Improvement of the Physico-Chemical Properties, Nutritional, and Antioxidant Compounds of Pomegranate Fruit cv. ‘Wonderful’ Using Integrated Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Irrigation Solution Composition
2.2. Fruits Measurements
2.3. Statistical Analysis
3. Results
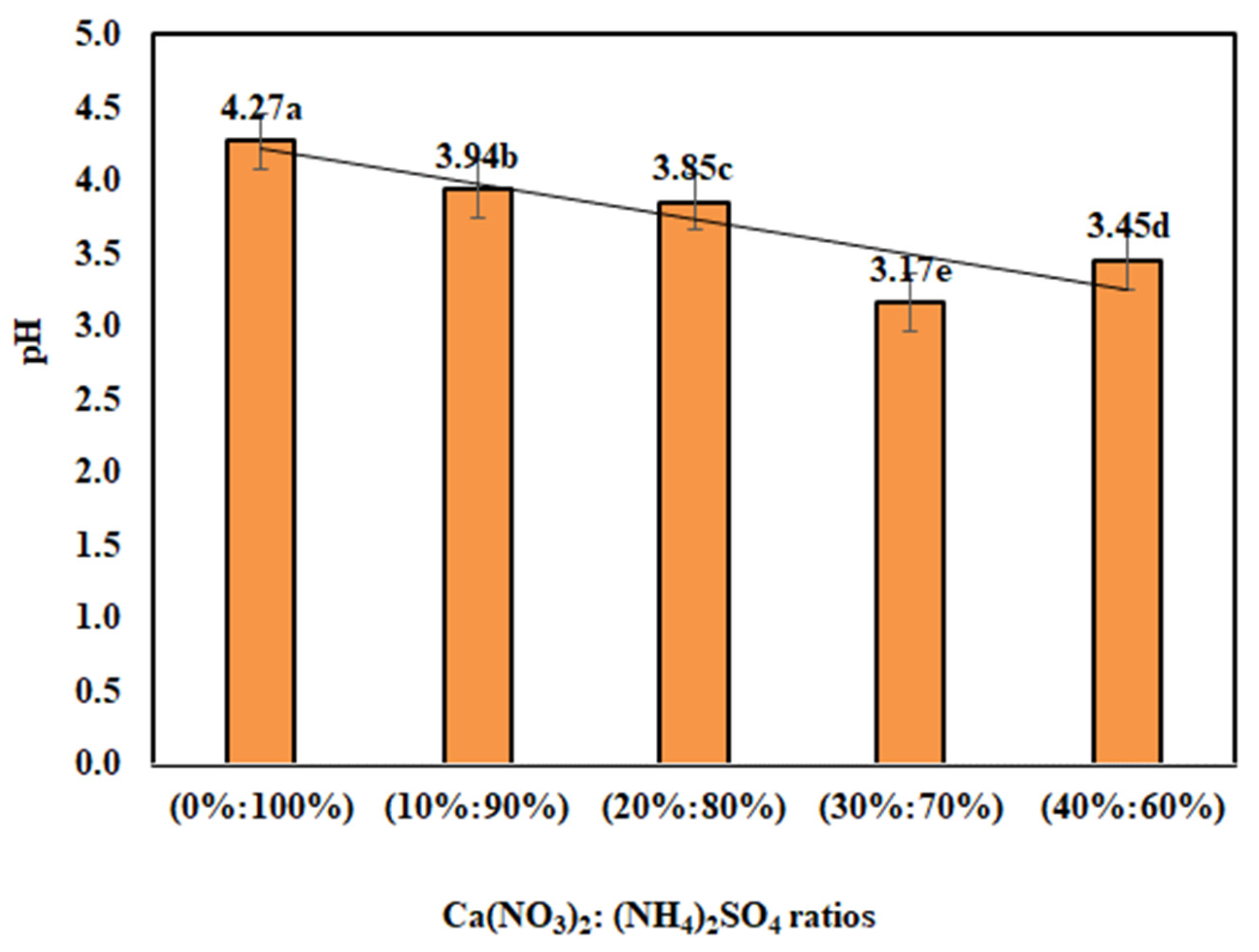
3.1. Impact of Ca(NO3)2: (NH4)2SO4 Proportions on Physico-Chemical Characteristics
3.2. Impact of Ca(NO3)2:(NH4)2SO4 Proportions on Fruit Color Values
3.3. Impact of Ca(NO3)2: (NH4)2SO4 Ratios on Moisture Content, Total, Reducing, and Non-Reducing Sugar Contents
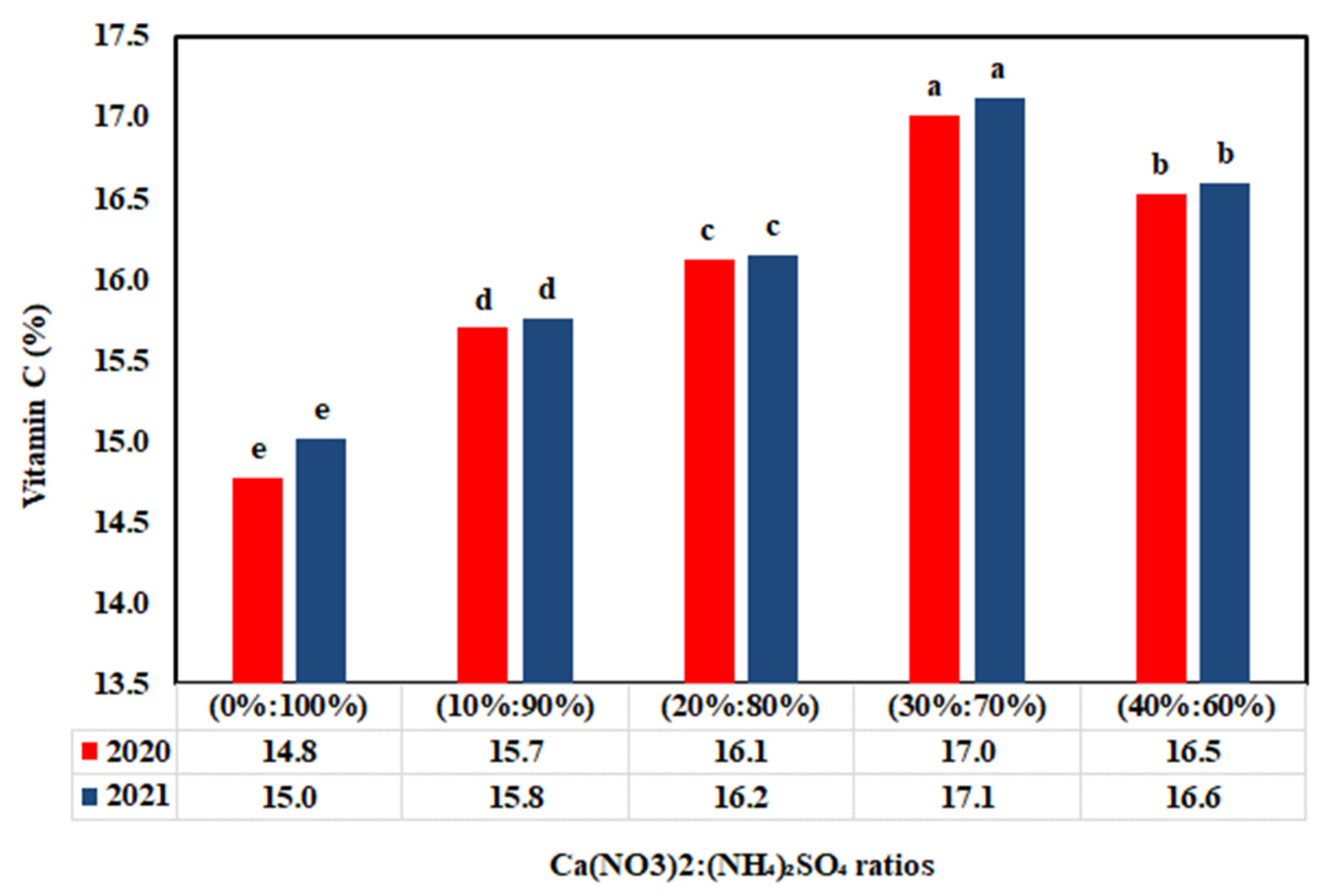
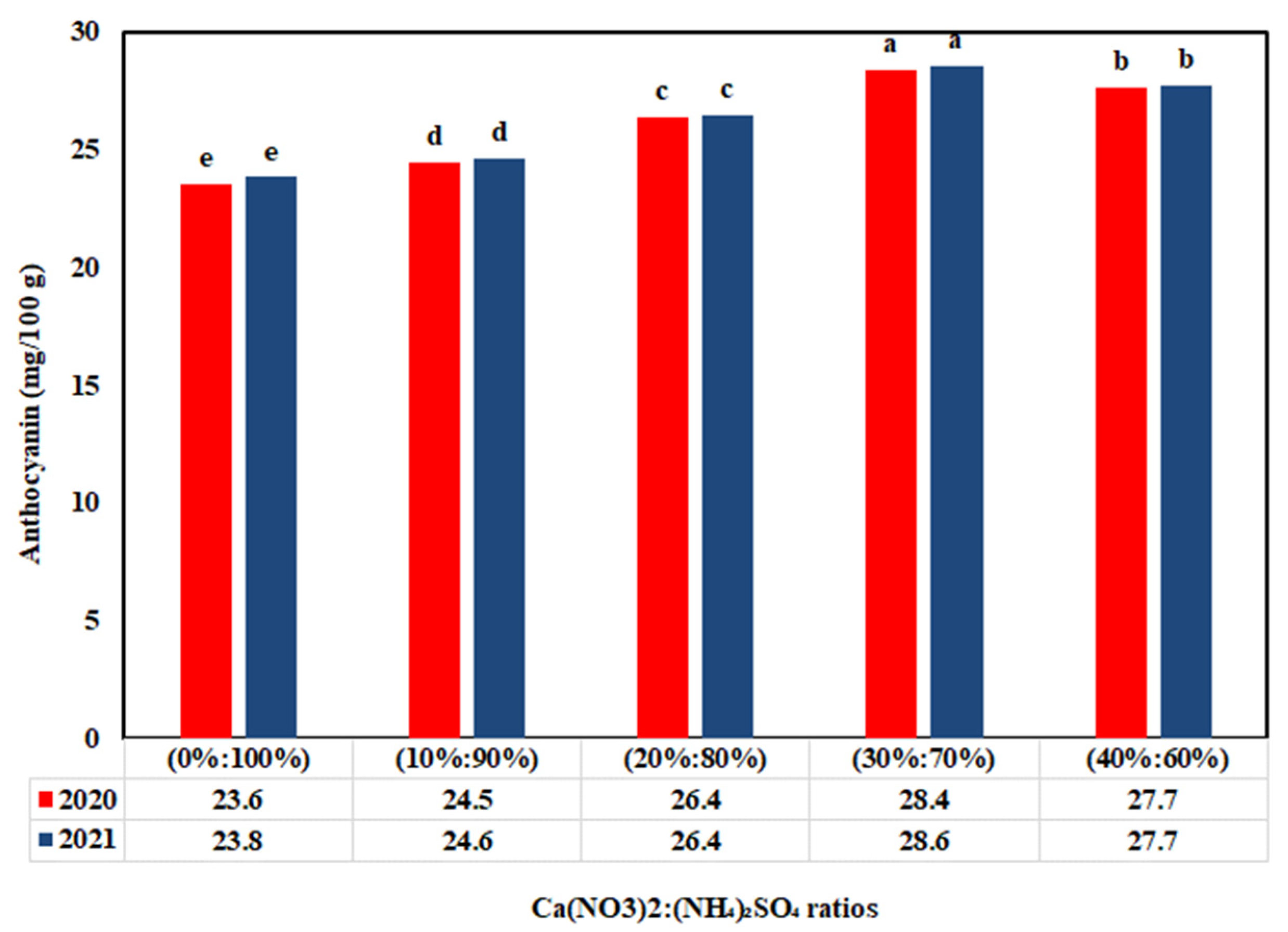
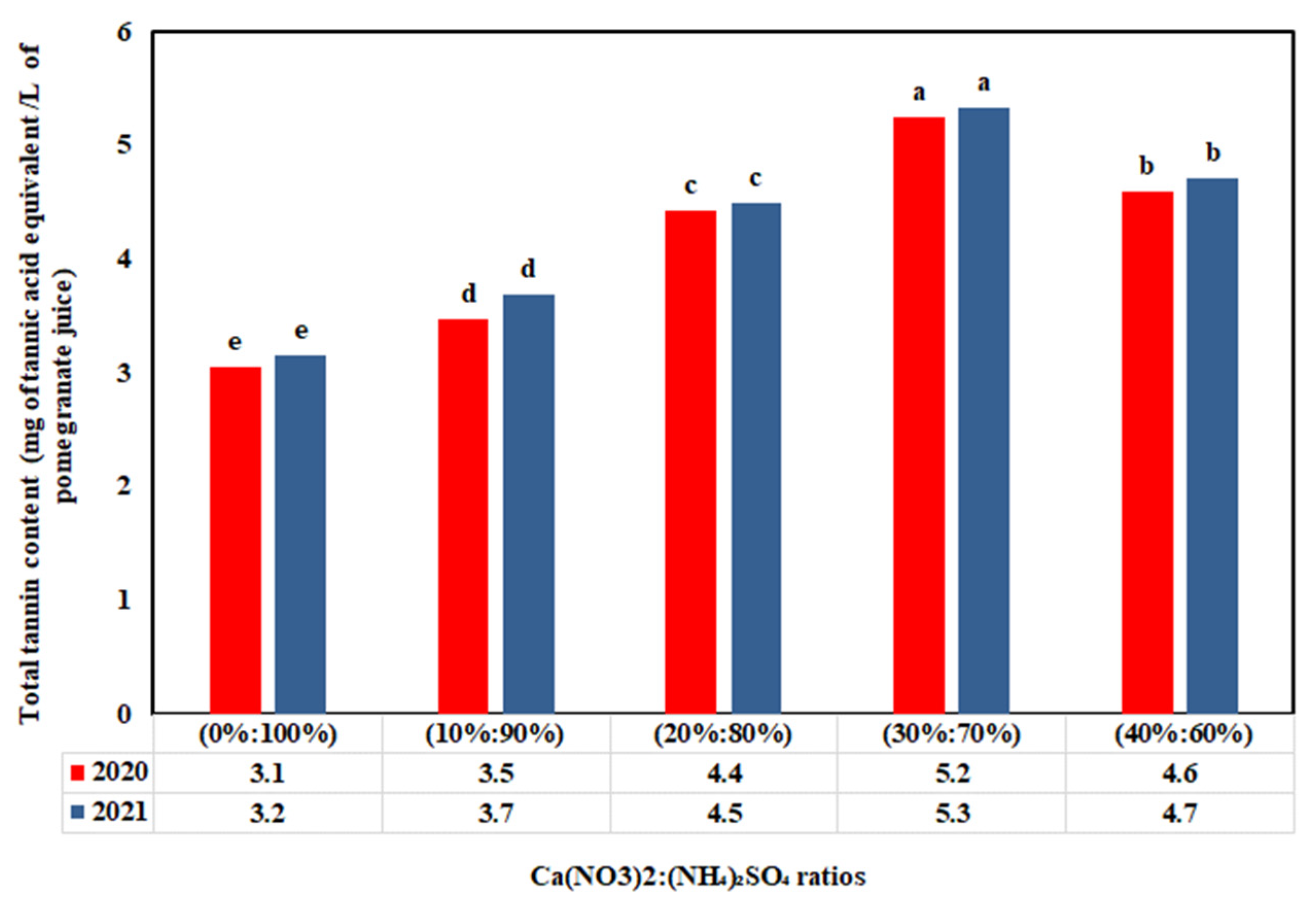
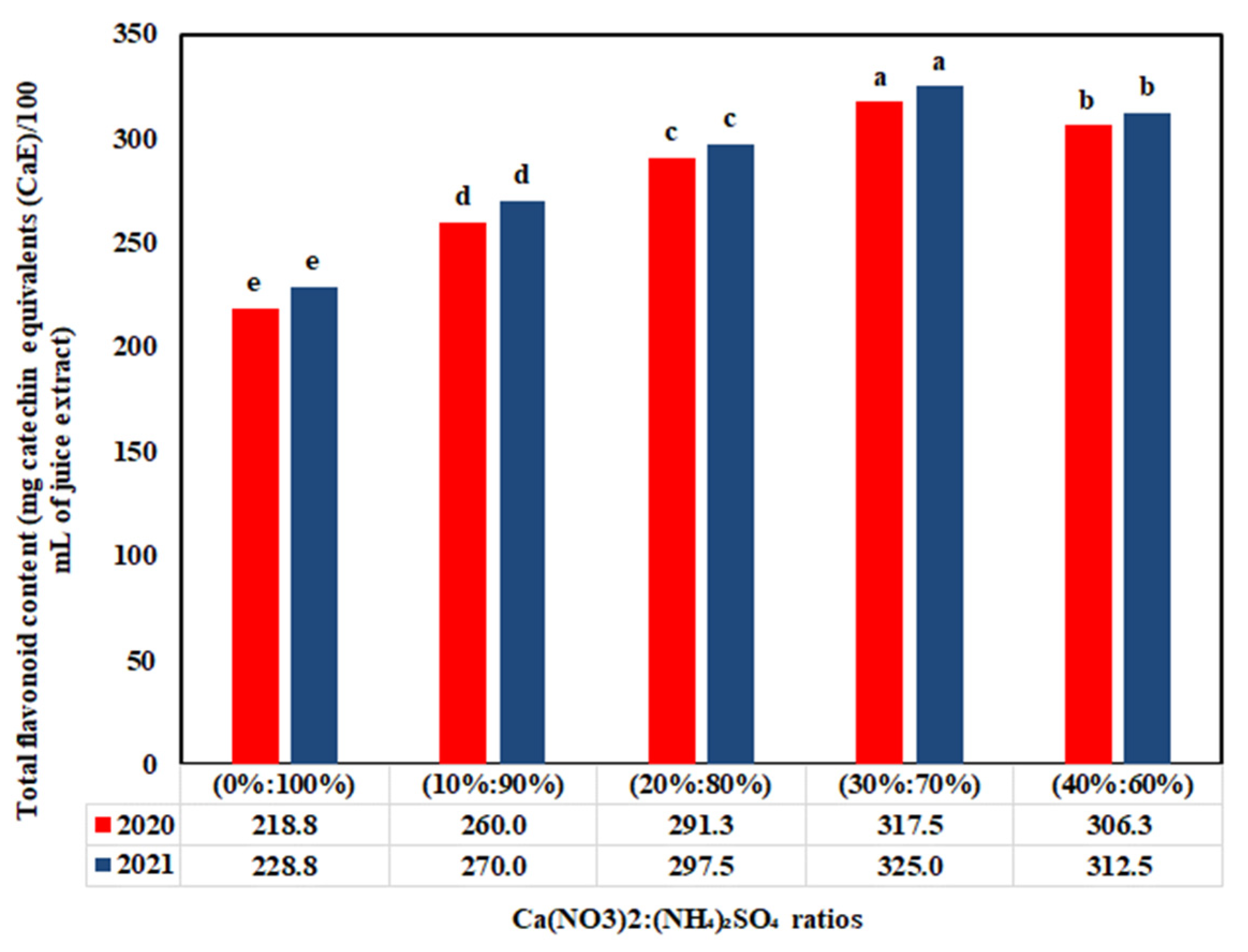
3.4. Impact of Ca(NO3)2:(NH4)2SO4 Ratios on Antioxidant Compounds
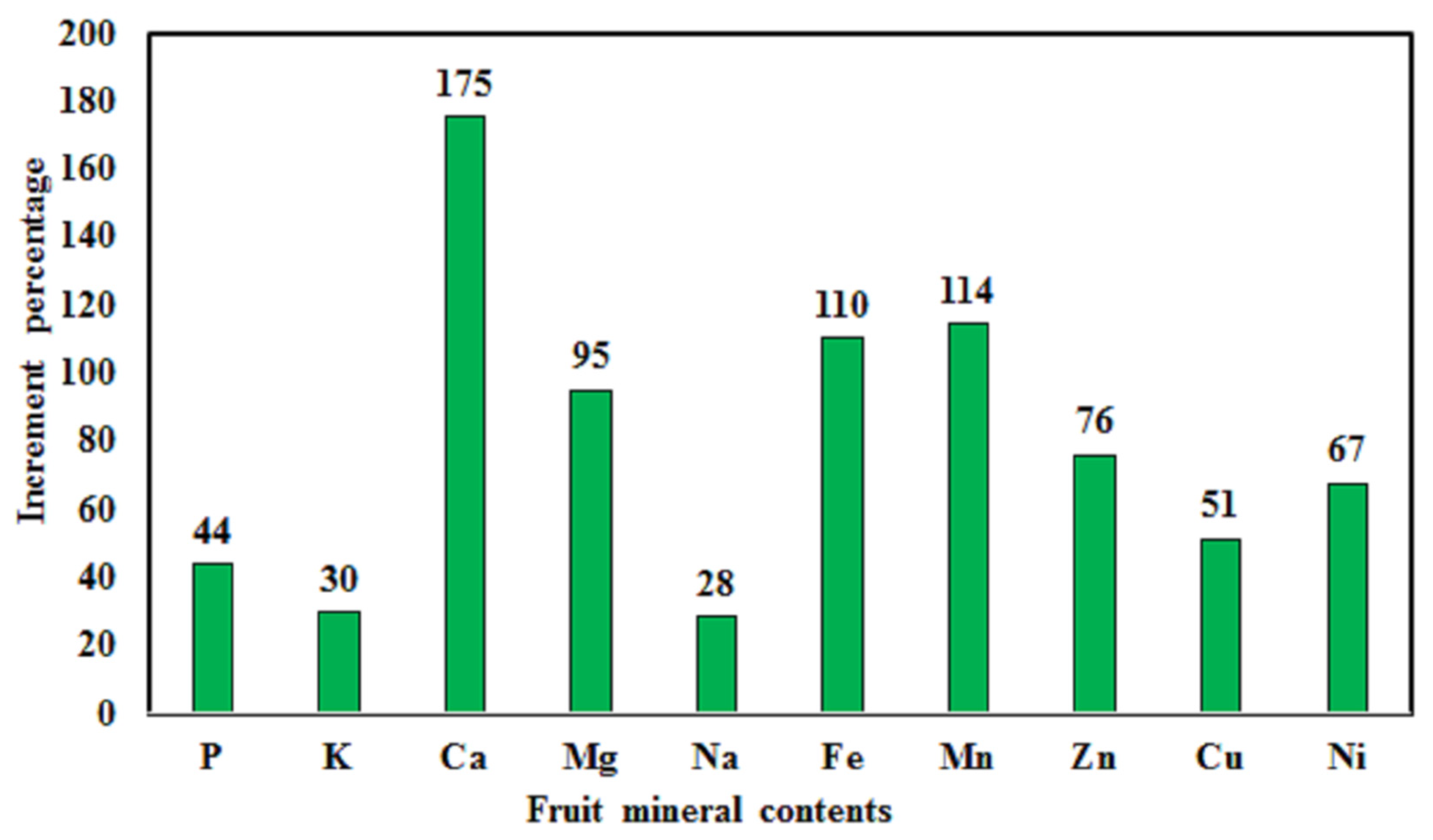
3.5. Impact of Ca(NO3)2:(NH4)2SO4 Ratios on Mineral Content
3.6. Correlation Analysis
4. Discussion
4.1. Fertilization Treatment Effects on the Physico-Chemical Characteristics of the Pomegranate Fruit of cv. ‘Wonderful’
4.2. Fertilization Treatment Effect on Color Characteristics of the Pomegranate Fruit of cv. ‘Wonderful’
4.3. Fertilization Treatment Effect on Moisture Content, Total, Reducing, and the Non-Reducing Sugar Content of Pomegranate Fruit of the cv. ‘Wonderful’
4.4. Fertilization Treatment Effect on Antioxidant Compounds
4.5. Fertilization Treatments Effect on Mineral Contents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Sattar, M.; Almutairi, K.F.; Aboukarima, A.M.; El-Mahrouky, M. Impact of organic manure on fruit set, fruit retention, yield, and nutritional status in pomegranate (Punica granatum L. “Wonderful”) under water and mineral fertilization deficits. PeerJ 2021, 9, e10979. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, horticulture, breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- Pokharkar, V.; Gulave, C.; Yadav, D. Economic impact of pomegranate research and extension on farm economy. Ann. Hortic. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Al-Dosary, N.M.N.; Abdel-Sattar, M.; Aboukarima, A.M. Effect of ammonium sulphate incorporated with calcium nitrate fertilizers on nutritional status, fruit set and yield of pomegranate trees cv. Wonderful. Agronomy 2022, 12, 971. [Google Scholar] [CrossRef]
- Morittu, V.M.; Mastellone, V.; Tundis, R.; Loizzo, M.R.; Tudisco, R.; Figoli, A.; Cassano, A.; Musco, N.; Britti, D.; Infascelli, F.; et al. Antioxidant, Biochemical, and In-Life Effects of Punica granatum L. Natural Juice vs. Clarified Juice by Polyvinylidene Fluoride Membrane. Foods 2020, 9, 242. [Google Scholar] [CrossRef]
- Martínez, J.; Melgarejo, P.; Hernández, F.; Salazar, D.; Martínez, R. Seed characterisation of five new pomegranate (Punica granatum L.) varieties. Sci. Hortic. 2006, 110, 241–246. [Google Scholar] [CrossRef]
- Hunter, D.; Foster, M.; McArthur, J.O.; Ojha, R.; Petocz, P.; Samman, S. Evaluation of the micronutrient composition of plant foods produced by organic and conventional agricultural methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 571–582. [Google Scholar] [CrossRef]
- Almutairi, K.F.; Abdel-Sattar, M.; Mahdy, A.M.; El-Mahrouky, M.A. Co-application of mineral and organic fertilizers under deficit irrigation improves the fruit quality of the Wonderful pomegranate. PeerJ 2021, 9, e11328. [Google Scholar] [CrossRef]
- Deshmukh, M.S.; Kachave, T.R.; Kanase, P.M. Evaluation of macro and micro nutrient status of pomegranate orchards from Maharashtra region by soil and leaf analysis. J. Pharmacogn. Phytochem. 2020, 9, 1378–1382. [Google Scholar]
- Hamouda, H.A.; El-Dahshouri, M.F.; Shaaban, S.H.A.; El-Saady, A.M. Nutritional status of Wonderful pomegranate during the growing season. EurAsian J. Bio. Sci. 2019, 13, 1771–1776. [Google Scholar]
- Pomegranate Fertilization Program. Available online: https://biofertilize.com/pomegranate-fertilization-program/ (accessed on 15 November 2022).
- Torshiz, A.O.; Goldansaz, S.H.; Motesharezadeh, B.; Askari, M.A.; Zarei, A. The influence of fertilization on pomegranate susceptibility to infestation by ectomyelois ceratoniae. Int. J. Fruit Sci. 2020, 20, S1156–S1173. [Google Scholar] [CrossRef]
- Heidari, M.; Mohammad, M.M. Effect of rate and time of nitrogen application on fruit yield and accumulation of nutrient elements in Momordica charantia. J. Saudi Soc. Agric. Sci. 2012, 11, 129–133. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hortic. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Hasani, M.; Zamani, Z.; Savaghebi, G.; Sofla, H.S. Effect of foliar and soil application of urea on leaf nutrients concentrations, yield and fruit quality of pomegranate. J. Plant Nutr. 2015, 39, 749–755. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780824759049. [Google Scholar]
- Tanari, N.; Ramegowda, S.; Thottan, A.; Girigowda, M. Effect of fertigation of primary nutrients on pomegranate (Punica granatum L.) fruit productivity and quality. Trop. Plant Res. 2019, 6, 424–432. [Google Scholar] [CrossRef]
- Gajbhiye, B.R.; Patil, V.; Kachave, T.R. Effect of integrated nutrient management (INM) on available micro nutrients of pomegranate (Punica granatum L.) orchard soil. Int. J. Chem. Stud. 2020, 8, 1900–1903. [Google Scholar] [CrossRef]
- Nasser, M.A. Yield and fruit quality of Wonderful pomegranate trees under three levels of chemical and organic nitrogen fertilizers. Middle East J. Agric. Res. 2018, 7, 1856–1866. [Google Scholar]
- Khalaj, M.A.; Noroozisharaf, A. Efficiency of ammonium and nitrate ratios on macronutrient content and morphological properties of Gerbera jamesonii cut flower. Agric. Conspec. Sci. 2020, 85, 281–289. [Google Scholar]
- Jeyabaskaran, K.J.; Shirgure, P.S.; Pandey, V.; Srivastava, A.K.; Uma, S. Fertigation in horticulture: A guarantee to economized quality production. Indian J. Fertil. 2021, 17, 364–383. [Google Scholar]
- Briones, A.M.; Okabe, S., Jr.; Umemiya, Y.; Ramsing, N.B.; Reichardt, W.; Okuyama, H. Ammonia-oxidizing bacteria on root biofilms and their possible contribution to N use efficiency of different rice cultivars. Plant Soil 2003, 250, 335–348. [Google Scholar] [CrossRef]
- Vasileva, V.; Ilieva, A. Chemical composition, nitrate reductase activity and plastid pigments content in lucerne under the influence of ammonium and nitrate form mineral nitrogen. Agron. Res. 2011, 9, 357–364. [Google Scholar]
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef]
- Hu, L.; Yu, J.; Liao, W.; Zhang, G.; Xie, J.; Lv, J.; Xiao, X.; Yang, B.; Zhou, R.; Bu, R. Moderate ammonium nitrate alleviates low light intensity stress in mini Chinese cabbage seedling by regulating root architecture and photosynthesis. Sci. Hortic. 2015, 186, 143–153. [Google Scholar] [CrossRef]
- Abbasi, H.N.; Vasileva, V.; Lu, X. The influence of the ratio of nitrate to ammonium nitrogen on nitrogen removal in the economical growth of vegetation in hybrid constructed Wetlands. Environments 2017, 4, 24. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, B.; Hao, Y.; Liu, H.; Sun, G.; Chen, R.; Song, S. Appropriate NH4+/NO3− ratio triggers plant growth and nutrient uptake of flowering chinese cabbage by optimizing the pH value of nutrient solution. Front. Plant Sci. 2021, 12, 656144. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Villarreal, R.; Valdez-Aguilar, L.A.; Sandoval-Rangel, A.; Robledo-Torres, V.; Benavides-Mendoza, A. Tolerance of lisianthus to high ammonium levels in Rockwool culture. J. Plant Nutr. 2014, 38, 73–82. [Google Scholar] [CrossRef]
- Da Silva, G.B.; Prado, R.D.M.; Silva, S.L.O.; Campos, C.N.S.; Castellanos, L.G.; Dos Santos, L.C.N.; Barreto, R.F.; Teodoro, P.E. Nitrogen concentrations and proportions of ammonium and nitrate in the nutrition and growth of yellow passion fruit seedlings. J. Plant Nutr. 2020, 43, 2533–2547. [Google Scholar] [CrossRef]
- Rebelo, M.C.; Nunes, M.A.; Ramalho, J.C.; Santos, M.E.; Antunes, M.L. Effects of calcium deficiency on Coffea arabica. Nutrient changes and correlation of calcium levels with some photosynthetic parameters. Plant Soil 1995, 172, 87–96. [Google Scholar] [CrossRef]
- Eticha, D.; Kwast, A.; Chiachia, T.R.D.S.; Horowitz, N.; Stützel, H. Calcium nutrition of orange and its impact on growth, nutrient uptake and leaf cell wall. Citrus Res. Technol. 2017, 38, 62–70. [Google Scholar] [CrossRef]
- Song, Q.; Liu, Y.; Pang, J.; Yong, J.W.H.; Chen, Y.; Bai, C.; Gille, C.; Shi, Q.; Wu, D.; Han, X.; et al. Supplementary calcium restores peanut (Arachis hypogaea) growth and photosynthetic capacity under low nocturnal temperature. Front. Plant Sci. 2020, 10, 1637. [Google Scholar] [CrossRef]
- Bolan, N.S.; Loganathan, P.; Saggar, S. Calcium and magnesium in soils. In Encyclopedia of Soils in the Environment, 1st ed.; Hillel, D., Ed.; Academic Press: New York, NY, USA, 2007; pp. 149–154. [Google Scholar] [CrossRef]
- Xu, T.; Li, T.; Qi, M. Calcium requirement for ethylene-induced abscission. J. Plant Nutr. 2009, 32, 351–366. [Google Scholar] [CrossRef]
- Stebbins, R.L.; Dewey, D.H.; Shull, V.E. Calcium crystals in apple stem, petiole and fruit tissue1. Hortscience 1972, 7, 492–493. [Google Scholar] [CrossRef]
- Sarrwy, S.M.A.; Gadalla, E.G.; Mostafa, E.A.M. Effect of calcium nitrate and boric acid sprays on fruit set, yield and fruit quality of cv. Amhat date palm. World J. Agric. Sci. 2012, 8, 506–515. [Google Scholar] [CrossRef]
- Naphun, W.; Kawada, K.; Matsui, T.; Yoshida, Y.; Kusunoki, M. Effects of calcium spray on the quality of ‘Nyoho’ strawberries grown by peat-bag-substrate bench culture. Kasetsart J. Nat. Sci. 1997, 32, 9–14. [Google Scholar]
- Modaihsh, A.S.; Sallam, A.; Ghoneim, A.M.; Mahjoub, M.O. Assessing salt-affected degraded soils using remote sensing. Case study: Al-Qassim region, Saudi Arabia. J. Food Agric. Environ. 2014, 12, 383–388. [Google Scholar]
- Tehranifar, A.; Zarei, M.; Esfandiyari, B.; Nemati, Z. Physicochemical properties and antioxidant activities of pomegranate fruit (Punica granatum) of different cultivars grown in Iran. Hortic. Environ. Biotechnol. 2010, 51, 573–579. [Google Scholar]
- Papadakis, S.E.; Abdul-Malek, S.; Kamdem, R.E.; Yam, K.L. A versatile and inexpensive technique for measuring color of foods. Food Technol. 2000, 54, 48–51. [Google Scholar]
- McGuire, R.G. Reporting of Objective Color Measurements. Hortscience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Granato, D.; Masson, M.L. Instrumental color and sensory acceptance of soy-based emulsions: A response surface approach. Food Sci. Technol. 2010, 30, 1090–1096. [Google Scholar] [CrossRef]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 445–449. ISBN 978-0470049044. [Google Scholar]
- Klement, I.; Vilkovský, P.; Vilkovská, T.; Orłowski, K.A.; Barański, J.; Chuchala, D.; Suchta, A. The influence of drying temperature on color change of hornbeam and maple wood used as surface and inner layers of wood composites. Appl. Sci. 2021, 11, 10673. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Ranganna, S. Sugar estimation. In Handbook of Analysis and Quality Control for Fruit and Vegetable Products, 2nd ed.; Ranganna, S., Ed.; Tata McGraw-Hill: New Delhi, India, 2001; Volume 2, pp. 12–17. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analyses: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Orak, H.H.; Yagar, H.; Isbilir, S.S. Comparison of antioxidant activities of juice, peel, and seed of pomegranate (Punica granatum L.) and inter-relationships with total phenolic, Tannin, anthocyanin, and flavonoid contents. Food Sci. Biotechnol. 2012, 21, 373–387. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Ayanmanesh, M.; Babadayei-Samani, R.; Javid, A.; Sanaeifard, M.; Vitalini, S.; Iriti, M. Total anthocyanin, flavonoid, polyphenol and tannin contents of seven pomegranate cultivars grown in Iran. Acta Sci. Pol. Technol. Aliment. 2015, 17, 211–217. [Google Scholar] [CrossRef]
- Park, Y.-S.; Jung, S.-T.; Kang, S.-G.; Heo, B.G.; Arancibia-Avila, P.; Toledo, F.; Drzewiecki, J.; Namiesnik, J.; Gorinstein, S. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008, 107, 640–648. [Google Scholar] [CrossRef]
- Ozgen, M.; Durgaç, C.; Serçe, S.; Kaya, C. Chemical and antioxidant properties of pomegranate cultivars grown in the Mediterranean region of Turkey. Food Chem. 2008, 111, 703–706. [Google Scholar] [CrossRef]
- SAS Institute Inc. The SAS System for Windows, version 9.13; SAS Institute Inc.: Cary, NC, USA, 2008.
- Anon, B. Food Standards Code 2987; Autralian Govt. Public Service: Canberra, Australia, 1987.
- FAO/WHO Expert Committee on Food Additives. Summary and conclusion. In Proceedings of the 53rd Meeting Joint FAO/WHO Expert Committee on Food Additives, Rome, Italy,, 1–10 June 1999. [Google Scholar]
- Mahdavian, S.E.; Somashekar, R.K. Heavy metal contamination of vegetables and fruits from Bangalore city. Nat. Env. Poll. Tech. 2009, 8, 829–834. [Google Scholar]
- Kumar, J.; Chandel, J.S. Effect of different levels of N, P and K on growth and yield of pear cv. Red Bartlett. Progress. Hortic. 2004, 36, 202–206. [Google Scholar]
- Wani, J.A.; Raina, S.-K.S.K.; Bhat, M.Y.; Dar, M.A. Effect of available nutrients on yield and quality of pear fruit Bartlett in Kashmir Valley India. J. Environ. Biol. 2012, 33, 1011. [Google Scholar]
- Hamouda, H.A.; El-Dahshouri, M.F.; Hafez, O.M.; Zahran, N.G. Response of Le Cont pear performance, chlorophyll content and active iron to foliar application of different Iron sources under the newly reclaimed soil conditions. Int. J. ChemTech Res. 2015, 8, 1446–1453. [Google Scholar]
- Galindo, A.; Calín-Sánchez, A.; Collado-González, J.; Ondoño, S.; Hernández, F.; Torrecillas, A.; Carbonell-Barrachina, A.A. Phytochemical and quality attributes of pomegranate fruits for juice consumption as affected by ripening stage and deficit irrigation. J. Sci. Food Agric. 2013, 94, 2259–2265. [Google Scholar] [CrossRef]
- Abd-Ella, E.E.K.; Mervate, S.S.; Wafaa, A.Z. Effect of some organic and mineral fertilizer applications on growth and productivity of pomegranate trees. Alex. Sci. Exch. J. 2010, 31, 296–304. [Google Scholar]
- Li, N.; Wang, X.X.; Lu, B.L. Study of the effect of apple liquid fertilizer on the growth and fruit development of Starkrimson apple variety. (in Chinese). China Fruits 1999, 4, 20–21. [Google Scholar]
- Itoo, B.A.; Manivannan, K. Effect of macro and micronutrients in different forms in comparison with humic acid on growth, yield and quality of tomato (Lycopersicon esculentum Mill) cv. Annapurna. South Indian Hortic. 2004, 52, 342–346. [Google Scholar]
- Meena, M.K. Effect and Economic Feasibility of Preharvest Spray of Calcium Nitrate, Boric Acid and Zinc Sulphate on Yield Attributing Characters of Nagpur Mandarin (Citrus reticulata Blanco). Hortic. Int. J. 2017, 1, 23–28. [Google Scholar] [CrossRef]
- Kazemi, M. Influence of foliar application of iron, calcium and zinc sulfate on vegetative growth and reproductive characteristics of strawberry cv. ‘Pajaro’. Trakia J. Sci. 2014, 1, 21–26. [Google Scholar]
- Thor, K. Calcium—Nutrient and Messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Eissa, A.M.; El-Shazly, S.M.; Alabd, A.S. Improving quality of Wonderful pomegranate by using bagging and different agrochemical treatments. Alex. J. Agric. Sci. 2017, 2017, 103–109. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Ravid, N.; Wilkins, E.; Martinelli, A. A Morphological and histological characterization of bisexual and male flower types in pomegranate. J. Am. Soc. Hortic. Sci. 2011, 136, 83–92. [Google Scholar] [CrossRef]
- Abdel-salam, F.F.; Moharram, Y.G.; Esmat, M.E. Physical properties and some bioactive compounds of four pomegranate cultivars (Punica granatum L.) grown in Egypt. Alex. J. Food Sci. Technol. 2018, 15, 77–88. [Google Scholar] [CrossRef]
- Passafiume, R.; Perrone, A.; Sortino, G.; Gianguzzi, G.; Saletta, F.; Gentile, C.; Farina, V. Chemical–physical characteristics, polyphenolic content and total antioxidant activity of three Italian-grown pomegranate cultivars. NFS J. 2019, 16, 9–14. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Ali, A.A.M.; Nassar, H.N.; Elsawy, A.M. Performance of manfalouty and wonderful pomegranate cultivars under four Egyptian climate regions. J. Hortic. Sci. Ornam. Plants 2020, 12, 169–181. [Google Scholar] [CrossRef]
- Tarantino, A.; Frabboni, L.; Disciglio, G. Water-yield relationship and vegetative growth of Wonderful young pomegranate trees under deficit irrigation conditions in Southeastern Italy. Horticulturae 2021, 7, 79. [Google Scholar] [CrossRef]
- Cadze, J.S.; Voca, S.; Cmelik, Z. Physicochemical characteristics of main pomegranate (Punica granatum L.) cultivars grown in Dalmatia region of Coratia. J. Appl. Bot. Food Qual. 2011, 85, 202–206. [Google Scholar]
- Kashyap, P.; Pramanick, K.K.; Meena, K.K.; Meena, V. Effect of N and K application on yield and quality of pomegranate cv. Ganesh under rainfed conditions. Indian J. Hortic. 2012, 69, 322–327. [Google Scholar]
- Al-Saif, A.M.; Mosa, W.F.A.; Saleh, A.A.; Ali, M.M.; Sas-Paszt, L.; Abada, H.S.; Abdel-Sattar, M. Yield and fruit quality response of pomegranate (Punica granatum) to foliar spray of potassium, calcium and kaolin. Horticulturae 2022, 8, 946. [Google Scholar] [CrossRef]
- Hegazi, A.; Samra, N.R.; El-Baz, E.E.T.; Khalil, B.M.; Gawish, M.S. Improving fruit quality of Manfaloty and Wonderful pomegranates by using bagging and some spray treatments with gibberellic acid, calcium chloride and kaolin. J. Plant Prod. 2014, 5, 779–792. [Google Scholar]
- Mignani, I.; Greve, L.C.; Ben-Arie, R.; Stotz, H.U.; Li, C.; Shackel, K.A.; Labavitch, J.M. The effects of GA3 and divalent cations on aspects of pectin metabolism and tissue softening in ripening tomato pericarp. Physiol. Plant. 1995, 93, 108–115. [Google Scholar] [CrossRef]
- Jackman, R.L.; Stanley, D.W. Perspectives in the textural evaluation of plant foods. Trends Food Sci. Technol. 1995, 6, 187–194. [Google Scholar] [CrossRef]
- Ben-Arie, R.; Segal, N.; Guelfat-Reich, S. The maturation and ripening of the ‘Wonderful’ pomegranate. J. Am. Soc. Hortic. Sci. 1984, 109, 898–902. [Google Scholar] [CrossRef]
- Ferrara, G.; Cavoski, I.; Pacifico, A.; Tedone, L.; Mondelli, D. Morpho-pomological and chemical characterization of pomegranate (Punica granatum L.) genotypes in Apulia region, Southeastern Italy. Sci. Hortic. 2011, 130, 599–606. [Google Scholar] [CrossRef]
- Karav, S.; Ardic, O.A.; Eksi, A. Effect of cold storage of various pomegranate cultivars fruit juices on health promoting compounds and their activities. J. Food Nutr. Res. 2015, 3, 593–598. [Google Scholar] [CrossRef]
- Mohamed, A.K.A.; Ibrahim, R.; Abdel-Salam, M.M.; Abd-El-Ghany, A.M.M. Physico-chemical and antioxidant contents during developmental stages in three pomegranates cultivars under Assiut condition. Assiut J. Agric. Sci. 2015, 46, 77–96. [Google Scholar]
- Abbas, F.; Ali, N.; Abbas, Y.; Ali, A.; Hussain, N.; Abbas, T. Physicochemical analysis of pomegranate of Gilgit Baltistan, Pakistan. Agric. For. Fish. 2015, 4, 246–251. [Google Scholar] [CrossRef][Green Version]
- Akbarpour, V.; Hemmati, K.; Sharifani, M. Physical and chemical properties of pomegranate (Punica granatum L.) fruit in maturation stage. Am.-Eurasian J. Agric. Environ. Sci. 2009, 6, 411–416. [Google Scholar]
- Paul, M.J.; Driscoll, S.P. Sugar repression of photosynthesis: The role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ. 1997, 20, 110–116. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.; White, P.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Serna, M.D.; Borrás, R.; Legaz, F.; Primo-Millo, E. The influence of nitrogen concentration and ammonium/nitrate ratio on N-uptake, mineral composition and yield of citrus. Plant Soil 1992, 147, 13–23. [Google Scholar] [CrossRef]
- Tanan, T.T.; da Silva, A.L.; de Oliveira, U.C.; Neto, L.P.G.; Nascimento, M.N.D. Effect of nitrogen sources on fruit characteristics and seed physiological quality of Physalis angulata L. Pesqui. Agropecuária Trop. 2019, 49, 1–9. [Google Scholar] [CrossRef]
- Al- Hmadawi, A.M.S.; Al-Numani, R.M.H.; AL-Shemmery, W.H.M. Effect of pruning and spraying with N, Ca and GA3 on some characters of fruits and percentage of cracking of fig cv. Asowd Diala. Euphrates J. Agric. Sci. 2011, 3, 37–44. [Google Scholar]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Pizzolongo, F.; Romano, R.; Di Matteo, M. Study of pomological traits and physico-chemical quality of pomegranate (Punica granatum L.) genotypes grown in Italy. Eur. Food Res. Technol. 2018, 244, 1427–1438. [Google Scholar] [CrossRef]
- Abdulrahman, A.B.M.; Mhamad, H.J.; Talb, S.S.; Aljabary, A.M.A.O. Physicochemical Properties and Phenolic Contents of Fresh and Concentrated Juice of Four Pomegranate Cultivars in Iraq. IOP Conf. Series: Earth Environ. Sci. 2021, 910, 012093. [Google Scholar] [CrossRef]
- Zarei, M.; Azizi, M.; Bashir-Sadr, Z. Evaluation of physicochemical characteristics of pomegranate (Punica granatum L.) fruit during ripening. Fruits 2011, 66, 121–129. [Google Scholar] [CrossRef]
- Lyu, Y.; Porat, R.; Yermiyahu, U.; Heler, Y.; Holland, D.; Dag, A. Effects of nitrogen fertilization on pomegranate fruit, aril and juice quality. J. Sci. Food Agric. 2019, 100, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, L.M.; Benjamin, O.; Porat, R. Sensory and nutritional attributes of pomegranate juices extracted from separated arils and pressed whole fruits. J. Sci. Food Agric. 2015, 96, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, J.; Dawuda, M.M.; Xie, J.; Yu, J.; Li, J.; Zhang, X.; Tang, C.; Wang, C.; Gan, Y. Appropriate Ammonium-Nitrate Ratio Improves Nutrient Accumulation and Fruit Quality in Pepper (Capsicum annuum L.). Agronomy 2019, 9, 683. [Google Scholar] [CrossRef]
- Radunić, M.; Špika, M.J.; Ban, S.G.; Gadže, J.; Díaz-Pérez, J.C.; MacLean, D. Physical and chemical properties of pomegranate fruit accessions from Croatia. Food Chem. 2015, 177, 53–60. [Google Scholar] [CrossRef]
- Chater, J.M.; Duong, F.V.; Alas, M.G.; Yip, M.K.; Zib, A.N.; Luu, I.J.; Chu, C.; Carrillo-Kashani, A.; Wang, S.; Li, R.; et al. Postharvest Quality of Imported, Domestic, and Minimally Processed Pomegranate Fruit. Int. J. Fruit Sci. 2020, 20, S337–S351. [Google Scholar] [CrossRef]
- Onias, E.A.; Araújo, R.H.C.R.; Ferreira, A.P.N.; de Oliveira, A.M.F.; Teodosio, A.E.M.M.; Sarmento, D.H.A.; Alves, K.A.; de Queiroga, T.B. Genotype characterization of pomegranate trees grown in Tabuleiro de Russas–ce/caracterização de genótipos de romãzeira produzidos em tabuleiro de russas-ce. Braz. J. Dev. 2021, 7, 37199–37213. [Google Scholar] [CrossRef]
- Mashavhathakha, K.L. Yield and Quality of Pomegranate on Selected Geographical Areas in Western Cape Province, South Africa. Master’s Thesis, University of South Africa, Pretoria, South Africa, 2014. [Google Scholar]
- Fawole, O.A.; Opara, U.L.; Theron, K.I. Chemical and Phytochemical Properties and Antioxidant Activities of Three Pomegranate Cultivars Grown in South Africa. Food Bioprocess Technol. 2011, 5, 2934–2940. [Google Scholar] [CrossRef]
- Ferrara, G.; Giancaspro, A.; Mazzeo, A.; Giove, S.L.; Matarrese, A.M.S.; Pacucci, C.; Punzi, R.; Trani, A.; Gambacorta, G.; Blanco, A.; et al. Characterization of pomegranate (Punica granatum L.) genotypes collected in Puglia region, Southeastern Italy. Sci. Hortic. 2014, 178, 70–78. [Google Scholar] [CrossRef]
- Durgac, C.; Ozgen, M.; Simsek, O.; Kacar, Y.A.; Kiyda, Y.; Celebi, S.; Gunduz, K.; Serce, S. Molecular and pomological diversity among pomegranate (Punica granatum L.) cultivars in Eastern Mediterranean region of Turkey. Afr. J. Biotechnol. 2008, 7, 1294–1301. [Google Scholar]
- Mellisho, C.; Egea, I.; Galindo, A.; Rodríguez, P.; Conejero, W.; Romojaro, F.; Torrecillas, A. Pomegranate (Punica granatum L.) fruit response to different deficit irrigation conditions. Agric. Water Manag. 2012, 114, 30–36. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Effects of maturity status on biochemical content, polyphenol composition and antioxidant capacity of pomegranate fruit arils (cv. ‘Bhagwa’). S. Afr. J. Bot. 2013, 85, 23–31. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Opara, U.L. Influence of storage temperature and duration on postharvest physico-chemical and mechanical properties of pomegranate fruit and arils. CyTA J. Food 2014, 12, 389–398. [Google Scholar] [CrossRef]
- Labbé, M.; Ulloa, P.A.; López, F.; Sáenz, C.; Peña, Á.; Salazar, F.N. Characterization of chemical compositions and bioactive compounds in juices from pomegranates (‘Wonderful’, ‘Chaca’ and ‘Codpa’) at different maturity stages. Chil. J. Agric. Res. 2016, 76, 479–486. [Google Scholar] [CrossRef]
- Sharma, R.R.; Sharma, V.P. The Strawberry; JCAR: New Delhi, India, 2004; ISBN 13:9798171640187. [Google Scholar]
- Poovaiah, B.W. Role of calcium in ripening and senescence. Commun. Soil Sci. Plant Anal. 1979, 10, 83–88. [Google Scholar] [CrossRef]
- Cheéour, F.; Willemot, C.; Arul, J.; Desjardins, Y.; Makhlouf, J.; Charest, P.; Gosselin, A. Foliar application of calcium chloride delays postharvest ripening of strawberry. J. Am. Soc. Hortic. Sci. 1990, 115, 789–792. [Google Scholar] [CrossRef]
- Shwartz, E.; Glazer, I.; Bar-Ya’Akov, I.; Matityahu, I.; Bar-Ilan, I.; Holland, D.; Amir, R. Changes in chemical constituents during the maturation and ripening of two commercially important pomegranate accessions. Food Chem. 2009, 115, 965–973. [Google Scholar] [CrossRef]
- Türkmen, I.; Ekşi, A. Brix degree and sorbitol/xylitol level of authentic pomegranate (Punica granatum) juice. Food Chem. 2011, 127, 1404–1407. [Google Scholar] [CrossRef]
- Vardin, H.; Fenercioǧlu, H. Study on the development of pomegranate juice processing technology: Clarification of pomegranate juice. Food/Nahr. 2003, 47, 300–303. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; MacLean, D.; Goreta, S.; Workman, S.; Smith, E.; Sidhu, H.S.; Gunawan, G.; Bateman, A.; Bautista, J.; Lovett, W.; et al. Physical and Chemical Attributes of Pomegranate (Punica granatum L.) Cultivars Grown in Humid Conditions in Georgia. Hortscience 2019, 54, 1108–1114. [Google Scholar] [CrossRef]
- Weerakkody, P.; Jobling, J.; Infante, M.M.V.; Rogers, G. The effect of maturity, sunburn and the application of sunscreens on the internal and external qualities of pomegranate fruit grown in Australia. Sci. Hortic. 2010, 124, 57–61. [Google Scholar] [CrossRef]
- Saenz, C.; Seguel, J.; Gorena, T.; Sepulveda, E. Effect of the concentration temperature on some bioactives compounds and rheological properties of pomegranate juices. In Proceedings of the International Conference Food Innovation, Valencia, Spain, 25–29 October 2010; pp. 1–4. [Google Scholar]
- Zarei, M.; Azizi, M.; Bashiri-Sa, Z. Studies on physico-chemical properties and bioactive compounds of six pomegranate cultivars grown in Iran. J. Food Technol. 2010, 8, 112–117. [Google Scholar] [CrossRef]
- Li, X.; Wasila, H.; Liu, L.; Yuan, T.; Gao, Z.; Zhao, B.; Ahmad, I. Physicochemical characteristics, polyphenol compositions and antioxidant potential of pomegranate juices from 10 Chinese cultivars and the environmental factors analysis. Food Chem. 2014, 175, 575–584. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Lopéz-Cortés, I.; Salazar, D.M.; Ramalhosa, E.C.D. Physicochemical changes and antioxidant activity of juice, skin, pellicle and seed of pomegranate (cv “Mollar De Elche”) at different stages of ripening. Food Technol. Biotechnol. 2015, 53, 397–406. [Google Scholar] [CrossRef]
- Chater, J.M. The Effects of Foliar Nutrient Applications on Split, Yield, and Internal Fruit Quality of ‘Wonderful’ Pomegranate (Punica granatum L.). Master’s Thesis, Science in Agriculture, Faculty of California Polytechnic State University, San Luis Obispo, CA, USA, December 2015. [Google Scholar]
- Radwan, M.A.; Salama, A.K. Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem. Toxicol. 2006, 44, 1273–1278. [Google Scholar] [CrossRef]
- Sobukola, O.P.; Adeniran, O.M.; Odedairo, A.A.; Kaji Hausa, O.E. Heavy metal levels of some fruits and leafy vegetables from selected Markets in Lagos, Nigeria. Afr. J. Food Sci. 2010, 4, 389–393. [Google Scholar]
- Meighani, H.; Ghasemnezhad, M.; Bakshi, D. Evaluation of biochemical composition and enzyme activities in browned arils of pomegranate fruits. Int. J. Hort. Sci. Tech. 2014, 1, 53–65. [Google Scholar] [CrossRef]
- What Is the Role of Calcium in Plant Growth? Available online: https://www.vaniperen.com/role-of-calcium-in-plant-growth (accessed on 1 December 2022).
- Wang, G.; Wang, J.; Han, X.; Chen, R.; Xue, X. Effects of Spraying Calcium Fertilizer on Photosynthesis, Mineral Content, Sugar–Acid Metabolism and Fruit Quality of Fuji Apples. Agronomy 2022, 12, 2563. [Google Scholar] [CrossRef]

| Parameters | Soil Depth (cm) | Average | |
|---|---|---|---|
| 0–30 | 30–60 | ||
| Sand (%) | 57.9 | 50.3 | 54.1 |
| Clay (%) | 14.9 | 15.9 | 15.4 |
| Silt (%) | 27.2 | 33.8 | 30.5 |
| Soil texture | Sandy loam | Loam | Sandy loam |
| pH | 8.3 | 7.9 | 8.1 |
| Electrical conductivity (dS/m) | 2.47 | 1.11 | 1.79 |
| CaCO3 (%) | 3.6 | 3.9 | 3.8 |
| Na+ (meq/L) | 2.0 | 4.3 | 3.2 |
| Ca2+ (meq/L) | 9.6 | 3.6 | 6.6 |
| Mg2+ (meq/L) | 13.0 | 2.4 | 7.7 |
| K+ (meq/L) | 0.1 | 0.8 | 0.45 |
| HCO3− (meq/L) | 1.3 | 2.1 | 1.7 |
| Cl− (meq/L) | 13.3 | 6.0 | 9.65 |
| SO42− (meq/L) | 10.1 | 3.0 | 6.55 |
| Organic matter (%) | 0.30 | 0.21 | 0.26 |
| Ca (NO3)2:(NH4)2SO4 Ratio | (NH4)2SO4 from the Commercial Ammonium Sulphate (21-0-0+24 S) (Ammonisul) | Ca (NO3)2 from Torofert Calcium (15-0-0+27 Ca) | Total Application Rate | |||||
|---|---|---|---|---|---|---|---|---|
| Amount (kg) | Application Rate | Amount (kg) | Application Rate | |||||
| N (kg/ha) | CaO (kg/ha) | N (kg/ha) | CaO (kg/ha) | N (kg/ha) | CaO (kg/ha) | |||
| (0%:100%); control | 952.38 | 200 | 0 | 0 | 0 | 0 | 200 | 0 |
| (10%:90%) | 857.14 | 180 | 0 | 133.33 | 20 | 36 | 200 | 36 |
| (20%:80%) | 761.90 | 160 | 0 | 266.66 | 40 | 72 | 200 | 72 |
| (30%:70%) | 666.67 | 140 | 0 | 400.00 | 60 | 108 | 200 | 108 |
| (40%:60%) | 571.43 | 120 | 0 | 533.32 | 80 | 144 | 200 | 144 |
| Season | Ca(NO3)2: (NH4)2SO4 Ratio | Physical Attributes | Chemical Attributes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fruit Weight | Fruit Volume | Fruit Length | Fruit Diameter | Juice pH | TSS | Titratable Acidity | Maturity Index | ||
| (g) | (ml) | (cm) | (cm) | (-) | (%) | (%) | (%) | ||
| 2020 | (0%:100%) | 303.25e | 344.25e | 7.72e | 8.41e | 4.32a | 15.45d | 1.14a | 13.6e |
| (10%:90%) | 313.75d | 352.00d | 7.79d | 8.62d | 3.96b | 16.30c | 1.00b | 16.3d | |
| (20%:80%) | 320.25c | 364.25c | 7.82c | 8.74c | 3.78c | 16.80b | 0.94c | 17.8c | |
| (30%:70%) | 336.25a | 386.25a | 7.94a | 9.04a | 3.19e | 17.23a | 0.91d | 18.9b | |
| (40%:60%) | 326.25b | 373.25b | 7.86b | 8.94b | 3.44d | 16.85b | 0.85e | 19.9a | |
| LSD (5%) | 2.87 | 5.01 | 0.03 | 0.05 | 0.03 | 0.14 | 0.01 | 0.24 | |
| 2021 | (0%:100%) | 305.75e | 346.00e | 7.74e | 8.49b | 4.22a | 15.55d | 1.13a | 13.8e |
| (10%:90%) | 316.50d | 353.25d | 7.81d | 8.69d | 3.93b | 16.35c | 0.99b | 16.5d | |
| (20%:80%) | 323.50c | 367.00c | 7.85c | 8.78c | 3.84c | 16.85b | 0.93c | 18.1c | |
| (30%:70%) | 338.50a | 385.75a | 7.96a | 9.05a | 3.14e | 17.25a | 0.90d | 19.2b | |
| (40%:60%) | 327.50b | 371.75b | 7.88b | 8.94e | 3.46d | 16.90b | 0.85e | 19.9a | |
| LSD (5%) | 2.44 | 2.27 | 0.02 | 0.02 | 0.02 | 0.09 | 0.01 | 0.22 | |
| Season | Ca(NO3)2: (NH4)₂SO₄ Ratio | Luminosity or Lightness (L*) | Color Variation from Green to Red (a*) | Color Variation from Blue to Yellow (b*) | Chroma | Color Difference (ΔE*) |
|---|---|---|---|---|---|---|
| 2020 | (0%:100%) | 48.80e | 43.50e | 22.35e | 48.90e | |
| (10%:90%) | 52.07d | 45.36d | 23.23d | 50.96d | 3.9 | |
| (20%:80%) | 56.49c | 47.59b | 25.44b | 53.96b | 9.2 | |
| (30%:70%) | 59.04b | 49.62a | 26.22a | 56.12a | 12.5 | |
| (40%:60%) | 61.78a | 46.53c | 24.68c | 52.66c | 13.5 | |
| LSD (5%) | 0.81 | 0.48 | 0.27 | 0.53 | ||
| 2021 | (0%:100%) | 49.64e | 43.72e | 22.70e | 49.26e | |
| (10%:90%) | 52.73d | 45.69d | 23.55d | 51.40d | 3.8 | |
| (20%:80%) | 56.87c | 47.80b | 25.67b | 54.26b | 8.8 | |
| (30%:70%) | 59.28b | 49.62a | 26.31a | 56.16a | 11.9 | |
| (40%:60%) | 63.25a | 46.74c | 24.54c | 52.79c | 14.1 | |
| LSD (5%) | 0.38 | 0.27 | 0.21 | 0.32 |
| Season | Ca(NO3)2: (NH4)2SO4 Ratio | Fruit Moisture Content | Total Sugar | Reducing Sugar | Non-Reducing Sugar |
|---|---|---|---|---|---|
| (%, wb) | (%) | (%) | (%) | ||
| 2020 | (0%:100%) | 83.0a | 12.85d | 11.81e | 1.04c |
| (10%:90%) | 82.3b | 12.88d | 11.88d | 1.00c | |
| (20%:80%) | 81.5d | 13.57b | 12.03c | 1.54a | |
| (30%:70%) | 81.0e | 13.79a | 12.44a | 1.36b | |
| (40%:60%) | 82.2c | 13.23c | 12.21b | 1.02c | |
| LSD (5%) | 0.04 | 0.05 | 0.04 | 0.07 | |
| 2021 | (0%:100%) | 83.0 | 12.88e | 11.83e | 1.05c |
| (10%:90%) | 82.3 | 12.92d | 11.90d | 1.02c | |
| (20%:80%) | 82.2 | 13.59b | 12.05c | 1.54a | |
| (30%:70%) | 82.0 | 13.84a | 12.46a | 1.37b | |
| (40%:60%) | 82.1 | 13.26c | 12.22b | 1.04c | |
| LSD (5%) | 0.01 | 0.03 | 0.03 | 0.04 |
| Season | Ca(NO3)2:(NH4)2SO4 Ratio | P | K | Ca | Mg | Na |
|---|---|---|---|---|---|---|
| (g/kg) | ||||||
| 2020 | (0%:100%) | 1.51e | 8.08e | 0.43e | 0.49e | 0.29d |
| (10%:90%) | 1.64d | 8.58d | 0.68d | 0.65d | 0.32c | |
| (20%:80%) | 1.75c | 9.53b | 0.94c | 0.77c | 0.33b | |
| (30%:70%) | 2.14a | 10.50a | 1.14a | 0.92a | 0.38a | |
| (40%:60%) | 1.84b | 9.23c | 1.07b | 0.87b | 0.34b | |
| LSD (5%) | 0.03 | 0.15 | 0.03 | 0.02 | 0.02 | |
| 2021 | (0%:100%) | 1.53e | 8.20e | 0.41e | 0.48e | 0.29d |
| (10%:90%) | 1.77d | 8.75d | 0.71d | 0.66d | 0.32c | |
| (20%:80%) | 1.85c | 9.63b | 0.95c | 0.79c | 0.33b | |
| (30%:70%) | 2.23a | 10.60a | 1.17a | 0.95a | 0.38a | |
| (40%:60%) | 1.85b | 9.35c | 1.09b | 0.88b | 0.34b | |
| LSD (5%) | 0.02 | 0.12 | 0.02 | 0.02 | 0.02 | |
| Season | Ca(NO3)2:(NH4)2SO4 Ratio | Fe | Mn | Zn | Cu | Ni |
|---|---|---|---|---|---|---|
| (mg/kg) | ||||||
| 2020 | (0%:100%) | 9.0d | 4.50c | 8.25d | 5.5d | 0.21d |
| (10%:90%) | 12.5c | 5.50c | 11.0c | 6.0c | 0.28c | |
| (20%:80%) | 15.0b | 7.50b | 13.25b | 7.0b | 0.32b | |
| (30%:70%) | 18.0a | 9.25a | 14.25a | 8.0a | 0.35a | |
| (40%:60%) | 15.5b | 8.00b | 13.75b | 7.25b | 0.33b | |
| LSD (5%) | 1.19 | 1.01 | 0.9 | 0.49 | 0.01 | |
| 2021 | (0%:100%) | 8.5d | 4.25d | 8.3d | 5.25d | 0.22d |
| (10%:90%) | 12.75c | 5.75c | 11.5c | 6.00c | 0.29c | |
| (20%:80%) | 15.5b | 7.50b | 13.5b | 7.25b | 0.33b | |
| (30%:70%) | 18.75a | 9.50a | 14.8a | 8.25a | 0.36a | |
| (40%:60%) | 15.75b | 8.25b | 13.8b | 7.50b | 0.33b | |
| LSD (5%) | 0.86 | 0.81 | 0.9 | 0.67 | 0.01 | |
| Anon [53] | 150 | 10 | ||||
| FAO/WHO [54] | 70 | |||||
| Pomegranate fruits [55] | 80.54 | 34.56 | 22.19 | 4.51 | 26.87 | |
| Fruit Macro- and Micronutrients Content | Fruit Quality Attributes | |||||
|---|---|---|---|---|---|---|
| Vitamin C | Anthocyanin | Total Phenolic Content | Total Tannin Content | Total Flavonoid Content | ||
| P | Pearson Correlation | 0.933 ** | 0.872 ** | 0.904 ** | 0.940 ** | 0.875 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
| K | Pearson Correlation | 00.926 ** | 0.941 ** | 0.938 ** | 0.960 ** | 0.898 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
| Ca | Pearson Correlation | 0.973 ** | 0.959 ** | 0.979 ** | 0.975 ** | 0.984 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
| Mg | Pearson Correlation | 0.985 ** | 0.947 ** | 0.971 ** | 0.974 ** | 0.986 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
| Na | Pearson Correlation | 0.914 ** | 0.878 ** | 0.904 ** | 0.919 ** | 0.880 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
| Fe | Pearson Correlation | 0.962 ** | 0.946 ** | 0.948 ** | 0.961 ** | 0.957 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
| Mn | Pearson Correlation | 0.944 ** | 0.930 ** | 0.953 ** | 0.961 ** | 0.929 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
| Zn | Pearson Correlation | 0.934 ** | 0.938 ** | 0.933 ** | 0.932 ** | 0.965 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
| Cu | Pearson Correlation | 0.916 ** | 0.906 ** | 0.922 ** | 0.935 ** | 0.908 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
| Ni | Pearson Correlation | 0.957 ** | 0.946 ** | 0.949 ** | 0.948 ** | 0.972 ** |
| Significant (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| No. of observations | 40 | 40 | 40 | 40 | 40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Sattar, M.; Al-Obeed, R.S.; Aboukarima, A.M.; Górnik, K.; Eshra, D.H. Improvement of the Physico-Chemical Properties, Nutritional, and Antioxidant Compounds of Pomegranate Fruit cv. ‘Wonderful’ Using Integrated Fertilization. Horticulturae 2023, 9, 195. https://doi.org/10.3390/horticulturae9020195
Abdel-Sattar M, Al-Obeed RS, Aboukarima AM, Górnik K, Eshra DH. Improvement of the Physico-Chemical Properties, Nutritional, and Antioxidant Compounds of Pomegranate Fruit cv. ‘Wonderful’ Using Integrated Fertilization. Horticulturae. 2023; 9(2):195. https://doi.org/10.3390/horticulturae9020195
Chicago/Turabian StyleAbdel-Sattar, Mahmoud, Rashid S. Al-Obeed, Abdulwahed M. Aboukarima, Krzysztof Górnik, and Dalia H. Eshra. 2023. "Improvement of the Physico-Chemical Properties, Nutritional, and Antioxidant Compounds of Pomegranate Fruit cv. ‘Wonderful’ Using Integrated Fertilization" Horticulturae 9, no. 2: 195. https://doi.org/10.3390/horticulturae9020195
APA StyleAbdel-Sattar, M., Al-Obeed, R. S., Aboukarima, A. M., Górnik, K., & Eshra, D. H. (2023). Improvement of the Physico-Chemical Properties, Nutritional, and Antioxidant Compounds of Pomegranate Fruit cv. ‘Wonderful’ Using Integrated Fertilization. Horticulturae, 9(2), 195. https://doi.org/10.3390/horticulturae9020195





