The Parasitoid Hyposoter didymator Can Transmit a Broad Host Range Baculovirus in a Two Host System
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Baculovirus Isolates
2.3. Improvements of H. didymator Rearing on S. littoralis Host in Laboratory
2.4. Preference of H. didymator for S. exigua and S. littoralis Hosts
2.5. Transmission of AcMNPV by H. didymator to S. exigua and S. littoralis Hosts
2.6. Statistical Analysis
3. Results
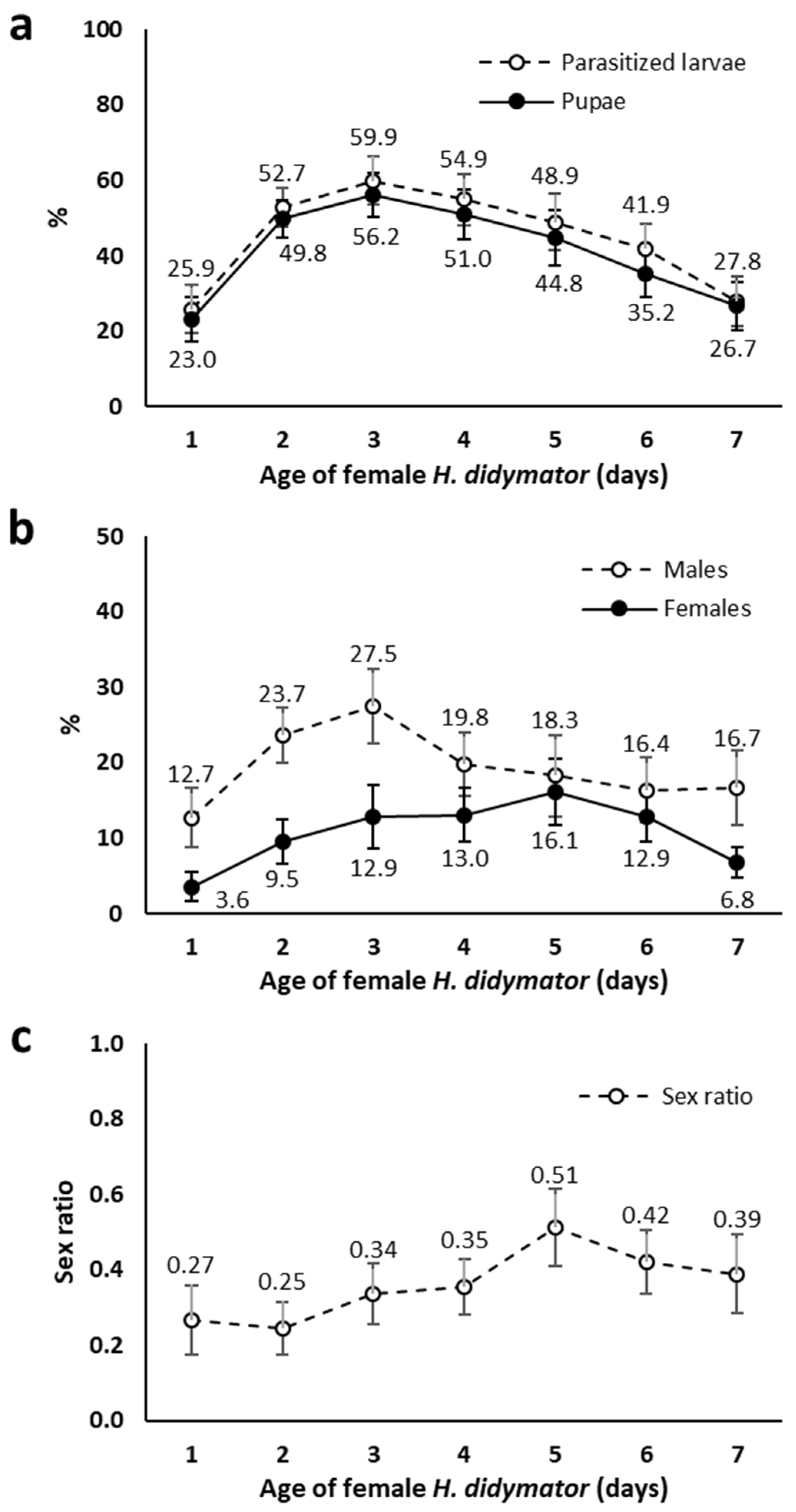
3.1. Improvements of H. didymator Rearing on S. littoralis Host in Laboratory
3.2. Preference of H. didymator for S. exigua and S. littoralis Hosts and Transmission of AcMNPV
4. Discussion
4.1. Improvements of H. didymator Rearing on S. littoralis Host in Laboratory
4.2. Transmission of AcMNPV by H. didymator to S. exigua and S. littoralis Hosts
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szewczyk, B.; de Souza, M.L.; de Castro, M.E.B.; Moscardi, M.L.; Moscardi, F. Baculovirus biopesticides. In Pesticides: Formulations, Effects, Fate; Stoytcheva, M., Ed.; IntechOpen: London, UK, 2011; pp. 25–36. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009, establishing a framework for Community action to achieve the sustainable use of pesticides. OJEU 2009, 309, 71–86. [Google Scholar]
- Czaja, K.; Góralczyk, K.; Strucinski, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides-Towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- APRD. Arthropod Pesticide Resistance Database. Michigan State University. Available online: http://www.pesticideresistance.org (accessed on 15 September 2022).
- CABI. Invasive Species Compendium. Spodoptera exigua (beet armyworm). Available online: https://www.cabi.org/isc/datasheet/29808 (accessed on 7 April 2022).
- CABI. Invasive Species Compendium. Spodoptera littoralis (cotton leafworm). Available online: https://www.cabi.org/isc/datasheet/51070 (accessed on 7 April 2022).
- Morales, J.; Medina, P.; Viñuela, E. The influence of two endoparasitic wasps, Hyposoter didymator and Chelonus inanitus, on the growth and food consumption of their host larva Spodoptera littoralis. BioControl 2007, 52, 145–160. [Google Scholar] [CrossRef]
- Hafeez, M.; Ullah, F.; Khan, M.M.; Li, X.; Zhang, Z.; Shah, S.; Imran, M.; Assiri, M.A.; Fernández-Grandon, G.M.; Desneux, N.; et al. Metabolic-based insecticide resistance mechanism and ecofriendly approaches for controlling of beet armyworm Spodoptera exigua: A review. Environ. Sci. Pollut. Res. Int. 2022, 29, 1746–1762. [Google Scholar] [CrossRef]
- García-Martín, M.; Gámez, M.; Torres-Ruiz, A.; Cabello, T. Functional response of Chelonus oculator (Hymenoptera: Braconidae) to temperature and its consequences to parasitism. Community Ecol. 2008, 9, 45–51. [Google Scholar] [CrossRef]
- Khafagi, W.E.; Hegazi, E.M. Does superparasitism improve host suitability for parasitoid development? A case study in the Microplitis rufiventris-Spodoptera littoralis system. BioControl 2008, 53, 427–438. [Google Scholar] [CrossRef]
- Garay, J.; Sebestyén, Z.; Varga, Z.; Gámez, M.; Torres, A.; Belda, J.E.; Cabello, T. A new multistage dynamic model for biological control exemplified by the host–parasitoid system Spodoptera exigua–Chelonus oculator. J. Pest Sci. 2015, 88, 343–358. [Google Scholar] [CrossRef]
- Li, S.J.; Huang, J.P.; Chang, Y.Y.; Quan, S.Y.; Yi, W.T.; Chen, Z.S.; Liu, S.Q.; Cheng, X.W.; Huang, G.H. Development of Microplitis similis (Hymenoptera: Braconidae) on two candidate host species, Spodoptera litura and Spodoptera exigua (Lepidoptera: Noctuidae). Fla. Entomol. 2015, 98, 736–741. [Google Scholar] [CrossRef]
- Agbodzavu, M.K.; Lagat, Z.O.; Gikungu, M.; Rwomushana, I.; Ekesi, S.; Fiaboe, K.K.M. Performance of the newly identified endoparasitoid Cotesia icipe Fernandez-Triana & Fiaboe on Spodoptera littoralis (Boisduval). J. Appl. Entomol. 2018, 142, 646–653. [Google Scholar] [CrossRef]
- Mohamed, H.O. Efficacy of the parasitoid, Trichogrammatoidea bactrae Nagaraja (Hymenoptera: Trichogrammatidae) on the cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) egg masses. Egypt. J. Biol. Pest Control 2021, 31, 20. [Google Scholar] [CrossRef]
- Hegazi, E.M.; Khafagi, W.E. Reduction of larval resistance and adult reproductive capacity in Spodoptera littoralis at different degrees of superparasitization by Microplitis rufiventris. Physiol. Entomol. 2022, 47, 117–125. [Google Scholar] [CrossRef]
- Magaña, A.J.; Dáder, B.; Sancho, G.; Adán, A.; Morales, I.; Viñuela, E. Comparison of the parasitization of Chelonus inanitus L. (Hymenoptera: Braconidae) in two Spodoptera pests and evaluation of the procedure for its production. Insects 2022, 13, 99. [Google Scholar] [CrossRef]
- Moscardi, F.; de Souza, M.L.; de Castro, M.E.B.; Moscardi, M.L.; Szewczyk, B. Baculovirus pesticides: Present state and future perspectives. In Microbes and Microbial Technology; Ahmad, I., Ahmad, F., Pichtel, J., Eds.; Springer: New York, NY, USA, 2011; pp. 415–445. [Google Scholar]
- Eberle, K.E.; Jehle, J.A.; Huber, J. Microbial control of crop pests using insect viruses. In Integrated Pest Management: Principles and Practice; Abrol, D.P., Shankar, U., Eds.; CAB International: Wallingford, UK, 2012; pp. 281–298. [Google Scholar]
- Beas-Catena, A.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. Baculovirus biopesticides: An overview. J. Ani. Plant Sci. 2014, 24, 362–373. [Google Scholar]
- Haase, S.; Sciocco-Cap, A.; Romanowski, V. Baculovirus insecticides in Latin America: Historical overview, current status and future perspectives. Viruses 2015, 7, 2230–2267. [Google Scholar] [CrossRef]
- Abbas, M.S.T.; Boucias, D.G. Interaction between nuclear polyhedrosis virus-infected Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae and predator Podisus maculiventris (Say) (Hemiptera: Pentatomidae). Environ. Entomol. 1984, 13, 599–602. [Google Scholar] [CrossRef]
- Abbas, M.S.T. Interactions between nuclear polyhedrosis virus, host and predators. J. Plant Dis. Prot. 1988, 95, 606–610. [Google Scholar]
- Lee, Y.; Fuxa, J.H. Transport of wild-type and recombinant nucleopolyhedroviruses by scavenging and predatory arthropods. Microb. Ecol. 2000, 39, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Castillejos, V.; Cisneros, J.; Goulson, D.; Cave, R.D.; García, L.; Caballero, P.; Williams, T. The potential of Chrysoperla rufilabris and Doru taeniatum as agents for dispersal of Spodoptera frugiperda nucleopolyhedrovirus in maize. Entomol. Exp. Appl. 2001, 98, 353–359. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gani, M.; Jasrotia, P.; Srivastava, K. Development of the predator Eocanthecona furcellata on different proportions of nucleopolyhedrovirus infected Spodoptera litura larvae and potential for predator dissemination of virus in the field. BioControl 2013, 58, 543–552. [Google Scholar] [CrossRef]
- Gutiérrez-Cárdenas, O.G.; Adán, A.; Beperet, I.; Medina, P.; Caballero, P.; Garzón, A. The role of Chrysoperla carnea (Steph.) (Neuroptera: Chrysopidae) as a potential dispersive agent of noctuid baculoviruses. Insects 2020, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Cossentine, J.E. The parasitoid factor in the virulence and spread of lepidopteran baculoviruses. Virol. Sin. 2009, 24, 305–314. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, J.X.; Chen, Y.J.; Wang, J.Y.; Ji, X.Y.; Wan, N.F. Contribution of a parasitoid species to multiplication and transmission of a multiple nucleopolyhedrovirus in caterpillars. J. Appl. Entomol. 2020, 144, 308–314. [Google Scholar] [CrossRef]
- Washburn, J.O.; Haas-Stapleton, E.J.; Tan, F.F.; Beckage, N.E.; Volkman, L.E. Co-infection of Manduca sexta larvae with polydnavirus from Cotesia congregata increases susceptibility to fatal infection by Autographa californica M nucleopolyhedrovirus. J. Insect Physiol. 2000, 46, 179–190. [Google Scholar] [CrossRef]
- Escribano, A.; Williams, T.; Goulson, D.; Cave, R.D.; Chapman, J.W.; Caballero, P. Consequences of interspecific competition on the virulence and genetic composition of a nucleopolyhedrovirus in Spodoptera frugiperda larvae parasitized by Chelonus insularis. Biocontrol Sci. Technol. 2001, 11, 649–662. [Google Scholar] [CrossRef]
- Cai, Y.; Fan, J.; Sun, S.; Wang, F.; Yang, K.; Li, G.; Pang, Y. Interspecific interaction between Spodoptera exigua multiple nucleopolyhedrovirus and Microplitis bicoloratus (Hymenoptera: Braconidae: Microgastrina) in Spodoptera exigua (Lepidoptera: Noctuidae) larvae. J. Econ. Entomol. 2012, 105, 1503–1508. [Google Scholar] [CrossRef]
- Irabagon, T.A.; Brooks, W.M. Interaction of Campoletis sonorensis and a nuclear polyhedrosis virus in larvae of Heliothis virescens. J. Econ. Entomol. 1974, 67, 229–331. [Google Scholar] [CrossRef]
- Azam, A.; Kunimi, Y.; Inoue, M.N.; Nakai, M. Effect of granulovirus infection of Spodoptera litura (Lepidoptera: Noctuidae) larvae on development of the endoparasitoid Chelonus inanitus (Hymenoptera: Braconidae). Appl. Entomol. Zool. 2016, 51, 479–488. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Z.; Lu, Z.; Li, J.; Yang, Y.; Zhang, Q.; Liu, X. Interaction between endoparasitoid Microplitis mediator (Hymenoptera: Braconidae) and nucleopolyhedrovirus in larvae of Helicoverpa armigera (Lepidoptera: Noctuidae). Biol. Control 2017, 115, 152–156. [Google Scholar] [CrossRef]
- Rivkin, H.; Kroemer, J.A.; Bronshtein, A.; Belausov, E.; Webb, B.A.; Chejanovsky, N. Response of immunocompetent and immunosuppressed Spodoptera littoralis larvae to baculovirus infection. J. Gen. Virol. 2006, 87, 2217–2225. [Google Scholar] [CrossRef]
- Miranda-Fuentes, P.; Quesada-Moraga, E.; Aldebis, H.K.; Yousef-Naef, M. Compatibility between the endoparasitoid Hyposoter didymator and the entomopathogenic fungus Metarhizium brunneum: A laboratory simulation for the simultaneous use to control Spodoptera littoralis. Pest Manag. Sci. 2020, 76, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.S.; Myers, J.H. The ecology and evolution of insect baculoviruses. Annu. Rev. Ecol. Evol. 2003, 34, 239–272. [Google Scholar] [CrossRef]
- Belda, I.M.; Beperet, I.; Williams, T.; Caballero, P. Genetic variation and biological activity of two closely related alphabaculoviruses during serial passage in permissive and semi-permissive heterologous hosts. Viruses 2019, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.I.; Viñuela, E. Improvements in rearing method for Hyposoter didymator (Hymenoptera: Ichneumonidae), considering sex allocation and sex determination theories used for Hymenoptera. Biol. Control 2007, 43, 271–277. [Google Scholar] [CrossRef]
- Hatem, A.E.; Shawer, D.M.; Vargas-Osuna, E. Parasitism and optimization of Hyposoter didymator (Hymenoptera: Ichneumonidae) rearing on Spodoptera littoralis and Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 2016, 109, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Elvira, S.; Gorría, N.; Muñoz, D.; Williams, T.; Caballero, P. A simplified low-cost diet for rearing Spodoptera exigua (Lepidoptera: Noctuidae) and its effect on S. exigua nucleopolyhedrovirus production. J. Econ. Entomol. 2010, 103, 17–24. [Google Scholar] [CrossRef]
- Dáder, B.; Aguirre, E.; Caballero, P.; Medina, P. Synergy of lepidopteran nucleopolyhedroviruses AcMNPV and SpliNPV with insecticides. Insects 2020, 11, 316. [Google Scholar] [CrossRef]
- Statgraphics Technologies Inc. Statgraphics Centurion 18; Statgraphics Technologies Inc.: The Plains, VA, USA, 2020. [Google Scholar]
- Bahena, F.; González, M.; Viñuela, E.; del Estal, P. Establecimiento de la especie huésped óptima para la cría en laboratorio del parasitoide de noctuidos Hyposoter didymator (Thunberg). Bol. San. Veg. Plagas 1998, 24, 465–472. [Google Scholar]
- Miranda-Fuentes, P.; Yousef-Yousef, M.; Valverde-García, P.; Rodríguez-Gómez, I.M.; Garrido-Jurado, I.; Quesada-Moraga, E. Entomopathogenic fungal endophyte-mediated tritrophic interactions between Spodoptera littoralis and its parasitoid Hyposoter didymator. J. Pest Sci. 2021, 94, 933–945. [Google Scholar] [CrossRef]
- Queiroz-Santos, L.; Casagrande, M.M.; Specht, A. Morphological characterization of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae: Heliothinae). Neotrop. Entomol. 2018, 47, 517–542. [Google Scholar] [CrossRef]
- Mackauer, M.; Sequeira, R. Patterns of development in insect parasite. In Parasites and Pathogens of Insects; Beckage, N.E., Thompson, S.N., Federici, B.A., Eds.; Academic: New York, NY, USA, 1993; pp. 1–23. [Google Scholar]
- Schneider, M.I. Optimización del Empleo en Lucha Biológica de Hyposoter didymator (Thunberg) y Evaluación Ecotoxicológica de Modernos Plaguicidas en Laboratorio. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 10 December 2002. [Google Scholar]
- Dorémus, T.; Jouan, V.; Urbach, S.; Cousserans, F.; Wincker, P.; Ravallec, M.; Wajnberg, E.; Volkoff, A.N. Hyposoter didymator uses a combination of passive and active strategies to escape from the Spodoptera frugiperda cellular immune response. J. Insect Physiol. 2013, 59, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.A.; Hutchinson, P.; Dutch, M.E.; Lawrence, P.J.; Michael, P.J. An efficient method of mass rearing two introduced parasitoids of Noctuids (Lepidoptera: Noctuidae). Aust. J. Entomol. 1993, 32, 79–80. [Google Scholar] [CrossRef]
- Bahena, F.; Adán, A.; González, M.; Viñuela, E.; del Estal, P. Método de cría de Hyposoter didymator (Thunberg) (Hymenoptera: Ichneumonidae), parasitoide de noctuidos de importancia agrícola. Bol. San. Veg. Plagas 1998, 24, 975–984. Available online: https://www.mapa.gob.es/ministerio/pags/Biblioteca/Revistas/pdf_plagas%2FBSVP24-04-Adenda-0975-0984.pdf (accessed on 7 April 2022).
- Hegazi, E.M.; El-Aziz, G.M.A.; El-Shazly, A.Y.; Khafagi, W.E. Influence of host age and host deprivation on longevity, egg load dynamics and oviposition rate in Microplitis rufiventris. Insect Sci. 2007, 14, 485–495. [Google Scholar] [CrossRef]
- Khatri, D.; He, X.; Wang, Q. Mating behaviour and egg maturation in Diadegma semiclausum Hellen (Hymenoptera: Ichneumonidae). N. Z. Plant Prot. 2009, 62, 174–178. [Google Scholar] [CrossRef]
- Murillo, H.; Hunt, D.W.; VanLaerhoven, S.L. Fecundity and life table parameters of Campoletis sonorensis (Hymenoptera: Ichneumonidae), an endoparasitoid of the cabbage looper Trichoplusia ni Hübner (Lepidoptera: Noctuidae), under laboratory conditions. Biocontrol Sci. Technol. 2012, 22, 125–134. [Google Scholar] [CrossRef]
- Korenko, S.; Potopová, V.; Satrapová, J.; Pekár, S. Life history of the spider parasitoid Zatypota percontatoria (Hymenoptera: Ichneumonidae). Entomol. Sci. 2016, 19, 104–111. [Google Scholar] [CrossRef]
- Adel-Sattar, M.M.; El-Malla, M.A.; Ghoneim, Y.F.; Singab, M. Pyrethroids and biocides resistance in field strains of the cotton leaf worm, Spodoptera littoralis (Boisd.) during 2006-2008 cotton seasons. Aust. J. Basic Appl. Sci. 2012, 6, 305–308. [Google Scholar]
- Elmenofy, W.; Salem, R.; Osman, E.; Yasser, N.; Abdelmawgod, A.; Saleh, M.; Zaki, A.; Hanafy, E.; Tamim, S.; Amin, S.; et al. Evaluation of two viral isolates as a potential biocontrol agent against the Egyptian cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Lasa, R.; Pagola, I.; Ibanez, I.; Belda, J.E.; Williams, T.; Caballero, P. Efficacy of Spodoptera exigua multiple nucleopolyhedrovirus as a biological insecticide for beet armyworm control in greenhouses of southern Spain. Biocontrol Sci. Technol. 2007, 17, 221–232. [Google Scholar] [CrossRef]
- El-Sheikh, E.A. Efficacy of Spodoptera littoralis nucleopolyhedrovirus on Spodoptera frugiperda (JE Smith) and Spodoptera exigua (Hübner): Virulence, biological effects, and inhibition of juvenile hormone esterase. Egypt. J. Biol. Pest Control 2015, 25, 587–595. [Google Scholar]
- Woolhouse, M.E.; Taylor, L.H.; Haydon, D.T. Population biology of multihost pathogens. Science 2001, 292, 1109–1112. [Google Scholar] [CrossRef]
- Goulson, D. Can host susceptibility to baculovirus infection be predicted from host taxonomy or life history? Environ. Entomol. 2003, 32, 61–70. [Google Scholar] [CrossRef]
- Reudler Talsma, J.H.; Elzinga, J.A.; Harvey, J.A.; Biere, A. Optimum and maximum host sizes at parasitism for the endoparasitoid Hyposoter didymator (Hymenoptera: Ichneumonidae) differ greatly between two host species. Environ. Entomol. 2007, 36, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Frayssinet, M.; Audiot, P.; Cusumano, A.; Pichon, A.; Malm, L.E.; Jouan, V.; Vabre, M.; Malavieille, S.; Delalande, M.; Vargas-Osuna, E.; et al. Western European populations of the Ichneumonid wasp Hyposoter didymator belong to a single taxon. Front. Ecol. Evol. 2019, 7, 20. [Google Scholar] [CrossRef]
- Flanders, S.E. The sex-ratio in the Hymenoptera: A function of the environment. Ecology 1942, 23, 120–121. [Google Scholar] [CrossRef]
- Moreau, G.; Eveleigh, E.S.; Lucarotti, C.J.; Morin, B.; Quiring, D.T. Opposing effects of mortality factors on progeny operational sex ratio may thwart adaptive manipulation of primary sex ratio. Ecol. Evol. 2017, 7, 4973–4981. [Google Scholar] [CrossRef] [PubMed]
- Nusawardani, T.; Ruberson, J.R.; Obrycki, J.J.; Bonning, B.C. Effects of a protease-expressing recombinant baculovirus insecticide on the parasitoid Cotesia marginiventris (Cresson). Biol. Control 2005, 35, 46–54. [Google Scholar] [CrossRef]


| S. littoralis Age | S. littoralis Parasitized Larvae (%) | H. didymator Pupae (%) | H. didymator Adults (%) | H. didymator Females (%) | H. didymator Males (%) | Sex Ratio 3 |
|---|---|---|---|---|---|---|
| Young L2 1 | 48.85 ± 3.52 | 47.80 ± 3.53 | 42.24 ± 3.56 | 7.98 ± 1.59 a | 34.26 ± 3.68 | 0.24 ± 0.05 |
| Mature L2 | 51.70 ± 3.22 | 50.51 ± 3.17 | 42.79 ± 3.01 | 10.44 ± 1.79 a | 32.36 ± 2.82 | 0.25 ± 0.04 |
| Young L3 2 | 52.10 ± 3.54 | 50.67 ± 3.55 | 42.02 ± 2.82 | 13.65 ± 2.30 ab | 28.37 ± 2.70 | 0.30 ± 0.04 |
| Mature L3 | 58.17 ± 3.38 | 55.31 ± 3.34 | 44.60 ± 3.09 | 17.70 ± 2.44 b | 26.90 ± 3.02 | 0.39 ± 0.05 |
| Length of parasitization (h) | ||||||
| 2 | 42.14 ± 4.90 a | 37.14 ± 4.68 a | 30.71 ± 4.18 a | 5.71 ± 2.41 a | 25.00 ± 4.38 | 0.20 ± 0.08 |
| 24 | 72.86 ± 4.90 b | 66.43 ± 5.70 b | 42.14 ± 5.02 ab | 17.14 ± 4.22 b | 25.00 ± 4.13 | 0.37 ± 0.08 |
| 48 | 64.08 ± 4.57 b | 60.97 ± 4.45 b | 43.27 ± 4.10 b | 15.37 ± 3.78 ab | 27.90 ± 4.70 | 0.34 ± 0.07 |
| Type of parasitization | ||||||
| Individual | 58.93 ± 4.37 a | 57.01 ± 4.28 a | 39.00 ± 3.60 a | 7.53 ± 1.82 | 31.47 ± 3.07 | 0.38 ± 0.12 |
| Collective | 70.82 ± 3.14 b | 69.39 ± 3.26 b | 48.76 ± 3.04 b | 10.97 ± 1.60 | 37.79 ± 2.91 | 0.34 ± 0.07 |
| Parameter (%) | Host Species | Hyposoter didymator | HS | Hd | HS × Hd | |||
|---|---|---|---|---|---|---|---|---|
| Non-Contaminated | AcMNPV-Contaminated | Mean | ||||||
| Natural mortality 1 | S. exigua | 7.60 ± 1.31 | 2.22 ± 0.92 | 5.27 ± 0.94 | F | 1.99 | 16.22 | 0.31 |
| S. littoralis | 5.29 ± 1.54 | 1.38 ± 0.61 | 3.40 ± 0.91 | p | 0.16 | <0.001 | 0.58 | |
| Mean | 6.60 ± 1.00 A | 1.81 ± 0.55 B | ||||||
| Noctuid adults 2 | S. exigua | 36.27 ± 6.28 | 28.87 ± 7.20 | 33.07 ± 4.71 a | F | 21.01 | 0.49 | 3.81 |
| S. littoralis | 51.83 ± 4.33 | 67.50 ± 5.45 | 59.41 ± 3.69 b | p | <0.001 | 0.49 | 0.06 | |
| Mean | 43.00 ± 4.18 | 47.57 ± 5.70 | ||||||
| Parasitoid pupae 3 | S. exigua | 56.13 ± 6.02 | 35.67 ± 8.25 | 47.28 ± 5.15 | F | 2.68 | 8.04 | 0.32 |
| S. littoralis | 42.88 ± 3.91 | 29.12 ± 5.36 | 36.22 ± 3.47 | p | 0.11 | 0.01 | 0.57 | |
| Mean | 50.40 ± 3.92 A | 32.50 ± 4.94 B | ||||||
| Parasitoid adults 4 | S. exigua | 48.75 ± 5.53 | 26.03 ± 7.69 | 37.39 ± 3.80 | F | 2.80 | 7.08 | 2.32 |
| S. littoralis | 31.37 ± 2.77 | 25.21 ± 4.61 | 28.29 ± 3.87 | p | 0.10 | 0.01 | 0.13 | |
| Mean | 40.06 ± 3.58 A | 25.61 ± 4.09 B | ||||||
| Sex ratio 5 | S. exigua | 0.13 ± 0.04 | 0.38 ± 0.10 | 0.26 ± 0.05 | F | 0.63 | 22.17 | 0.41 |
| S. littoralis | 0.14 ± 0.05 | 0.48 ± 0.08 | 0.31 ± 0.04 | p | 0.43 | <0.001 | 0.53 | |
| Mean | 0.13 ± 0.04 A | 0.43 ± 0.05 B | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morel, A.; Leigh, B.; Muñoz, D.; Caballero, P.; Medina, P.; Dáder, B. The Parasitoid Hyposoter didymator Can Transmit a Broad Host Range Baculovirus in a Two Host System. Horticulturae 2023, 9, 170. https://doi.org/10.3390/horticulturae9020170
Morel A, Leigh B, Muñoz D, Caballero P, Medina P, Dáder B. The Parasitoid Hyposoter didymator Can Transmit a Broad Host Range Baculovirus in a Two Host System. Horticulturae. 2023; 9(2):170. https://doi.org/10.3390/horticulturae9020170
Chicago/Turabian StyleMorel, Ariel, Brendan Leigh, Delia Muñoz, Primitivo Caballero, Pilar Medina, and Beatriz Dáder. 2023. "The Parasitoid Hyposoter didymator Can Transmit a Broad Host Range Baculovirus in a Two Host System" Horticulturae 9, no. 2: 170. https://doi.org/10.3390/horticulturae9020170
APA StyleMorel, A., Leigh, B., Muñoz, D., Caballero, P., Medina, P., & Dáder, B. (2023). The Parasitoid Hyposoter didymator Can Transmit a Broad Host Range Baculovirus in a Two Host System. Horticulturae, 9(2), 170. https://doi.org/10.3390/horticulturae9020170








