1 and 62.3%, respectively) in control. Carbendazim outperformed all treatments recording the lowest rate of damping-off, root rot, and wilt.

Table 6.
Effect of treatments applied as seed soaking and foliar spraying in reducing root rot and wilt of marigold during the 2019/2020 and 2020/2021 seasons under field conditions.
2.7. Morphological and Yield Characteristics
As shown in Figure 1, marigold treated with carbendazim, S. aromaticum EO, C. zeylanicum EO, and Z. officinales PE, recorded the best plant height, 92.2, 87.2, 84.4, and 78 cm, respectively, with significant differences, while, the lowest value was recorded with L. camara PE (59.3) compared to 47.3 in control plants (Figure 1A). Regarding the number of branches/plant, the highest values were observed with S. aromaticum EO (14), carbendazim (13.1), and C. zeylanicum EO (12.1), and showed no significant differences, followed by Z. officinales PE (11.3), C. citratus PE (10.2), and M. piperita EO (9.2). The lowest number of branches/plant were observed with L. camara PE (8.1) compared to 5.3 in control (Figure 1B). The highest number of leaves/plant was achieved with treatments of carbendazim (140.1) and S. aromaticum EO (131.3), and showed no significant differences, followed by C. zeylanicum EO (114.1), Z. officinales PE (103.1), and C. citratus PE (96.3). M. piperita EO had an intermediate value (84.2). The lowest number of leaves/plant was with L. camara PE (77.2) compared to 65.2 in control (Figure 1B). In addition, treatments of carbendazim, S. aromaticum EO, C. zeylanicum EO, and Z. officinales PE increased the number of inflorescences/plant significantly, recording 179.1, 157.1, 139.2, and 122.1, respectively. The lowest inflorescences/plant were noted with L. camara PE (100.2) compared to 83.2 in control (Figure 1B). Moreover, S. aromaticum EO and carbendazim led to the highest diameter of inflorescences (5.7 and 5.5 cm, respectively) without significant differences, followed by Z. officinales PE (5 cm), C. zeylanicum EO (4.9 cm), C. citratus PE (4.3 cm), and M. piperita EO (4 cm), while the lowest value was recorded with L. camara PE (3.8 cm) compared to 3.4 cm in control (Figure 1C). Concerning the fresh and dry weight of inflorescences/plant, the best results were obtained with carbendazim, S. aromaticum EO, C. zeylanicum EO, and Z. officinales PE, with significant differences. These recorded 574.2, 523.6, 476.3, and 417.6 g, respectively, in the fresh weight, and 143.1, 128.5, 117.1, and 104.4 g, respectively, in the dry weight, while, the lowest values were recorded with L. camara PE (315.3 and 74.2 g) in the fresh and dry weight of inflorescences, respectively (Figure 1D).
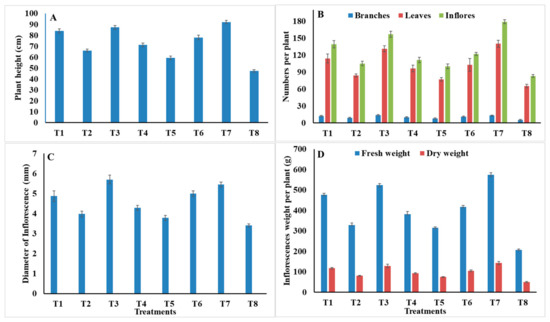
Figure 1.
Effect of treatments on (A) plant height; (B) number of branches/plant, total number of leaves/plant, and total number of inflorescences/plant; (C) diameter of inflorescences; and (D) fresh and dry weight of inflorescences/plant of marigold. Data are mean of two repeated trials during the 2019/2020 and 2020/2021 growing seasons, each with three replicates.
2.8. Measurement of Phytochemical Parameters
As shown in Figure 2, the highest content of total chlorophyll was recorded in plants treated with S. aromaticum EO, carbendazim, Z. officinales PE, and C. zeylanicum EO, without significant differences 11.74, 11.23, 10.64, and 10.38 mg/g FW, respectively. The lowest chlorophyll value was noted with L. camara PE (7.56) compared to 5.28 in control (Figure 2A). The carotenoids in leaves and petals were increased significantly by all treatments. The highest carotenoid content in leaves was recorded with C. zeylanicum EO, S. aromaticum EO, and carbendazim, without significant differences 0.92, 0.89, and 0.84 mg/g FW, respectively, while the lowest carotenoid content in the leaves was recorded with L. camara PE (0.66) compared to 0.47 in control. The highest carotenoid content in petals was recorded with S. aromaticum EO, C. zeylanicum EO, carbendazim, Z. officinales PE, and C. citratus PE, and showed no significant differences. The values were 5.43, 5.24, 5.07, 4.83, and 4.55 mg/g FW, respectively. In converse, the lowest carotenoid content was recorded with L. camara PE (3.89) compared to 2.56 in control (Figure 2B). Moreover, plants treated with C. zeylanicum EO and S. aromaticum EO contained the highest amounts of total phenols, without significant differences, followed by carbendazim, Z. officinales PE, C. citratus PE, and M. piperita EO. The TPC values were 334, 322, 302, 289, 270, and 258 mg/100 g DW, respectively. While the lowest TPC amount was recorded with L. camara PE (231) compared to 117 in control (Figure 2D). The results also show that treatments of C. zeylanicum EO and S. aromaticum EO, significantly increased the total anthocyanin content, with significant differences, followed by Z. officinales PE, carbendazim, M. piperita EO, and C. citratus PE. The total anthocyanin values were 269, 254, 244, 239, 231, and 225 μmol/g DW, respectively, while the lowest value was recorded by L. camara PE (206) compared to 143 in control (Figure 2D). Plants treated with S. aromaticum EO, C. zeylanicum EO, and carbendazim had also a higher content of total flavonoids than those treated with other treatments. The corresponding TFC values were 174, 170, and 164 mg/100 g DW, respectively, while plants treated with L. camara PE contained the lowest content (125) compared to 85 in control (Figure 2D).
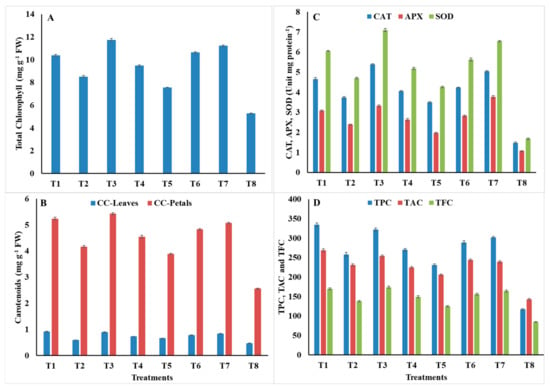
Figure 2.
(A) total chlorophyll; (B) carotenoids content; (C) activity of the enzymes catalase (CAT), ascorbate peroxidase (APX), and superoxide dismutase (SOD) in root rot/wilt-infected marigold; and (D) total phenolic compounds (TPC; mg 100 g−1 DW), total anthocyanin (TAC, μmol g−1 DW), and total flavonoids (TFC; mg 100 g−1 DW) in response to the applied treatments. Data are mean of two repeated trials during the 2019/2020 and 2020/2021 growing seasons, each with three replicates. ± bars indicate the standard deviation of the mean.
2.9. Activity of Defense-Related Antioxidant Enzymes
As shown in Figure 2C, the highest CAT activity was recorded with the treatments of S. aromaticum EO, carbendazim, and C. zeylanicum EO 5.381, 5.042, and 4.659 Unit mg protein−1 min−1, respectively, compared to 1.479 in control, while the intermediate activities of 4.236, 4.058, and 3.740 Unit mg protein−1 min−1 were recorded with Z. officinales PE, C. citratus PE, and M. piperita EO, respectively. Conversely, plants treated with L. camara PE recorded the lowest activity (3.495). Moreover, maximum APX activities of 3.763, 3.324, and 3.084 μmol ascorbate mg protein−1 min−1 were recorded with carbendazim, S. aromaticum EO, and C. zeylanicum EO, respectively, compared to 1.065 in control. Plants treated with L. camara PE recorded the minimum value (1.963). Regarding SOD, the highest activity was recorded with S. aromaticum EO, carbendazim, C. zeylanicum EO, and Z. officinales PE. The corresponding SOD activities were 7.095, 6.547, 6.051, and 5.633 Unit mg protein−1 min−1, respectively, compared to 1.683 in control, while L. camara PE recorded the lowest activity (4.259).
3. Discussion
Synthetic fungicides are the first line of defense against plant pathogens, however their use is greatly restricted internationally due to environmental and health risks and consumer demands for safe, natural alternatives [42]. The European Union has encouraged regulatory changes to ban many of these fungicides [43]. Therefore, scientists urgently need to search for alternative control strategies. In this manuscript, we investigated the antifungal ability of four essential oils and four plant extracts against root rot and wilt of marigolds in vitro and in vivo. Numerous studies have established the potential of medicinal and aromatic plants as biofungicides in the agricultural sector [44]. The idea behind discovering fungicides from plants is based on their ability to synthesize diverse secondary metabolites, which serve to protect plants from microbes, insects, and herbivores [45]. In addition, it is relatively safe, exhibits low toxicity to humans, and is easily biodegradable compared to fungicides [46].
Our results revealed that among seven soil-borne fungi isolated from rotted and wilted marigold, F. oxysporum, R. solani, F. solani, S. sclerotiorum, and P. splendens were the most frequent. This result was supported by [10]. Based on their pathogenicity, the fungi R. solani, S. sclerotiorum, F. oxysporum, and F. solani were the most destructive to marigold, recording 26.7%, 33.3%, 40%, and 46.7% surviving plants, respectively. These results are in line with those obtained by [8,10,47]. Plants have the ability to produce a wide range of molecules, which are known to perform a function in protecting plants against pathogens, due to their biological properties [48]. Among these molecules, more than 3000 essential oils have been identified, and they are an important source of bioactive compounds—antibacterial, insecticidal, fungicidal, nematicidal, and herbicidal [49]. These compounds include terpenes (mono-, sesqui-, and diterpene), alcohols, phenols, epoxides, aldehydes, ketones, esters, amines, ethers, and acids [50].
Our in vitro investigation showed that all EOs had potent mycelium growth inhibitory activity for pathogenic fungi. This finding has been supported by many previous studies; for example, Kishore et al. [51] reported that EOs of clove and cinnamon effectively inhibited the mycelium growth of fourteen pathogenic fungi. According to Barrera-Necha et al. [52], EOs of cinnamon, clove, and thyme completely inhibited the mycelium growth of F. oxysporum f. sp. gladioli. In addition, Sharma et al. [18] reported that clove EO was found to be more effective in inhibiting conidial germination and mycelium growth of F. oxysporum f. sp. lycopersici. In general, the antifungal properties of EOs may be attributed to their ability to disrupt the cell wall and membrane and coagulate the cytoplasm, which leads to damage to cellular organelles and allows the escape of macromolecules [53]. In addition, the lipophilic nature of EOs allows them to pass through the cell wall and damage the cytoplasmic membrane and disrupt layers of polysaccharide, phospholipids, and fatty acids, ultimately making them permeable [54]. The mechanism of action of EOs involves a loss of membrane integrity accompanied by the release of an intracellular substance and inhibition of cellular respiration, with a consequent inability to maintain homeostasis associated with changes in cell morphology [55]. According to Huang et al. [56], EOs inhibit mycelium growth and cause changes in mycelium morphology, including shrinking, deformity, fracture, and dryness. Similarly, Lagrouh et al. [14] reported that EOs can affect fungi through six mechanisms of action; inhibition of cell wall formation, disruption of the cell membrane by inhibition of ergosterol synthesis, inhibition of mitochondrial electron transport, inhibition of cell division, interference with nucleic acids synthesis and/or protein synthesis inhibition, and inhibition of efflux pumps. Our results showed that S. aromaticum EO at a concentration of 1 mL/L presented a greater antifungal ability against fungal mycelium growth, followed by C. zeylanicum and M. piperita, at the same concentration.
Many studies have investigated the antifungal ability of essential oils against a wide range of pathogenic fungi. Among these EOs, clove EO (S. aromaticum) [25,34,35], cinnamon EO (C. zeylanicum) [30,31,32], and peppermint EO (M. piperita) [19,23,33]. The antifungal activity of clove EO is probably due to the presence of a high concentration of eugenol (75–100%) [57]. Eugenol has a phenolic structure known for its high activity against microorganisms, which can denature proteins and interact with phospholipids of the cell membrane resulting in a change in permeability [58]. The antifungal properties of cinnamon EO can be attributed to the presence of different types of EOs such as trans-cinnamaldehyde, eugenol, cinnamyl acetate, L-borneol, caryophyllene oxide, L-bornyl acetate, b-caryophyllene, E-nerolidol, ᾳ-cubebene, terpinolene, ᾳ-terpineol, and ᾳ-thujene. These EOs affect various cellular structures of pathogens [59]. The inhibitory activity of peppermint EO has been attributed to the ability of its bioactive molecules to penetrate the cell wall and cytoplasmic membrane and destroy mitochondrial membranes [60]. In addition, it degrades the outer membrane of pathogens, which facilitates the entry of eugenol into the cytoplasm and its interaction with the protein [61]. Our results demonstrated the efficiency of EOs of S. aromaticum, C. zeylanicum, and M. piperita when used at concentration of 3 mL/L for seed soaking along with 1.5 mL/L for foliar spraying in suppressing root rot and wilt of marigold in vivo. These results are in agreement with those obtained by Abdel-Kader et al. [62] who reported that coating bean seeds with EOs of clove, caraway, thyme, and peppermint significantly decreased root rot at pre- and post-emergence. Similarly, Hamad et al. [63] reported that the use of clove EO at a concentration of 1500 ppm completely prevented the infection of guava by decline fungi. Further, Saltos-Rezabala et al. [64] tested the efficiency of thyme EO in reducing the severity of early blight of tomato and found that the infected leaf area and final disease index were reduced by 47.8% and 39%, respectively. Plant extracts have great potential given their diverse origins and abilities to inhibit the mycelium growth, spore germination, and control over viability of conidia [65]. Our investigation revealed that all methanol plant extracts showed strong activity against the mycelium growth of pathogenic fungi, except for O. basilicum at a 5% concentration. The highest inhibition of mycelium growth was achieved by Z. officinales, C. citratus, and L. camara, at a concentration of 20%. Several studies have demonstrated the antifungal potential of PEs against a large number of pathogenic fungi. Among these Pes were ginger extract (Z. officinales) [23,25,26], lemongrass extract (C. citratus) [18,19,20], and lantana extract (L. camara) [21,22]. The antimicrobial activity of ginger extract has been evaluated by several investigators including, for example, Rawal and Adhikari [66], who revealed that chloroform extract of ginger at a concentration of 750 mg/mL showed the highest zone of inhibition of 25.75 mm against Fusarium oxysporum f. sp. lycopersici. In addition, Gull et al. [67] reported that ginger methanol and ethanol extracts are more effective as antibacterial activity than ginger aqueous extracts. This activity may be due to the presence of different phytochemicals, such as flavonoids, saponins, carbohydrates, alkaloids, and triterpenes [68]. Lemongrass extract activity can be attributed to disrupting the plasma membrane, mitochondrial disorganization, and leakage of Ca2+, K+, and Mg2+ [69]. Loss of ions could further affect signal transduction and fungal germination. The inhibitory activity of lantana extract may be due to the fact that it contains various phytochemicals such as flavonoids, saponins, alkaloids, terpenoids, phlobatanins, tanins, and glycosides [70]. Our results demonstrated the efficacy of methanol plant extracts of Z. officinales, C. citratus, and L. camara when used at a concentration of 40% for seed soaking along with 20% for foliar spraying in reducing root rot and wilt of marigold in vivo.
Many studies have confirmed the efficiency of PEs in reducing the incidence of fungal diseases by performing directly or indirectly by inhibiting the growth and reproduction of fungi [65]. PEs have been shown to inhibit of pathogenic fungi through three modes of action: increasing fungal mortality (fungicidal effect), inhibiting fungal growth and development (fungistatic effect), and/or improving plant growth by inducing the defense responses of infected plants [36]. Moreover, PEs can kill fungi directly, inhibit their growth and development, or reduce the germination of their spores. This may be attributed to the fact that many of their bioactive compounds possess antimicrobial activity [71]. PES may also control fungal diseases because it can act as secondary messengers that enhance plant defense mechanisms [72]. It can also increase the activity of peroxidase, the accumulation of phenolic compounds, and the H2O2 concentration, all of which can reduce the severity of the disease in the affected plants [73]. The current study showed that carbendazim was superior in reducing disease over Topsin M. A similar result was reported by Sharma et al. [74] who found that carbendazim achieved greater control of F. oxysporum f. sp. lycopersici. The fungicidal action inhibits the growth of fungi by one or more of the following mechanisms of action: interfering with the biosynthesis of the cell wall and the formation of ion channels on the cell membrane [75], inhibiting the normal functions of topoisomerase enzymes, increasing the permeability of the cell wall, targeting the plasma membrane [76], interfering with the biosynthesis of sterols, and inhibiting the biosynthesis of ergosterol, leading to irreversible damage to the cell wall [77]. Our results indicated that the treatment with PEs and EOs led to significant improvement in the morphology and yield characteristics of marigold in both seasons. Moreover, these botanicals improved phytochemical parameters. Similar results have been obtained by previous investigations [20,78]. The increase in leaf pigments may be attributed to the stimulation of the increase in hormones, the uptake of minerals necessary for chlorophyll synthesis such as Fe and Me, and the formation of pyridoxal enzymes that play a role in beta-aminolevulinic compound as an essential compound in chlorophyll synthesis [79]. The present results also showed a significant increase in total phenolic compounds and flavonoids. These phytochemicals protect various components of the cell from damage and play a main role in plant growth and development by changing cellular processes [39]. Synthesis of phenols is a known defense response to pathogen attack, and it may occur prior to infection [80]. Phenols act as protection against pathogenic fungi by affecting pathogen physiology, morphology, and ultrastructure or indirectly by enhancing plant systemic resistance [81]. Because of their toxic nature, phenols, like phytoalexins, are activators of pathogen resistance genes and modifiers of pathogen toxicity [82]. Phenol is involved in the lignification of the cell wall, increasing the structural barrier that impedes the spread of the pathogen within the plant tissues. Lignin may also reduce the transfer of nutrients from the host cell to the pathogen [83]. Flavonoids are one of the largest classes of naturally occurring polyphenols, which possess a variety of biological activities including antioxidant, antibacterial, antifungal, and antiviral [84]. According to Mohamed et al. [26], fungal growth inhibition occurs mainly due to the presence of flavonoids. Our investigation showed a significant increase in the activity of the enzymes catalase, ascorbate peroxidase, and superoxide dismutase. These results are in agreement with those reported by [85]. Plants can evolve multiple defense mechanisms to recognize and resist fungal infection by activating complex defense responses [86]. One rapid response is the early generation of reactive oxygen species (ROS) including hydrogen peroxide (H2O2), hydroxyl radical (OH−), and superoxide anion (O2−) [87]. ROS scavenging enzymes, including catalase, ascorbate peroxidase, and superoxide dismutase are found in cell organelles and cytoplasm and play an essential role in regulating ROS levels and the extent of oxidative damage [88]. Catalase is responsible for the decomposition of H2O2 into H2O and O2, and for regulating the level of H2O2 in plant tissues. It also contributes to plant development and plays an important role in plant resistance to pathogens and aging processes [89]. Ascorbate peroxidase is the second most important hydrogen peroxide scavenging enzyme and is essential for protecting cell components from damage by H2O2 and hydroxyl (OH−) radicals [90]. Superoxide dismutase is the first line of defense against pathogen attack and protects plants against oxidative stress and plays a pivotal role in maintaining redox balance and defense response in plants under stress. Its function is to catalyze the decomposition of O2− and OH− to H2O2 and H2O [91].
4. Materials and Methods
4.1. Plant Material, Treatment Details, and Experimental Site Conditions
The current study was conducted in the laboratory, greenhouse, and experimental farm of the Faculty of Agriculture, Beni-Suef University, Egypt, to evaluate the activity of essential oils and plant extracts in vitro against the growth of root rot and wilt fungi, and in vivo against the progression of the diseases, in comparison with fungicides: carbendazim 50% WP and Topsin M 70% WP. The experimental site is located at 29° and 3° N latitude and 31° and 5° E longitude, and 32.4 m above sea level. Considering the climatic properties of the field area over a two-year period, found that the average annual precipitation was 15.85 mm, the temperature was 26.21 °C, the relative humidity was 56.30%, and the evaporation was 145.08 mm, as shown in (Supplementary Table S1).
The physical and chemical properties of the soil used in this study were analyzed according to the methods described by Page et al. [92] and Klute [93] (Supplementary Table S2). Soil samples were randomly collected from the experiment area at a depth of 0–30 cm before the start of the experiment and analyzed in the Central Laboratory for Fertilizers Analysis, ARC, Egypt. The chemical analysis of irrigation water was also carried out as presented in the same Supplementary Table according to the method given by Richards [94]. Marigold seeds (cv. Orange Flower) used in this study were provided by the Institute of Horticultural Research, ARC. Seeds were sown on 7–10 September, both in the greenhouse and in the field. During the trial period, agricultural practices were applied according to the approvals of the Ministry of Agriculture and Land Reclamation, Egypt.
4.2. Extraction of Essential Oils and Plant Extracts
4.2.1. Plant Samples Collection, Drying, and Grinding
Eight fresh and healthy plant species belonging to six plant families were collected from the experimental farm at the Faculty of Agriculture, Fayoum University. Plants have been identified taxonomically by the Botany Department. Plant information is documented in (Table 7). Samples were washed, disinfected in 2% sodium hypochlorite solution for 30 min, rinsed with sterile distilled water, and dried completely in shade at about 25 ± 3 °C for 15 to 23 days. The dried samples were ground into a fine powder using an electric grinder.

Table 7.
Common/scientific name, family/part used, and antifungal activity of medicinal and aromatic plants used as a source of EOs and PEs evaluated for their antifungal properties against marigold root rot and wilt fungi.
4.2.2. Essential Oils Extraction and Used Concentrations
Extraction of essential oils from selected plant species was performed using a modified Clevenger-type apparatus based on the procedure described by Phu et al. [95]. A 300 g of dry material was extracted in 1000 mL distilled water by hydro-distillation for 6 h. The resulting essential oil was dehydrated over anhydrous sodium sulfate to remove water, then placed in dark sealed vials and stored in a refrigerator at 4 °C for further studies. The resulting essential oils were cinnamon, peppermint, clove, and thyme, the relative density of these essential oils were 1.03, 0.898, 1.04, and 0.917 g/mL at 25 °C, respectively. The pure essential oil was mixed with 2 mL of Tween 80 [96], and then known quantities of sterile distilled water were added to it to obtain the final concentrations (0.5, 0.75, 1.0, 1.5, and 3.0 mL/L).
4.2.3. Preparation of Methanol Plant Extracts and Required Concentrations
Dry material was extracted by the method described by Kumar et al. [97]. A 50 mg powder was soaked in methanol 80% (v/v), Ricca Chemical, TX, USA (10 mL methanol/mg plant material) in a 500 mL conical flask three times every 24 h, flasks were shaken for 24 h and then left to stand for 5 h in dark glass bottles for tissue maceration. The extract was filtered through double layers of muslin, centrifuged at 9000 rpm/min for 10 min, and filtered again through Whatman No. 1 filter paper. The final extract was collected in dark glass bottles and exposed to 40 °C in a water bath for 30 min to vaporize the methanol. The extract was sterilized by filtration through a bacterial filter (0.22 μm pore size), then was preserved in sterile bottles at 5 °C until further use. The resulting crude extract was a standard solution which was diluted with sterile distilled water to give concentrations of 5%, 10%, 20%, and 40%.
4.3. In Vitro Assays
4.3.1. Culture Medium, Plant Sampling, and Pathogen Isolation
Marigold showing typical symptoms of root rot and wilt were collected from Beni-Suef, Ihnasia, and Somosta districts at Beni-Suef Governorate, Egypt, then transported to the laboratory to isolate microorganisms in the method devised by Sahi and Khalid [98]. The roots were washed, cut into small pieces 5–10 mm long and 3 mm thick, disinfected in 2% sodium hypochlorite solution, rinsed in sterile distilled water, and dried between sterile filter paper. Root pieces were transferred into Petri-dishes (9 cm in diameter) containing sterile PDA, consisting of 200 g of potato extract, 20 g of dextrose, 20 g of agar, and sterile distilled water to make 1000 mL [99]. To prevent bacterial contamination, PDA was supplemented with chloramphenicol (0.19 g/L) and the pH was adjusted to 6.5. All plates were incubated at 25 ± 2 °C for 3–7 days. Developed colonies were sub-cultured on fresh PDA by hyphal tip and/or single spore isolation technique proposed by Dhingra and Sinclair [100]. Purified isolates were identified according to Gilman [101], Reid et al. [102], and Barnett and Hunter [103]. Re-isolation from artificially diseased plants was performed. Developed colonies were sub-cultured on fresh PDA and identification was confirmed with the original isolates to achieve Koch’s postulations. Isolates were maintained on PDA slants, and refrigerated at 5 °C as stock cultures for further studies. The frequency of fungi was calculated according to the following equation:
4.3.2. Evaluation of the Antifungal Activity of Essential Oils and Plant Extracts against the Growth of Pathogenic Fungi
An in vitro assay was performed to evaluate the activity of essential oils and plant extracts against the mycelium growth of pathogenic fungi by the food poisoning technique described by Adjou et al. [104]. Various amounts of standard plant extracts and essential oils were added individually to conical flasks containing 100 mL of PDA prior to solidification and then gently shaken to give the required concentrations. A bactericide (chloramphenicol, 0.19 g/L) was added to the medium to avoid bacterial contamination. The poisoned medium was poured into sterile Petri dishes at a rate of 25 mL per plate and allowed to solidify. Plates were individually inoculated at the center with equal disks (7 mm) taken from 7-day-old fungal culture. Plates inoculated with fungi without botanicals were retained as control. Each treatment was repeated three times. All plates were incubated at 28 °C until the tested fungi reached full growth in control. Mycelium growth size was measured by averaging the two dimensions of each fungal colony. The mycelium growth inhibition was calculated according to the formula proposed by Pinto et al. [105]:
where dc = Mycelium growth size in control and dt = Mycelium growth size in treatment.
4.4. Greenhouse Evaluations
4.4.1. Soil Infestation and Pathogenicity Test
Pathogenicity was performed for the most frequent fungi, namely F. oxysporum, R. solani, F. solani, S. sclerotiorum, and P. splendens (based on in vitro isolation data) towards marigold. The inoculum was prepared on sterile sand-barley medium in 500 mL glass bottles. Each bottle was inoculated with 5 discs (7 mm in diameter), taken from 4-day-old fungal culture and incubated at 25 ± 1 °C, except for S. sclerotiorum (18 ± 2 °C), for 2 weeks. Sandy loam soil was sterilized in 5% formalin solution for 15 min, then covered with a polyethylene sheet for 7 days to retain gas and left to dry for 2 weeks until all traces of formaldehyde were gone [106]. Soil infestation was carried out by adding the inoculum to the soil at 3% and mixing it well one week before sowing. Infested soil was packed into sterile plastic pots (40 cm in diameter) and watered regularly three times one week before sowing. Marigold seeds were surface-disinfected in 1% sodium hypochlorite solution, washed with sterile water, dried between two sterile layers, and sown at a rate of 10 seeds per pot. Five pots were used as replicates. Pots containing non-infested soil were used as control. The diseases were assessed and survived plants were also calculated.
4.4.2. Pot Experimental Layout and Disease Control Approach
Pot experiments were conducted to evaluate the activity of treatments in reducing root rot and wilt. Inoculums were individually added to the soil at 3% and mixed thoroughly 1 week before sowing. Infested soil was packed into sterile plastic pots. The seeds were individually soaked in plant extracts, essential oils, and fungicides for 12 h, then left to air-dry and sown at a rate of 10 seeds per pot. Foliar spraying with the above botanicals was applied at 2–4 true leaf growth stage. Spraying was carried out three times with 7-day intervals at half the concentrations used for seed soaking. Three pots were used as replicates in a randomized complete block design (RCBD). Untreated pots were kept as control and diseases were assessed.
4.5. Field Trial Design and Management Strategy
A two-year field trial was carried out during the 2019/2020 and 2020/2021 seasons to evaluate the activity of botanicals against root rot and wilt of marigold. Trials were arranged in RCBD with 3 plots (3 × 5 m, with 1 m between plots) for each treatment, with 4 rows and 50 cm row spacing. The seeds were individually soaked in plant extracts (40%), essential oils (3 mL/L), and carbendazim (1 g/L) for 12 h, then left to air-dry, and sown at a rate of 3 seeds per hill and 25 cm apart. At half the concentrations used for seed soaking, foliar spraying with the above treatments was applied three times with 7-day intervals starting at 2–4 true leaf growth stage. Untreated seeds/plants were kept as control and diseases were measured.
4.5.1. In Vivo Measurements
Disease Assessments
Marigold grown under naturally and/or artificially infected soil were carefully examined to record the following pathological measurements:
- A.
- The incidence of damping-off at pre- and post-emergence stages was recorded 15 and 35 days after sowing, respectively, according to the following equations;
- B.
- The disease incidence of root rot/wilt was assessed 90 days after sowing according to the equation proposed by Chavdarov [107]:
- C.
- The disease incidence data was re-used to calculate the percentage disease reduction for each treatment as the following equation:
- D.
- The survived plants at the end of the experiment were calculated as the ratio of infected plants to the total number of plants as in the following equation:
Morphological Parameters
From the beginning of January to the end of April, all fully opened inflorescences (capitula) were plucked weekly by hand. The number, diameter (cm), and fresh and dry weight (g) of inflorescences/plant were recorded. At the end of the growing season, plant height (cm), number of branches, and number of leaves were noted. To measure all stated parameters, 10 plants were randomly selected from each replicate.
4.6. Biochemical Assays
4.6.1. Phytochemical Parameters
Preparation of Plant Material Extract
To measure phenols and flavonoids, the aerial parts (stems and leaves) of marigold were collected from plants grown in field trials in mid-February (at 150 days of plant age). The plant material was dried at 65 °C for 48 h and then stored at ambient temperature (25 °C) in the dark before extraction. The dried material was weighed and extracted in ethanol 70% (1.0 g plant material/10 mL ethanol). The mixture was shaken for 2 h at 120 rpm and 30 °C. The extract was centrifuged at 1013× g for 5 min. The supernatant was filtered and stored at −20 °C in dark glass bottles until further use [108].
Total Phenolic Compounds (TPC)
The TPC was determined by the Folin-Ciocalteu method optimized by Georgé et al. [109]. The absorbance was measured at 760 nm. The results were expressed as mg of gallic acid equivalent (GAE) per 100 g DW.
Total Flavonoid Content (TFC)
Quantification of TFC was based on Gurnani et al. [110]. Quercetin was used as a standard ranging from 50 to 500 mg L−1 (r = 0.9987). The reaction medium contained NaNO2 (5%), AlCl3 (10%), NaOH (4%), and 250 μL of the ethanolic extract. The absorbance was measured at 425 nm. The results were expressed as mg quercetin equivalents (QE) per 100 g DW.
Total Anthocyanin Content (TAC)
A 0.1 g of dried petals was crashed in a Chinese mortar with 10 mL of acidic methanol. The extract was poured into tubes placed in the dark for 24 h at 25 °C. The extract was centrifuged for 10 min at 4000 rpm and the absorbance was read at 550 nm. The extinction coefficient equation of ε = 33,000 mol−1 cm−1 was used to calculate the anthocyanin concentration [111] according the relation of (A = εbc) in μmol/g of petal DW (where A = sample absorption, b = cell width, and c = concentration of the desired solution).
4.6.2. Chlorophyll and Carotenoids Content
Photosynthetic pigments were measured using the method of Lichtenthaler [112]. A 0.2 g of fresh leaves (or ray flowers) was pulverized with a mortar in 10 mL of 80% acetone. The solution was filtered, then the volume was supplemented to 15 mL by adding acetone. A 3 mL of the solution was poured into a cuvette and its absorbance was measured at 663.2, 646.8, and 470 nm. The pigments were calculated using the following equations:
where V = volume of acetone (mL) and W = weight of sample (g).
Chlorophyll − a (mg/mL) = (12.5 A663.2) − (2.79 A653)
Chlorophyll − b (mg/mL) = (21.51 A646.8) − (5.1 A663.2)
Total Chlorophyll (mg/mL) = Chl. a + Chl. b
Carotenoids (mg/mL) = (1000 A470) − (1.8 Chl. a) − (85.02 Chl. b)
Chl./or Carot. (mg/g FW) = (Chl./or Carot. Conc. − V/1000 − W)
4.6.3. Antioxidant Enzyme Activities
Enzyme Extraction
Leaves extending fully from the middle of the main stem were taken, immersed in liquid nitrogen, transported directly to the laboratory, and stored at −80 °C until enzyme extraction. Protein extraction was performed with 500 mg of leaf samples. Treatments were carried out in three replicates. The total protein content of each enzyme extract was determined using the Bradford method and bovine serum albumin V (BSA V) was used as a standard [113].
Catalase (CAT) Activity
Catalase activity was measured using a modified Beer and Sizer method as described by Sohrabi et al. [114]. The enzymatic reaction was initiated by adding the protein extract. The reaction mixture consisted of 100 mmol L−1 phosphate buffer (pH 7.0), 0.1 mmol L−1 EDTA, 20 mmol L−1 H2O2, and 20 μL protein extract. After 1 min of reaction, a spectrophotometer at 240 nm monitored the decrease in H2O2 content. H2O2 was quantified using its molar extinction coefficient (36 mol L−1 cm). Results were expressed in CAT unit mg protein−1 min−1.
Ascorbate Peroxidase (APX) Activity
Ascorbate peroxidase activity was assessed using the method of Caverzan et al. [115]. The reaction mixture consisted of 50 mM potassium phosphate (pH 7.0), 0.5 mM ascorbic acid, 0.1 mM EDTA, 0.15 mM H2O2, and 50 μL protein extract. After 1 min of reaction, a spectrophotometer at 290 nm monitored the reduction of oxidation of ascorbic acid.
Superoxide Dismutase (SOD) Activity
Superoxide dismutase activity was measured using the method described by Caverzan et al. [115]. The reaction mixture consisted of 50 μM p-nitro blue tetrazolium chloride (NBT), 1.3 μM riboflavin, 13 mM methionine, 75 nM EDTA, 50 mM phosphate buffer (pH 7.8), and 50 μL of protein extract. The reaction mixture was irradiated under light at 78 μmol m−2 s−1 for 15 min, and its absorbance was read spectrophotometrically at 560 nm.
4.7. Statistical Data Analysis
Data were subjected to statistical analysis by ANOVA, using Web Agriculture Stat Package software (WASP 2.0, Central Coastal Agricultural Research Institute, Goa, India). The values shown are the means of all measurements. Combined analysis of data obtained from the two study seasons and the Duncan’s range test were used to compare significant differences between treatments at p ≤ 0.05.
5. Conclusions
Our study demonstrated the inhibitory activity of EOs and PEs on the mycelium growth of pathogenic fungi causing root rot and wilt of marigold in vitro. This was evident in lower rates of diseases in vivo when applied as seed soaking along with foliar spraying. This was accompanied by a significant improvement in morphological, yield, and phytochemical parameters of marigold as well as a significant increase in the activity of defense-related enzymes. These botanicals are easily accessible to smallholder farmers, easy to process, and eco-friendly. They can be used as possible parts of integrated control measures against soil-borne pathogens. In this study, botanicals were used separately. Further studies on their combined application are recommended to determine the synergistic relationship between them. It is also suggested that they be screened for their phytochemicals content and to investigate its possible phytotoxicity and its effects on soil and rhizosphere microbiome, especially on beneficial microorganisms. These proposed future approaches would permit the development of sustainable agricultural techniques, thus allowing for the reduction of harmful impacts on the environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9020222/s1, Table S1: Prevailing climatic data at the experimental site during the two growing seasons of marigold (C. officinalis L.) 2019/2020 and 2020/2021; Table S2: Physical and chemical properties of soil and chemical analysis of irrigation water used in the current study (average of two years).
Author Contributions
Conceptualization, H.F.A.A. and M.F.S.; methodology, H.F.A.A., I.A.A.M. and R.S.T.; software, H.F.A.A., M.F.S. and D.O.W.; formal analysis, H.F.A.A. and M.F.S.; investigation, H.F.A.A., M.F.S., I.A.A.M., R.S.T. and D.O.W.; resources, H.F.A.A.; data curation, M.F.S., I.A.A.M., R.S.T., D.O.W. and M.L.B.; writing—original draft preparation, H.F.A.A. and M.F.S.; writing—review and editing, H.F.A.A., M.F.S., I.A.A.M., R.S.T., D.O.W. and M.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project number (RSPD2023R751), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
All data are presented within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gazim, Z.C.; Rezende, C.M.; Fraga, S.R.; Svidzinski, T.I.E.; Cortez, D.A.G. Antifungal activity of the essential oil from Calendula officinalis L. (Asteraceae) growing in Brazil. Braz. J. Microbiol. 2008, 39, 613. [Google Scholar] [CrossRef]
- Golestani, M.; Dolatkhahi, A.; Kazemi, F. Effect of planting dates on flowering period of Calendula officinalis, Bellis perennis and Viola sp. Adv. Crop Sci. 2013, 3, 563–567. [Google Scholar]
- Verma, P.K.; Raina, R.; Agarwal, S.; Kour, H. Phytochemical ingredients and pharmacological potential of Calendula officinalis Linn. Pharm. Biomed. Res. 2018, 4, 1–17. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Nayagam, A.A.J.; Natarajan, R. Wound healing potentials of herbal ointment containing Calendula officinalis Linn. on the alteration of immunological markers and biochemical parameters in excision wounded animals. Clin. Phytosci. 2020, 77, 2–8. [Google Scholar] [CrossRef]
- Escher, G.B.; Borges, L.D.C.; Santos, J.S.; Cruz, T.M.; Marques, M.P.; do Carmo, M.A.V.; Azevedo, L.; Furtado, M.M.; Sant’Ana, A.S.; Wen, M.; et al. From the field to the pot: Phytochemical and functional analyses of Calendula officinalis L. flower for incorporation in an organic yogurt. Antioxidants 2019, 8, 559. [Google Scholar] [CrossRef]
- Sohi, H.S. Personal communication on disease of marigold; IIHR: Bangalore, India, 1983.
- Heibertshausen, D.; KortekampInfection, A. Cycle and biological control of Podosphaera xanthii, the powdery mildew of marigold (Calendula officinalis). Gesunde Pflanz. 2004, 56, 201–207. [Google Scholar] [CrossRef]
- Garibaldi, A.; Minuto, A.; Gullino, M.L. First report of Sclerotinia sclerotiorum on Calendula officinalis in Italy. Dis. Notes 2007, 85, 446. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, Y.; Kumar, P. Diseases of ornamental plants and their management. In Eco-Friendly Innovative Approaches in Plant Disease Management; Singh, V.K., Singh, Y., Singh, A., Eds.; Int. Book Distributors and Publisher: New Delhi, India, 2012; pp. 543–572. [Google Scholar]
- Abdel-Wahed, G.A. Application of some fungicides alternatives for management root rot and wilt fungal diseases of marigold (Calendula officinalis L.). Sci. J. Agric. Sci. 2020, 2, 31–41. [Google Scholar] [CrossRef]
- Kumar, V. Marigold Diseases and Its Control. Agropedia. 2012. Available online: http://agropedia.iitk.ac.in/content/marigold-diseases-its-control (accessed on 15 June 2022).
- Seleiman, M.F.; Santanen, A.; Mäkelä, P. Recycling sludge on cropland as fertilizer-Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Moreno-Ramírez, L.; Valdez-Salas, B.; Seleiman, M.F.; El-Hendawy, S.; Aldhuwaib, K.J.; Alotaibi, M.; González-Mendoza, D. New Bacillus subtilis Strains Isolated from Prosopis glandulosa Rhizosphere for Suppressing Fusarium Spp. and Enhancing Growth of Gossypium hirsutum L. Biology 2023, 12, 73. [Google Scholar] [CrossRef]
- Seepe, H.A.; Nxumalo, W.; Amoo, S.O. Natural products from medicinal plants against phytopathogenic fusarium species: Current research endeavors, challenges and prospects. Molecules 2021, 26, 6539. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Q.; Jacobsen, S.E.; Tang, Y. The impact and prospect of natural product discovery in agriculture. EMBO Rep. 2018, 19, e46824. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Sultana, S.; Akhtar, N.; Asif, H.M. Phytochemical screening and antipyretic effects of hydro-methanol extract of Melia azedarach leaves in rabbits. Bangladesh J. Pharmacol. 2013, 8, 214–217. [Google Scholar] [CrossRef]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef]
- Císarová, M.; Hleba, L.; Medo, J.; Tančinová, D.; Mašková, Z.; Čuboň, J.; Kováčik, A.; Foltinová, D.; Božik, M.; Klouček, P. The in vitro and in situ effect of selected essential oils in vapor phase against bread spoilage toxicogenic aspergilla. Food Control 2020, 110, 107007. [Google Scholar] [CrossRef]
- Moumni, M.; Allagui, M.B.; Mezrioui, K.; Ben Amara, H.; Romanazzi, G. Evaluation of seven essential oils as seed treatments against seed borne fungal pathogens of Cucurbita maxima. Molecules 2021, 26, 2354. [Google Scholar] [CrossRef]
- Passos, J.L.P.; Barbosa, L.C.A.; Demuner, A.J.; Alvarenga, E.S.; da Silva, C.M.; Barreto, R.W. Chemical characterization of volatile compounds of Lantana camara L. and L. radula Sw. and their antifungal activity. Molecules 2012, 17, 11447–11455. [Google Scholar] [CrossRef]
- Seepe, H.A.; Amoo, S.O.; Nxumalo, W.; Adeleke, R.A. Sustainable use of thirteen south African medicinal plants for the management of crop diseases caused by Fusarium species–An in vitro study. S. Afr. J. Bot. 2020, 130, 456–464. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Tarighi, S. Antifungal activity of various essential oils against Rhizoctonia solani and Macrophomina phaseolina as major bean pathogens. J. Appl. Microbiol. 2015, 118, 704–717. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crop Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Ibáñeza, M.D.; López-Gresab, M.P.; Lisónb, P.; Rodrigob, I.; Bellésb, J.M.; González-Masa, M.C.; Blázqueza, M.A. Essential oils as natural antimicrobial and antioxidant products in the agrifood industry. MOL2NET 2019, 5, 2624–5078. [Google Scholar]
- Mohamed, A.B.; El-Sheikh, A.M.M.; El-Sharkawy, R.M.I. Antifungal activity of bioagents and plant extracts against certain fungal diseases of potatoes. J. Phytopathol. Pest Manag. 2021, 8, 29–45. [Google Scholar]
- Nuzhat, T.; Vidyasagar, G.M. Antifungal investigations on plant essential oils. A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 19–28. [Google Scholar]
- United Nation Industrial Development Organisation FAO. Herbs, Spices and Essential Oils Post-Harvest Operations in Developing Countries; United Nation Industrial Development Organisation: Vienna, Austria, 2005; p. 9.
- Yu, J.; Su, J.; Li, F.; Gao, J.; Li, B.; Pang, M.; Lv, G.; Chen, S. Identification and quantification of pine needle essential oil from different habitats and species of China by GC-MS and GC method. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Ojaghian, M.R.; Sun, X.; Zhang, L.; Li, X.; Xie, G.L.; Zhang, J.; Wang, L. Effect of E-Cinnamaldehyde against Sclerotinia sclerotiorum on potato and induction of glutathione s-transferase genes. Physiol. Mol. Plant Pathol. 2015, 91, 66–71. [Google Scholar] [CrossRef]
- Císarová, M.; Tančinová, D.; Medo, J.; Kačániová, M. The in vitro effect of selected essential oils on the growth and mycotoxin production of Aspergillus species. J. Environ. Sci. Health Part B 2016, 51, 668–674. [Google Scholar] [CrossRef]
- Yeole, G.J.; Kotkar, H.M.; Teli, N.P.; Mendki, P.S. Herbal fungicide to control fusarium wilt in tomato plants. Biopestic. Int. 2016, 12, 25–35. [Google Scholar]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha piperita L. (peppermint) essential oils. J. King Saud Univ. Sci. 2019, 31, 528–533. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y. Antifungal activities of cinnamon oil against Rhizopus nigricans, Aspergillus flavus and Penicillium expansum in vitro and in vivo fruit test. Int. J. Food Sci. Technol. 2010, 45, 1837–1842. [Google Scholar] [CrossRef]
- Sempere-Ferre, F.; Asamar, J.; Castell, V.; Roselló, J.; Santamarina, M.P. Evaluating the antifungal potential of botanical compounds to control Botryotinia fuckeliana and Rhizoctonia solani. Molecules 2021, 26, 2472. [Google Scholar] [CrossRef]
- Draz, I.S.; Elkwaga, A.A.; Elzaawely, A.A.; El-Zahaby, H.M.; Ismail, A.W.A. Application of plant extracts as inducers to challenge leaf rust of wheat. Egypt. J. Biol. Pest Cont. 2019, 29, 6. [Google Scholar] [CrossRef]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Méd. 2017, 27, 303–311. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Van De Poel, B.; Höfte, M.; Ende, W.V.D. Sweet immunity: Inulin boosts resistance of lettuce (Lactuca sativa) against grey mold (Botrytis cinerea) in an ethylene-dependent manner. Int. J. Mol. Sci. 2019, 20, 1052. [Google Scholar] [CrossRef]
- De Pinto, M.C.; De Gara, L. Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J. Exp. Bot. 2004, 55, 2559–2569. [Google Scholar] [CrossRef]
- Walter, S.; Nicholson, P.; Doohan, F. Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 2009, 185, 54–66. [Google Scholar] [CrossRef]
- Barna, B.; Fodor, J.; Harrach, B.; Pogany, M.; Király, Z. The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol. Biochem. 2012, 59, 37–43. [Google Scholar] [CrossRef]
- Hewedy, O.A.; Abdel Lateif, K.S.; Seleiman, M.F.; Shami, A.; Albarakaty, F.M.; El-Meihy, M.R. Phylogenetic Diversity of Trichoderma Strains and Their Antagonistic Potential against Soil-Borne Pathogens under Stress Conditions. Biology 2020, 9, 189. [Google Scholar] [CrossRef]
- Hillocks, R. Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop. Prot. 2012, 31, 85–93. [Google Scholar] [CrossRef]
- Ribeiro, A.; Romeiras, M.M.; Tavares, J.; Faria, M.T. Ethnobotanical survey in Canhane village, district of Massingir, Mozambique: Medicinal plants and traditional knowledge. J. Ethnobiol. Ethnomed. 2010, 6, 33. [Google Scholar] [CrossRef]
- Ahmad, I.; Beg, A.Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J. Ethnopharmacol. 2001, 74, 113–123. [Google Scholar] [CrossRef]
- MartÍnez, J.A. Natural fungicides obtained from plants, fungicides for plant and animal diseases. In Fungicides for Plant and Animal Diseases; Dhanasekaran, D., Ed.; In Tech Open: Shanghai, China, 2012. [Google Scholar]
- Shurigin, V.; Alaylar, B.; Davranov, K.; Wirth, S.; Bellingrath-Kimura, S.D.; Egamberdieva, D. Diversity and biological activity of culturable endophytic bacteria associated with marigold (Calendula officinalis L.). AIMS Microbiol. 2021, 7, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.D.; Hogenhout, S.; Foyer, C.H. Mechanisms of plant-insect interaction. J. Exp. Bot. 2015, 66, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essen. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Zorzi-Tamazoni, G.; Schiavo-Griggio, E.; Pessin-Broilo, R.; Teresinha da silva Ribeiro, G.L.; Goncalvez-Soares; Schwambach, J. Screening for inhibitory activity of essential oils on fungal tomato pathogen Stemphylium solani Weber. Biocatal. Agric. Biotechnol. 2018, 16, 364–372. [Google Scholar] [CrossRef]
- Kishore, G.K.; Pande, S.; Harish, S. Evaluation of essential oils and their components for broadspectrum antifungal activity and control of late leaf spot and crown rot diseases in peanut. Plant Dis. 2007, 91, 375–379. [Google Scholar] [CrossRef]
- Barrera-Necha, L.L.; Garduño-Pizaña, C.; Garcīa-Bārrera, L.J. In vitro antifungal activity of essential oils and their compounds on mycelial growth of Fusarium oxysporum f. sp. gladioli (Massey) Snyder and Hansen. Plant Pathol. J. 2009, 8, 17–21. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- De Jesus, C.; Santos Frazo, G.G.; Blank, A.F.; De Aquino Santana, L.C.L. Myrcia ovata cambessedes essential oils: A proposal for a novel natural antimicrobial against foodborne bacteria. Microb. Pathog. 2016, 99, 142–147. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Huang, X.; Liu, T.; Zhou, C.; Huang, Y.; Liu, X.; Yuan, H. Antifungal activity of essential oils from three artemisia species against Colletotrichum gloeosporioides of Mango. Antibiotics 2021, 10, 1331. [Google Scholar] [CrossRef]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and Dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef]
- Bhuiyan, N.; Begum, J.; Nandi, N.; Akter, F. Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum). Afr. J. Plant Sci. 2010, 4, 451–454. [Google Scholar]
- Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of antimicrobial action of cinnamon and oregano oils, cinnamaldehyde, carvacrol, 2,5-dihydroxybenzaldehyde, and 2-hydroxy-5-methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods 2017, 6, 72. [Google Scholar] [CrossRef]
- Arnal-Schnebelen, B.; Hadji-Minaglou, F.; Peroteau, J.F.; Ribeyre, F.; de Billerbeck, V.G. Essential oils in infectious gynaecological disease: A statistical study of 658 cases. Int. J. Aromather. 2004, 14, 192–197. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Abdel-Kader, M.M.; El-Mougy, N.S.; Lashin, S.M. Essential oils and Trichoderma harzianum as an integrated control measure against Faba bean root rot pathogens. J. Plant Prot. Res. 2011, 51, 306–313. [Google Scholar] [CrossRef]
- Hamad, Y.K.; Fahmi, M.M.; Zaitoun, F.M.; Ziyada, S.M. Role of essential oils in controlling fungi that cause decline disease of guava. Int. J. Pure App. Biosci. 2015, 3, 143–151. [Google Scholar] [CrossRef]
- Saltos-Rezabala, L.A.; Silveira, P.R.D.; Tavares, D.G.; Moreira, S.I.; Magalhães, T.A.; Botelho, D.M.D.S.; Alves, E. Thyme essential oil reduces disease severity and induces resistance against Alternaria linariae in tomato plants. Horticulture 2022, 8, 919. [Google Scholar] [CrossRef]
- Aravena, R.; Besoain, X.; Riquelme, N.; Salinas, A.; Valenzuela, M.; Oyanedel, E.; Barros, W.; Olguin, Y.; Madrid, A.; Alvear, M.; et al. Antifungal nanoformulation for biocontrol of tomato root and crown rot caused by Fusarium oxysporum f. sp. radicis-lycopersici. Antibiotics 2021, 10, 1132. [Google Scholar] [CrossRef]
- Rawal, P.; Adhikari, R.S. Evaluation of antifungal activity of Zingiber officinale against Fusarium oxysporum f. sp. lycopersici. Adv. Appl. Sci. Res. 2016, 7, 5–9. [Google Scholar]
- Gull, I.; Saeed, M.; Shaukat, H.; Aslam, S.M.; Samra, Z.O.; Athar, A.M. Inhibitory effect of Allium sativum and Zingiber officinales extracts on clinically important drug resistant pathogenic bacteria. Ann. Clinic. Microbiol. Antimicrob. 2012, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Iotsor, B.I.; Iseghohi, F.; Oladoja, O.E.; Raji, O.R.; Yusuf, Z.; Oyewole, O.A. Antimicrobial activities of garlic and ginger extracts on some clinical isolates. Int. J. Biotechnol. 2019, 8, 59–65. [Google Scholar]
- Helal, G.A.; Sarhan, M.M.; Shahla, A.N.K.A.; Abou El-Khair, E.K. Effects of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2-strain. J. Basic Microbiol. 2007, 47, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Godbole, S. Antifungal and phytochemical analysis of Lantana camara, Citrus limonum (lemon), Azadirachta indica (neem) and Hibiscus rosasinensis (China rose). J. Pharmacy Res. 2015, 9, 476–479. [Google Scholar]
- Rongai, D.; Milano, F.; Scio, E. Inhibitory effect of plant extracts on conidial germination of the phytopathogenic fungus Fusarium oxysporum. Am. J. Plant Sci. 2012, 3, 1693–1698. [Google Scholar] [CrossRef]
- Morsy, K.M.; Abdel-Monaim, M.F.; Mazen, M.M. Use of abiotic and biotic inducers for controlling fungal diseases and improving growth of alfalfa. World J. Agric. Sci. 2011, 7, 566–576. [Google Scholar]
- El-Shahir, A.A.; El-Wakil, D.A.; Latef, A.A.H.A.; Youssef, N.H. Bioactive compounds and antifungal activity of leaves and fruit methanolic extracts of Ziziphus spina-christi L. Plants 2022, 11, 746. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, N.K.; Srivastava, A.; Kataria, A.; Dubey, S.; Sharma, S.; Kundu, B. Clove and lemongrass oil based non-ionic nanoemulsión for suppressing the growth of plant pathogenic Fusarium oxysporum f. sp. lycopersici. Ind. Crops Prod. 2018, 123, 353–362. [Google Scholar] [CrossRef]
- Hu, L.B.; Zhou, W.; Zhang, T.; Yang, Z.M.; Xu, J.H.; Shi, Z.Q. Mechanism of inhibition to Fusarium moniliforme by antimicrobial peptide Fengycins. Microbiol. China 2010, 37, 251–255. [Google Scholar]
- Kawakami, K.; Inuzuka, H.; Hori, N.; Takahashi, N.; Ishida, K.; Mochizuki, K.; Ohkusu, K.; Muraosa, Y.; Watanabe, A.; Kamei, K. Inhibitory effects of antimicrobial agents against Fusarium species. Med. Mycol. 2015, 53, 603–611. [Google Scholar] [CrossRef]
- Nene, Y.L.; Thapliyal, P.N. Fungicides in Plant Disease Control, 3rd ed.; Oxford and IBH Publishing: New Delhi, India, 1993; pp. 311–348. [Google Scholar]
- Ahmed, H.F.A.; Seleiman, M.F.; Al-Saif, A.M.; Alshiekheid, M.A.; Battaglia, M.L.; Taha, R.S. Biological control of celery powdery mildew disease caused by Erysiphe heraclei DC in vitro and in vivo conditions. Plants 2021, 10, 2342. [Google Scholar] [CrossRef]
- Adil, W.; Ahlam, M.; Muneeb, R.; Seema, A.; Hussain, M. Bee Propolis (Bee’s Glue): A phytochemistry review. J. Crit. Rev. 2017, 4, 9–13. [Google Scholar]
- Goodman, R.N.; Király, Z.; Wood, K.R. The Biochemistry and Physiology of Plant Disease; University of Missouri Press: Columbia, MO, USA, 1986. [Google Scholar]
- Mohamed, M.S.M.; Saleh, A.M.; Abdel-Farid, I.B.; El-Naggar, A.A. Growth, hydrolases and ultrastructure of Fusarium oxysporum as affected by phenolic rich extracts from several xerophytic plants. Pestic. Bioch. Physiol. 2016, 141, 57–64. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Ann. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Özçelik, B.; Deliorman Orhan, D.; Özgen, S.; Ergun, F. Antimicrobial activity of flavonoids against extended-spectrum ß-Lactamase (ES_L)-producing Klebsiella pneumonia. Trop. J. Pharm. Res. 2008, 7, 1151–1157. [Google Scholar] [CrossRef]
- Pastuszak, J.; Szczerba, A.; Dziurka, M.; Hornyák, M.; Kope’c, P.; Szklarczyk, M.; Płażek, A. Physiological and biochemical response to Fusarium culmorum infection in three durum wheat genotypes at seedling and full anthesis stage. Int. J. Mol. Sci. 2021, 22, 7433. [Google Scholar] [CrossRef]
- Dangl, J.; Jones, J.D.G. Plant pathogens and integrated defense responses to pathogens. Nature 2001, 411, 826–834. [Google Scholar] [CrossRef]
- Patykowski, J.; Urbanek, H. Activity of enzymes related to H2O2 generation and metabolism in leaf apoplastic fraction of tomato leaves infected with Botrytis cinerea. J. Phytopathol. 2003, 151, 153–161. [Google Scholar] [CrossRef]
- Niu, J.H.; Cao, Y.; Lin, X.G.; Leng, Q.Y.; Chen, Y.M.; Yin, J.M. Field and laboratory screening of Anthurium cultivars for resistance to foliar bacterial blight and the induced activities of defense-related enzymes. Folia Hort. 2018, 30, 129–137. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proc. Natl. Acad. Sci. USA 2002, 99, 4097–4102. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xia, M.X.; Chen, J.; Yuan, R.; Deng, F.N.; Shen, F. Gene expression characteristics and regulation mechanisms of superoxide dismutase and its physiological roles in plants under stress. Biochemistry 2016, 81, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Page, A.I.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis: Part. 2—Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Klute, A. Methods of Soil Analysis: Part. 1—Physical and Mineralogical Methods, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar]
- Richards, L.F. Diagnosis and Improvement of Saline and Alkaline Soils (NO. 60); Soil and Water Conservative Research Branch, Agricultural Research Service, US Department of Agriculture: Washington, DC, USA, 1954.
- Phu, N.D.; Thy, L.H.P.; Lam, T.D.; Yen, V.H.; Lan, N.T.N. Extraction of jasmine essential oil by hydro-distillation method and applications on formulation of natural facial cleansers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 542, 1–6. [Google Scholar]
- Reuveni, M.; Agapov, V.; Reuveni, M. Controlling powdery mildew caused by Sphaerotheca fuliginea in cucumber by foliar sprays of phosphate and potassium salts. Crop Prot. 1996, 15, 49–53. [Google Scholar] [CrossRef]
- Kumar, A.K.; Ramachandra, S.S.; Narsu, L. Pharmacognostic and phytochemical investigations of roots of Hibiscus micranthus Linn. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 324–337. [Google Scholar]
- Sahi, I.Y.; Khalid, A.N. In vitro biological control of Fusarium oxysporum causing wilt in Capsicum annuum. Mycopathol. 2007, 5, 85–88. [Google Scholar]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Methods, 2nd ed.; CRC-Press, Inc.: Boca Raton, FL, USA, 1995. [Google Scholar] [CrossRef]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Methods; CRC Press Inc.: Boca Raton, FL, USA, 1985; p. 355. [Google Scholar]
- Gilman, J.C. A Manual of Soil Fungi, 2nd ed.; Iowa State Univ. Press: Ames, IA, USA, 1957; p. 450. [Google Scholar]
- Reid, D.A.; Hayward, A.C.; Waterston, J.M. CMI descriptions of pathogenic fungi and bacteria. Kew Bull. 1965, 19, 414. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; Macmillan Publishing Co.: New York, NY, USA, 1986. [Google Scholar]
- Adjou, E.S.; Kouton, S.; Dahouenon-Ahoussi, E.; Sohounhloue, C.K.; Soumanou, M.M. Antifungal activity of Ocimum canum essential oil against toxinogenic fungi isolated from peanut seeds in post-harvest in Benin. Int. Res. J. Biol. Sci. 2012, 1, 20–26. [Google Scholar]
- Pinto, C.M.F.; Maffia, L.A.; Casali, V.W.D.; Cardoso, A.A. In vitro effect of plant leaf extracts on mycelial growth and sclerotial germination of Sclerotium cepivorum. J. Phytopathol. 1998, 146, 421–425. [Google Scholar] [CrossRef]
- Whitehead, M.D. Sorghum, a medium suitable for the increase of inoculum for studies of soil-borne and certain other fungi. Phytopathology 1957, 47, 450. [Google Scholar]
- Chavdarov, P. Evaluation of lentil germplasm for disease resistance to fusarium wilt (Fusarium oxysporum f. sp. lentis). Cent. Eur Agric. 2006, 7, 121–126. [Google Scholar]
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Maleki, H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Int. J. Food Prop. 2018, 21, 452–470. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. and Food Chemistry 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Gurnani, N.; Gupta, M.; Mehta, D.; Mehta, B.K. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chili seeds (Capsicum frutescens L.). J. Taibah Univ. Sci. 2016, 10, 462–470. [Google Scholar] [CrossRef]
- Wagner, G.J. Content and vacuole/extra vacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979, 64, 88–93. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–380. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Heidari, G.; Weisany, W.; Golezani, K.G.; Mohammadi, K. Changes of antioxidative enzymes, lipid peroxidation and chlorophyll content in chickpea types colonized by different Glomus species under drought stress. Symbiosis 2012, 56, 5–18. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).